Abstract
Head and neck squamous cell carcinoma (HNSCC) remains a challenging clinical problem, due to the persisting high rate of local and distant failure due to the acquisition of chemo and radio-resistance. In this study, we examined if treatment with sorafenib, a potent inhibitor of Raf kinase and VEGFR, could reverse the resistant phenotype in tumor and tumor-associated endothelial cells thereby enhancing the therapeutic efficacy of currently used chemo-radiation treatment. We used both in vitro and in vivo models to test the efficacy of sorafenib either as a single agent or in combination with chemo-radiation. Sorafenib as a single agent demonstrated anti-tumor and angiogenesis properties, but the effects were more pronounced when used in combination with chemo-radiation treatment. Sorafenib significantly enhanced the anti-proliferative effects of chemo-radiation treatment by down-regulating DNA repair proteins (ERCC-1 and XRCC-1) in a dose dependent manner. In addition, combination treatment significantly inhibited tumor cell colony formation, tumor cell migration and tumor cell invasion. Combination treatment was also very effective in inhibiting VEGF-mediated angiogenesis, in vitro. In a SCID mouse xenograft model, combination treatment was very well tolerated and significantly inhibited tumor growth and tumor angiogenesis. Interestingly, following combination treatment, low dose sorafenib treatment alone was highly effective as a maintenance regimen. Taken together, our results suggest a potentially novel strategy to use sorafenib to overcome chemo and radio-resistance in tumor and tumor-associated endothelial to enhance the effectiveness of the chemo-radiation therapy.
Keywords: Sorafenib, ERCC-1, XRCC-1, chemo-radiation, HNSCC, angiogenesis
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most frequent cancer worldwide and five-year survival rate (<50%) is among the lowest of the major cancers (1, 2). Although advancements in the techniques for surgery, radiation and chemotherapy have increased the local control of HNSCC, the overall survival rates have not improved significantly over the last three decades. This poor outcome becomes even worse (20%, 5-year survival rate) for advanced stage HNSCC patients whose tumors are not amenable for surgery (3). Concurrent chemoradiation (CRT) regimen, generally employed for the treatment of advanced cases, is often associated with acute toxicity (4). In addition, the response rate is still poor and overall survival being measured in months (5). Therefore, it is imperative that new therapeutic strategies are developed to increase the long-term survival of these patients as well as decrease the adverse effects associated with CRT.
In order to develop tumor specific therapies, recent research efforts have attempted to exploit the biological differences that may exist between normal and malignant cells. One such therapeutic target for advanced head and neck tumors is EGFR, which is overexpressed in >80% of patients tumors as compared to normal mucosa (6). In addition, EGFR expression directly correlates with decreased survival in HNSCC patients (7-9). EGFR predominantly mediates its survival and proliferative function via the activation of Ras-Raf-MAPK and PI3K/Akt signaling pathways (10). HNSCC tumors are also shown to overexpress vascular endothelial growth factor (VEGF) and this overexpression has been associated with lymph node metastasis and poor survival (11, 12). We have recently shown that VEGF induces chemo and radio-resistance in endothelial cells by upregulating Bcl-2 protein (13). Moreover, Bcl-2 protects endothelial and tumor cells against radiation-induced apoptosis by upregulating the expression of survivin via the Raf-MEK-ERK pathway (14). MAPK pathway is also involved in the upregulation of DNA repair proteins particularly ERCC-1 and XRCC-1 (15) and a number of recent studies have highlighted the role of these repair proteins in the acquisition of chemo and radioresistance (16-18). ERCC-1 plays a key role in nucleotide excision repair by forming a complex with xeroderma pigmentosum complementation group F and this complex is required for the excision of damaged DNA (19). Similarly, XRCC-1 protein plays an important role in the repair of ionizing radiation-mediated double strand DNA breaks, single strand breaks or recombination repair (20, 21). Therefore, we hypothesize that targeting of Raf-kinase in head and neck cancer may enhance the therapeutic efficacy of CRT by modulating the expression of DNA repair proteins and inhibiting the acquisition of chemo and radio-resistance by tumor and tumor-associated endothelial cells.
Sorafenib is an oral multikinase inhibitor (22, 23) currently being used in clinics to treat patients with advanced renal cell carcinoma (RCC), hepatocellular carcinoma (HCC) and thyroid cancer (24-26). Initially, sorafenib was identified as a potent inhibitor of Raf serine/threonine kinase isoforms, in vitro. Sorafenib since has been shown to have potent inhibitory effects on other Raf isoforms as well (27, 28). In addition to targeting Raf kinase, sorafenib also inhibits proangiogenic mediators including VEGF receptor (VEGFR)-1, VEGFR-2, VEGFR-3 and platelet-derived growth factor receptor-β (PDGFR-β) (27). In a phase II clinical trial for patients with HNSCC, sorafenib was well tolerated but showed only modest anti-cancer activity (29). However, recent studies have shown that the anti-cancer activity of sorafenib is significantly enhanced when combined with chemotherapy or signal transduction inhibitors in advanced HCC and gastric cancer patients (30, 31). Therefore, full clinical activity of sorafenib may be achieved by combining it with CRT or other signal transduction inhibitors.
In this study, we used both in vitro and in vivo models to investigate if treatment with sorafenib could enhance the anti-tumor and anti-angiogenesis effects of CRT in HNSCC. Taken together, our results demonstrate that sorafenib can be successfully combined with low dose chemo-radiation regimen to potentiate its anti-tumor and anti-angiogenesis activities. Our study provides a scientific rationale to evaluate this or a similar combination strategy for clinical trials.
MATERIALS AND METHODS
Cell culture and reagents
Primary human dermal microvascular endothelial cells (ECs) were purchased from Lonza (Walkersville, MD) and were characterized by immunofluorescent staining for von Willebrand’s antigen (positive) and smooth muscle α-actin (negative). ECs were maintained in Endothelial Cell Basal Medium-2 (EBM-2) containing 5% FBS and growth supplements. CAL27 was obtained from ATCC and UM-SCC-74A was obtained from Dr. Thomas E. Carey, University of Michigan. Both these HNSCC cell lines were authenticated by genotyping and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS. ERCC-1 antibody for Western blotting (clone FL297) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and ERCC-1 antibody for immunohistochemistry was purchased from Lab Vision (Fremont, CA). pAkt, pERK1/2, tubulin and XRCC-1 antibodies were purchased from Cell Signaling (Danvers, MA).
Transfection with siRNA
Tumor cells were transfected with siRNA for ERCC-1 or XRCC-1 or ERCC-1 and XRCC-1 together using siGENOME SMART pool siRNAs from Dharmacon (Lafayette, CO) according to the manufacturer’s instructions. Seventy two hours post transfection, cells were either used for proliferation experiments or whole cell lysates were prepared for Western blotting.
Cell proliferation assay
HDMEC, CAL27 and UM-SCC-74A cells were treated with different concentrations of sorafenib (sorafenib tosylate, LC Laboratories, Woburn, MA), cisplatin (Sigma, Saint Louis, MO) or radiation. For combination treatment, cells were treated with sorafenib (5 μM), cisplatin (2 μM) and radiation (7.5 Gy) with a gap of 1 hour in between each. After 72 hrs, cell proliferation was assessed using a MTT assay Kit (Roche Diagnostics, Indianapolis, IN). The percentage cell growth inhibition for each group was calculated by adjusting the control group to 100%.
Tumor cell colony formation assay
Colony formation assay was performed in 35mm culture petri dishes as described previously (32). Tumor cells were treated with sorafenib (5 μM), cisplatin (2 μM) or radiation (7.5 Gy) alone or in combination. After 14 days of culture, colonies were stained with crystal violet (0.005%) for 1 hr and counted using Nikon Eclipse Ti microscope with DS-Fi1 camera at 40x magnification.
Western Blot Analysis
Whole cell lysates were separated by 4-12% NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred onto PVDF membranes Nonspecific binding was blocked by incubating the blots with 3% BSA in Tris buffered saline containing 0.1% Tween-20 (TBST) for 1 hr at room temperature (RT). The blots were then incubated with primary antibody in TBST + 3% BSA at 4°C overnight. After washing with TBST, the blots were incubated with horseradish peroxidase-conjugated sheep anti-mouse IgG (1:10,000) or with donkey anti-rabbit IgG (1:10,000) for 1 hr at RT. An ECL-plus detection system (Amersham Life Sciences, Piscataway, NJ) was used to detect specific protein bands. Protein loading in all the experiments was normalized by stripping the blots and then re-probing with anti-tubulin antibody. Alpha Innotech (San Leandro, CA) imaging software was used to quantify Western blot bands.
Tumor and endothelial cell motility assay
Cell motility assay was performed in 6-well plates. A fine scratch in the form of groove was made using a sterile pipette tip in about 90% confluent cells. Cells were then treated with sorafenib (5 μM), cisplatin (2 μM) or radiation (7.5 Gy) alone or in combination. The migration of cells were monitored microscopically using Nikon Eclipse Ti microscope with DS-Fi1 camera.
Tumor cell invasion assays
Tumor cells invasion was performed on Matrigel coated 24-well plate inserts (8 μM pore size, BD Biosciences) as described previously (33). The number of cells that had invaded through the Matrigel were counted in 5 random high power fields.
Matrigel in vitro endothelial tube formation assay
Endothelial cell tube formation was performed on Matrigel coated chamber slides as described previously (34). Each assay was photographed (Nikon Eclipse Ti microscope with DS-Fi1 camera) at 40x magnification and total area occupied by endothelial cell derived tubes in each chamber was calculated using software (NIS-Elements-Basic Research, Nikon, Melville, NY) and expressed as an angiogenic score.
SCID mouse flank xenograft model
6-8 week old SCID mice (NCI) were used in all the in vivo experiments (33). Tumor cells (UM-SCC-74A or CAL27, 1 × 106) and endothelial cells (1 × 106) were mixed with 100 μl of Matrigel and injected in the flanks of SCID mice. After 8 days, mice were stratified into different groups (five mice per group), so that the mean tumor volume in each group was comparable. At days 8, 11, 14, 17, 21, 24 and 28 animals were treated with sorafenib (10 mg/kg) and cisplatin (2 mg/kg) via I.P. injections. One day following sorafenib and/or cisplatin treatment, on days 9, 12, 15, 18, 22, 25 and 29 flank tumors were treated with radiation (3 Gy) while the rest of body was shielded from irradiation by a lead shield. Tumor volume measurements [volume (mm3) = L × W2/2 (length L, mm; width W, mm)] began on day 6 and continued twice a week until the end of the study. After 36 days, primary tumors were carefully removed and analyzed for tumor angiogenesis.
For sorafenib maintenance dose study, at day 36, ten animals from combination treatment group were stratified into two groups (five mice per group). One group was treated with sorafenib (10 mg/kg) twice a week and the other group was untreated control. Tumor volume was measured as described above.
Immunohistochemistry for angiogenesis, ERCC-1 and XRCC-1
Tumor sections were stained for angiogenesis (Von Willebrand factor), ERCC-1 and XRCC-1 as described previously (34). Microvessel density was calculated by counting 5 random high power fields (200x). Percentage positive cells for ERCC-1 or XRCC-1 were calculated by quantifying ERCC-1 and XRCC-1 positive cells and the total number of cells (positive and negative) present in 5 randomly selected high power fields (400x) of each tumor samples.
Statistical analysis
Data from all the experiments are expressed as mean ± SEM. Statistical differences were determined by two-way analysis of variance and Student’s t test. A p value of <0.05 was considered significant.
RESULTS
Sorafenib induces a dose dependent inhibition of tumor and endothelial cell proliferation
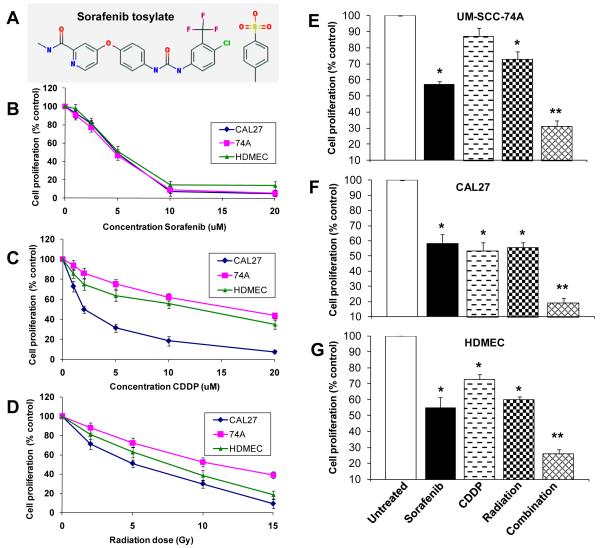
Head and neck tumor cell lines (UM-SCC-74A and CAL27) and endothelial cells (HDMEC) demonstrated similar sensitivities to sorafenib treatment (Fig. 1B). Sorafenib treatment for 72 hours resulted in 10%, 23%, 54%, 91%, and 95% growth inhibition for UM-SCC-74A, 7%, 18%, 51%, 93% and 95% growth inhibition for CAL27 cells and 2%, 18%, 49%, 86% and 87% growth inhibition for HDMEC at 1 μM, 2.5 μM, 5 μM, 10 μM and 20 μM respectively (Fig. 1B). In contrast, UM-SCC-74A cell line was highly resistant to both cisplatin (CDDP) and radiation treatment, whereas CAL27 cell line was quite sensitive to both cisplatin and radiation treatment (Fig. 1C-D).
Figure 1. Sorafenib enhances the anti-proliferative effects of chemo-radiation treatment.
A; Chemical structure of sorafenib tosylate. B-D; UM-SCC-74A, CAL27 or HDMECs were treated with different concentrations of sorafenib, cisplatin (CDDP) or radiation. E-G; Cells were treated with sorafenib, cisplatin or radiation alone or in combination. After 72 hrs, cell proliferation was assessed by MTT assay. The percentage cell growth inhibition for each group was calculated by adjusting the control group to 100%. *, represents a significant difference (p<0.05) as compared to the untreated control group and **, represents a significant difference (p<0.05) as compared to the single or double treatment groups.
Combination of low doses of sorafenib, cisplatin and radiation significantly inhibits endothelial cell and tumor cell proliferation
Combination treatment significantly inhibited cell growth (70%, 81% and 75% for UM-SCC-74A, CAL27 and HDMEC respectively, Fig. 1E-G). This cell growth inhibition by combination treatment was significantly higher than treatment with sorafenib alone (43%, 42% and 46% for UM-SCC-74A, CAL27 and HDMEC respectively), cisplatin alone (13%, 47% and 28% for UM-SCC-74A, CAL27 and HDMEC respectively) or radiation alone (28%, 45% and 41% for UM-SCC-74A, CAL27 and HDMEC respectively) or in dual combinations of sorafenib + cisplatin (45%, 57% and 54% for UM-SCC-74A, CAL27 and HDMEC respectively), sorafenib + radiation (49%, 59% and 53% for UM-SCC-74A, CAL27 and HDMEC respectively) or cisplatin + radiation (41%, 43% and 56% for UM-SCC-74A, CAL27 and HDMEC respectively).
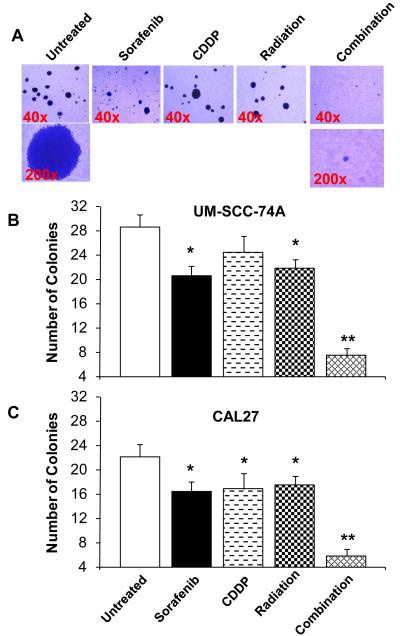
Combination treatment significantly inhibits UM-SCC-74A and CAL27 tumor cell colony formation in soft agar assay
We next investigated the effect of combination treatment on tumor cell colony formation. Similar to growth inhibition assay, combination treatment with sorafenib, cisplatin and radiation induced significant inhibition of tumor cell colony formation in both the cell lines (93% and 95% for UM-SCC-74A and CAL27 respectively, Fig. 2). Tumor cell colony formation inhibition by combination treatment was significantly higher than sorafenib alone (28% and 26% for UM-SCC-74A and CAL27, respectively), cisplatin alone (14% and 24% for UM-SCC-74A and CAL27, respectively) or radiation treatment alone (24% and 21% for UM-SCC-74A and CAL27, respectively) or in double combinations of sorafenib + cisplatin (43% and 46% for UM-SCC-74A and CAL27, respectively), sorafenib + radiation (54% and 47% for UM-SCC-74A and CAL27, respectively) or cisplatin + radiation (46% and 51% for UM-SCC-74A and CAL27, respectively). In addition, the size of colonies in combination treatment group was significantly smaller than no treatment, single treatment or double treatment groups (Fig. 2A).
Figure 2. Combination treatment significantly inhibits tumor cell colony formation.
Tumor cell colony formation assay was performed in low melting point agarose in 35mm culture Petri dishes. Tumor cells UM-SCC-74A (A-B) or CAL27 (C) were treated with sorafenib (5 μM), cisplatin (2 μM) or radiation (7.5 Gy) alone or in combination and cultured at 37°C. After 14 days, colonies were stained with crystal violet (0.005%) for 1 hr and counted. *, represents a significant difference (p<0.05) as compared to no treatment group and **, represents a significant difference (p<0.05) as compared to single or double treatment groups.
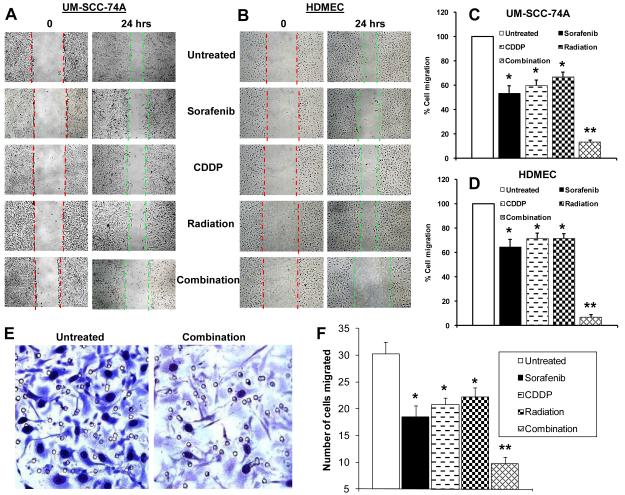
Combination treatment markedly reduces endothelial cell and tumor cell motility
Combination treatment effect on tumor (UM-SCC-74A, CAL27) and endothelial cell (HDMEC) motility was examined by scratch assay. Combination treatment significantly inhibited tumor and endothelial cell migration as compared to sorafenib, cisplatin or radiation alone or in double combinations (Fig. 3A-D). Similar migration inhibitory effect of combination treatment was observed in the second tumor cell line (CAL27, data not shown).
Figure 3. Combination treatment significantly inhibits tumor and endothelial cell motility and tumor cell invasion.
A-B; Tumor cell and endothelial cell motility was examined by scratch assay. C-D; Each assay was photographed and distances between the migrating cell edges were quantified and percentage cell migration calculated. Red dotted lines mark the edges at the start of experiments and green dotted lines mark the edges at the end of experiments. E-F; Tumor cell invasion was examined by Matrigel invasion assay. The number of tumor cells that had invaded through the Matrigel was counted in 5 high power fields. *, represents a significant difference (p<0.05) as compared to untreated group and **, represents a significant difference (p<0.05) as compared to single or double treatment groups.
Combination treatment significantly inhibits tumor cell invasion in Matrigel invasion assay
We used matrigel invasion assay to examine the effect of sorafenib combination treatment on tumor cell invasiveness. Sorafenib (5 μM), cisplatin (2 μM) or radiation (7.5 Gy) alone showed 39%, 32% and 27% inhibition of tumor cell (UM-SCC-74A) invasion through matrigel (Fig. 3C-D). Sorafenib in combination with cisplatin and radiation was highly effective in inhibiting tumor cell invasion (92%).
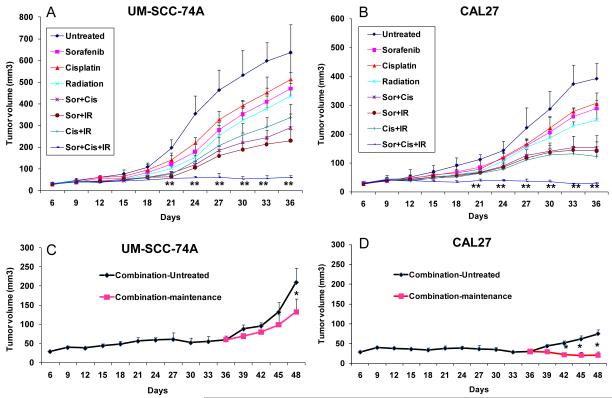
Combination treatment significantly inhibits tumor growth in vivo
The effect of combination treatment on tumor growth in vivo was examined using SCID mouse model for tumor growth and tumor angiogenesis (33). We performed two sets of experiments for tumor growth studies. In the first set of experiments, we investigated the effect of a combination therapy on tumor growth and tumor angiogenesis. Animals bearing UM-SCC-74A tumors treated with sorafenib, cisplatin or radiation alone showed 26%, 20%, and 32% decrease in tumor size (day 36), whereas animals bearing CAL27 tumors showed 27%, 22%, and 36% decrease in tumor size, respectively (Fig. 4A-B). Combination treatment with two agents together more than doubled the inhibition of tumor growth as compared to each of these agents given alone [(sorafenib + cisplatin; 55% and 62% for UM-SCC-74A and CAL27, respectively), (sorafenib + radiation; 64% and 63% for UM-SCC-74A and CAL27, respectively) (cisplatin + radiation; 48% and 64% for UM-SCC-74A and CAL27, respectively). Triple combination treatment with sorafenib, cisplatin, and radiation resulted in greater than 90% reduction in tumor size (91% for UM-SCC-74A and 93% for CAL27). This reduction in tumor size was significantly greater than treatment with single agent or dual agents. In addition, the combination treatment did not cause any animal mortality or induce significant decrease in body weight.
Figure 4. Combination treatment significantly inhibits tumor growth.
A-B; Tumor bearing animals were treated with sorafenib (10 mg/kg), cisplatin (2 mg/kg) or radiation (3 Gy) alone or in combination as described in methods. C-D; For sorafenib maintenance dose study, at day 36, ten animals from combination treatment group were stratified into two groups (five mice per group). One group was treated with sorafenib (combination-maintenance, 10 mg/kg) twice a week and the other group was untreated control (combination-untreated). *, represents a significant difference (p<0.05) as compared to untreated group and **, represents a significant difference (p<0.05) as compared to single or double treatment groups.
In the second set of experiments, we investigated if sorafenib treatment could be used as maintenance therapy after the completion of combination treatment. In this study, animals undergoing combination treatment were randomized into two groups on day 36 and one group received maintenance sorafenib treatment (combination-maintenance) and other group was untreated control (combination-untreated). Sorafenib as a single agent maintenance therapy was very effective and it completely prevented tumor recurrence in CAL27 (Fig. 4D) and significantly inhibited UM-SCC-74A tumor growth (63% inhibition at day 48, Fig. 4C).
Combination treatment significantly inhibits tumor angiogenesis
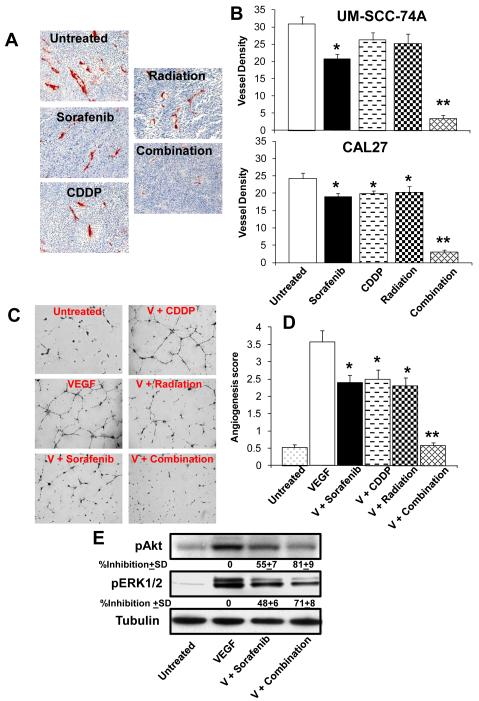
Tumor samples (UM-SCC-74A) from animals treated with sorafenib, cisplatin or radiation alone showed 27%, 16% and 19% decrease in tumor vessel density (Fig. 5A-B). Combination treatment with sorafenib, cisplatin and irradiation together was most effective by inhibiting more than 90% of tumor angiogenesis (Fig. 5A-B). Similar decrease in blood vessel density was observed in CAL27 tumors (Fig. 5B).
Figure 5. Combination treatment significantly inhibits tumor angiogenesis and VEGF mediated endothelial cell tube formation.
A; Representative photomicrographs of tumor blood vessel staining for untreated, sorafenib, cisplatin (CDDP) or radiation alone or combination groups for UM-SCC-74A tumors. B; Microvessel density in the tumor samples was calculated by counting 5 random fields (200x) and expressed as vessel density ± SE. C; Representative photomicrographs of in vitro tube formation assay for untreated, VEGF, VEGF + sorafenib (V + Sorafenib), VEGF + cisplatin (V + CDDP), VEGF + radiation (V + Radiation) and VEFG + combination treatment (V + Combination). D; quantitative data for tube formation expressed as angiogenic score ± SE from three independent experiments. *, represents a significant difference (p<0.05) as compared to VEGF group and **, represents a significant difference (p<0.05) as compared to VEGF or single treatment groups. E; Endothelial cells were treated with sorafenib (5 μM) or combination treatment (sorafenib + cisplatin + radiation) for 1 hour and then treated with VEGF for 30 minutes. Whole cell lysates from each group were Western blotted and probed for pAkt and pERK1/2. Equal sample loading was verified by stripping the blots and re-probing with anti-tubulin antibody. Band density of each protein was normalized with tubulin and expressed as % inhibition± SE as compared to controls from three independent experiments.
We next examined if sorafenib combination treatment mediates its anti-angiogenesis effects by inhibiting VEGF-mediated angiogenesis. VEGF treatment of endothelial cells significantly enhanced the tube formation on growth factor reduced Matrigel (Fig. 5C-D). Low dose combination of sorafenib (5 μM), cisplatin (2 μM) and radiation (7.5 Gy) completely inhibited VEGF-mediated tube formation (Fig. 5C-D), whereas sorafenib, cisplatin and radiation treatment alone showed 33%, 30% and 36% inhibition of endothelial cell tube formation, respectively (Fig. 5C-D). VEGF predominantly mediates angiogenesis via the activation of PI3K/Akt and MAPK signaling cascade (27) and combination treatment significantly inhibited Akt and ERK1/2 activation (81% and 71% respectively, Fig. 5 E).
Sorafenib enhances tumor cell chemo-radiation sensitization by down-regulating ERCC-1 and XRCC-1
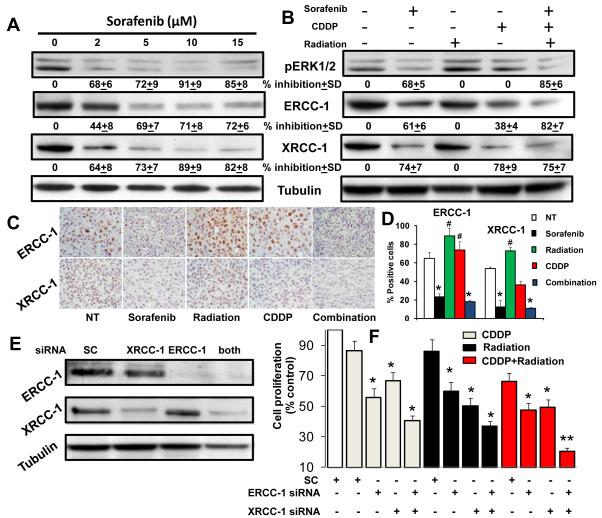
Tumor cells often develop chemo and radio-resistance by overexpressing DNA repair proteins, particularly ERCC-1 and XRCC-1 (16, 18). MAPK pathway is one of the key pathways involved in the upregulation of ERCC-1 and XRCC-1 (15). We therefore examined if sorafenib enhanced the anti-tumor effects of chemo-radiation by down-regulating these DNA repair proteins. Indeed, sorafenib inhibited ERK1/2 phosphorylation and down-regulated the expression of ERCC-1 and XRCC-1 in a dose dependent manner (Fig. 6A). In addition, combination treatment significantly decreased the expression of both ERCC-1 and XRCC-1 (Fig. 6B).
Figure 6. Sorafenib enhances chemo-radiation mediated anti-tumor effects by down-regulating DNA repair proteins ERCC-1 and XRCC-1.
A; UM-SCC-74A cells were treated with different concentrations of sorafenib for 24 hrs. B; UM-SCC-74A cell were treated with sorafenib (5 μM), cisplatin (2 μM) or radiation (7.5 Gy) alone or in combination for 24 hrs. Equal sample loading was verified by stripping the blots and re-probing with anti-tubulin antibody. Band density of each protein was normalized with tubulin and expressed as % inhibition± SE as compared to controls from three independent experiments. C; paraffin embedded UM-SCC-74A tumor samples were stained for ERCC-1 and XRCC-1 and representative photomicrographs (400x) of untreated (NT), sorafenib, radiation or cisplatin (CDDP) alone or combination groups. D; ERCC-1 and XRCC-1 positive cells were quantified in 5 high power fields (400x) of each tumor samples and % positive cells calculated. *, represents a significant decrease (p<0.05) and #, represents a significant increase (p<0.05) as compared to the untreated control group. E: ERCC-1, XRCC-1 or both (ERCC-1 and XRCC-1) proteins were knocked down in UM-SCC-74A cells by respective siRNA(s). Whole cell lysates from each experiment were separated using 4-12% NuPAGE Bis-Tris gels and probed for ERCC-1 and XRCC-1. Equal sample loading was verified by stripping the blots and re-probing with anti-tubulin antibody. E; Cell proliferation was measured in UM-SCC-74A cells after knocking down ERCC-1, XRCC-1 or both and treating with cisplatin (2 μM), radiation (7.5 Gy) or both (cisplatin and radiation) for 72 hours. The percentage cell growth inhibition for each group was calculated by adjusting the scramble control group to 100%. *, represents a significant difference (p<0.05) as compared to the scramble control group and **, represents a significant difference (p<0.05) as compared to the scramble control or single treatment groups.
We next stained tumor samples from our in vivo study to examine the effect of combination treatment on the expression of ERCC-1 and XRCC-1. Radiation treatment alone significantly increased ERCC-1 and XRCC-1 expression in vivo (7 fractionated radiation treatments over 21 days) whereas it did not significantly alter ERCC-1 and XRCC-1 levels in vitro (single radiation treatment and cell lysates prepared after 24 hours). These results suggest that tumor cells may require repeated exposure to radiation (fractionated doses) to upregulate ERCC-1 and XRCC-1 expression. As observed in our in vitro experiments, combination treatment with sorafenib, cisplatin and radiation significantly reduced ERCC-1 (82%) and XRCC-1 (89%) expression (Fig. 6C-D).
To further investigate the role of ERCC-1 and XRCC-1 in protection against chemo-radiation treatment, we selectively knocked down ERCC-1 or XRCC-1 or both of them together in tumor cells. Western blot analysis showed a complete knockdown of ERCC-1 and more than 80% knockdown of XRCC-1 in both the cell lines (Fig. 6E, data shown for UM-SCC-74A). Knockdown of ERCC-1 or XRCC-1 alone showed about 50% decrease in cell proliferation when treated with low doses of cisplatin (2 μM) and radiation (7.5 Gy), whereas knocking down of both ERCC-1 and XRCC-1 together decreased more than 80% of cell proliferation (Fig. 6F).
DISCUSSION
Chemo-radiation regimen is one of the most commonly used treatments for many head and neck cancer patients. However, this intense therapeutic regimen often results in significant toxicity leading to decreased quality of life. Therefore, there is an urgent need to develop combination treatment regimens that can improve the therapeutic efficacy of CRT while minimizing the toxic side effects. However, a major challenge for developing combination treatments is the identification of specific target molecule(s) that can provide the highest degree of synergistic anti-tumor activity with traditional therapies. One such target molecule for head and neck cancer is Raf kinase. Majority of head and neck tumors (>80%) overexpress EGFR and increased EGFR expression is directly correlated with worse prognosis, including advanced stage, poorly differentiated tumors and poor survival (6-9). EGFR expression in HNSCC also correlate positively with the acquisition of radio-resistance (35). Raf-MAPK is one of the key pathways EGFR uses to mediate its biological effects. RAF-MAPK pathway also plays an important role in mediating radio and chemo-resistance in tumor-associated endothelial cells (14). Therefore, we hypothesize that targeting of Raf kinases in advanced head and neck tumors could reverse the resistant phenotype in tumor cells as well as tumor-associated endothelial cells, thereby enhancing the therapeutic efficacy of standard CRT.
To test this hypothesis, we have performed combination treatment studies in vitro as well as in vivo, using our SCID mouse model. We selected sorafenib, a multi-kinase inhibitor, for this study because it is a potent anti-tumor agent as well as it is equally effective against tumor stroma components particularly tumor-associated endothelial cells (27). In addition, sorafenib has been successfully used in clinics for the treatment of advanced RCC, HCC and thyroid cancer (25, 26, 36). We selected two HNSCC cell lines (CAL27 and UM-SCC-74A) based on their chemo and radiation sensitivities for our in vitro and in vivo work. CAL27 is a relatively sensitive cell line to chemo and radiation treatment. In contrast, UM-SCC-74A is highly resistant to both chemo and radiation treatment. In addition, UM-SCC-74A contains wild-type p53, whereas CAL27 has mutant p53 gene. Interestingly, both these cell lines were equally sensitive to sorafenib treatment regardless of p53 mutational status, thereby suggesting that sorafenib mediates its anti-tumor effects independent of p53 status. This is important as more that 50% of head and neck tumors have mutant p53 and some of the targeted inhibitors selectively inhibit cell growth in cancer cells with wild-type p53 only (37, 38).
In the combination treatment studies, we selected doses of sorafenib (5 μM), cisplatin (2 μM), and radiation (7.5 Gy) that by themselves showed about 50% growth inhibition in chemo-radiation sensitive tumor cell line (CAL27). The rationale for selecting these low doses was to have the greatest possible synergistic anti-tumor effect without the serious side-effects that are often associated with the maximum tolerated doses of radiation and chemotherapy. Indeed, pretreatment with sorafenib significantly enhanced chemo-radiation mediated inhibition of tumor cell proliferation and colony formation. A number of studies have demonstrated that tumor cells acquire chemo and radio-resistance by increasing the expression of anti-apoptotic proteins and/or DNA repair proteins (16, 18). Efficient DNA repair in the cancer cells is an important mechanism of therapeutic resistance (39) and inhibition of DNA repair pathway would make tumor cells more sensitive to DNA damaging agents like chemotherapy and radiation treatment. In this study, we have shown for the first time that sorafenib can down-regulate the expression of DNA repair proteins ERCC-1 and XRCC-1 in a dose dependent manner. In addition, combination treatment was equally effective in inhibiting tumor cell. Sorafenib may be decreasing tumor cell invasion by inhibiting MMP production by blocking the Raf-MAPK pathway (40) which has been shown to induce the production of MMPs (41). Sorafenib in combination treatment also significantly inhibited VEGF-mediated endothelial cells tube formation (in vitro angiogenesis assay). This could be due to the direct inhibition of VEGFR2 and VEGFR3 signaling by sorafenib (27). Endothelial cell tube formation is a complex process that involves endothelial cell migration and/or proliferation. Our results suggest that combination treatment predominantly affects endothelial cell tube formation by inhibiting endothelial cell motility as combination treatment showed a similar inhibition of endothelial cell tube formation (91%) and endothelial cell migration (92%), whereas combination treatment effect on endothelial cell proliferation was significantly less (45%, data not shown) at 24 hours post treatment.
To determine if the observed in vitro synergy between sorafenib, cisplatin and ionizing radiation extends to the in vivo setting, we used a SCID mouse model to study the effect of combination treatment on tumor growth and tumor angiogenesis. Low doses of sorafenib (10 mg/kg), cisplatin (2 mg/kg) and radiation treatment (3 Gy, fractionated dose) exhibited even more pronounced anti-tumor effects. This marked inhibition of tumor growth by combination therapy particularly in chemo and radio-resistant cell line could be due to sorafenib-mediated inhibition of tumor proliferation via the Raf-MAPK pathway (40), down-regulation of DNA repair proteins and anti-apoptotic Mcl-1 protein (42) as well as reduction in the formation of new blood vessels by inhibiting VEGF signaling (27) as observed in tube formation assay. Sorafenib’s potent anti-angiogenesis effects may also be responsible for significant inhibition of tumor growth when sorafenib was used as a maintenance therapy. This combination treatment was very well tolerated in the animals. It did not cause any animal mortality or induced significant weight loss or induced any major systemic toxicity such as dry scaly skin or respiratory distress which has been reported in animals treated with high doses of chemo-radiation treatment or other small molecular weight inhibitors (43).
In conclusion, we have demonstrated that sorafenib significantly enhances the therapeutic efficacy of CRT by inhibiting tumor and endothelial cell survival, tumor cell invasiveness and angiogenesis. These results suggest a potentially novel strategy to enhance the therapeutic efficacy of CRT for head and neck cancers. Moreover, this strategy of using a combination of low doses of sorafenib, cisplatin and radiation has the potential of significantly decreasing side effects associated with the concurrent chemo-radiation treatment while maintaining their therapeutic efficacy.
Acknowledgments
Financial support: NIH/NCI-CA133250 (PK) and Joan’s fund Research Grant (BK and PK).
Abbreviations
- HNSCC
head and neck squamous cell carcinoma
- HDMEC
human dermal microvascular endothelial cell
- ERCC-1
excision repair cross-complementing group 1
- XRCC-1
X-Ray repair cross-complementing protein 1
- VEGF
vascular endothelial cell growth factor
- VEGFR
vascular endothelial cell growth factor receptor
- SCID
severe combined immunodeficiency
- CRT
concurrent chemoradiation
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
REFERENCES
- 1.Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86:104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 3.Kalavrezos N, Bhandari R. Current trends and future perspectives in the surgical management of oral cancer. Oral Oncol. 2010;46:429–32. doi: 10.1016/j.oraloncology.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Allen AM, Elshaikh M, Worden FP, Bradford CR, Teknos TN, Chepeha DB, et al. Acceleration of hyperfractionated chemoradiation regimen for advanced head and neck cancer. Head Neck. 2007;29:137–42. doi: 10.1002/hed.20495. [DOI] [PubMed] [Google Scholar]
- 5.Dimery IW, Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst. 1993;85:95–111. doi: 10.1093/jnci/85.2.95. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–6. [PubMed] [Google Scholar]
- 7.Maurizi M, Almadori G, Ferrandina G, Distefano M, Romanini ME, Cadoni G, et al. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br J Cancer. 1996;74:1253–7. doi: 10.1038/bjc.1996.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demiral AN, Sarioglu S, Birlik B, Sen M, Kinay M. Prognostic significance of EGF receptor expression in early glottic cancer. Auris Nasus Larynx. 2004;31:417–24. doi: 10.1016/j.anl.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, et al. Activated extracellular signal-regulated kinases: associat ion with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001;61:6500–10. [PubMed] [Google Scholar]
- 11.Teknos TN, Cox C, Yoo S, Chepeha DB, Wolf GT, Bradford CR, et al. Elevated serum vascular endothelial growth factor and decreased survival in advanced laryngeal carcinoma. Head Neck. 2002;24:1004–11. doi: 10.1002/hed.10163. [DOI] [PubMed] [Google Scholar]
- 12.Thomas GR, Nadiminti H, Regalado J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol. 2005;86:347–63. doi: 10.1111/j.0959-9673.2005.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar P, Miller AI, Polverini PJ. p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol Chem. 2004;279:43352–60. doi: 10.1074/jbc.M405777200. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Coltas IK, Kumar B, Chepeha DB, Bradford CR, Polverini PJ. Bcl-2 protects endothelial cells against gamma-radiation via a Raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res. 2007;67:1193–202. doi: 10.1158/0008-5472.CAN-06-2265. [DOI] [PubMed] [Google Scholar]
- 15.Yacoub A, Park JS, Qiao L, Dent P, Hagan MP. MAPK dependence of DNA damage repair: ionizing radiation and the induction of expression of the DNA repair genes XRCC1 and ERCC1 in DU145 human prostate carcinoma cells in a MEK1/2 dependent fashion. Int J Radiat Biol. 2001;77:1067–78. doi: 10.1080/09553000110069317. [DOI] [PubMed] [Google Scholar]
- 16.Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994;94:703–8. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed E. DNA damage and repair in translational oncology: an overview. Clin Cancer Res. 2010;16:4511–6. doi: 10.1158/1078-0432.CCR-10-0528. [DOI] [PubMed] [Google Scholar]
- 18.Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee SK, et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br J Cancer. 2008;99:167–72. doi: 10.1038/sj.bjc.6604464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 20.Green A, Prager A, Stoudt PM, Murray D. Relationships between DNA damage and the survival of radiosensitive mutant Chinese hamster cell lines exposed to gamma-radiation. Part 1: Intrinsic radiosensitivity. Int J Radiat Biol. 1992;61:465–72. doi: 10.1080/09553009214551221. [DOI] [PubMed] [Google Scholar]
- 21.Hoy CA, Fuscoe JC, Thompson LH. Recombination and ligation of transfected DNA in CHO mutant EM9, which has high levels of sister chromatid exchange. Mol Cell Biol. 1987;7:2007–11. doi: 10.1128/mcb.7.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opinion on Pharmacotherapy. 2010;11:1943–55. doi: 10.1517/14656566.2010.496453. [DOI] [PubMed] [Google Scholar]
- 24.Siegel AB, Olsen SK, Magun A, Brown RS. Sorafenib: Where do we go from here? Hepatology. 2010;52:360–9. doi: 10.1002/hep.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 26.Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–9. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 28.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 29.Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, et al. Phase II Trial of Sorafenib in Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck or Nasopharyngeal Carcinoma. Journal of Clinical Oncology. 2007;25:3766–73. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, Benson AB. Phase II Study of Sorafenib in Combination With Docetaxel and Cisplatin in the Treatment of Metastatic or Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: ECOG 5203. Journal of Clinical Oncology. 2010;28:2947–51. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, et al. Doxorubicin Plus Sorafenib vs Doxorubicin Alone in Patients With Advanced Hepatocellular Carcinoma. JAMA: The Journal of the American Medical Association. 2010;304:2154–60. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 32.Kumar P, Gao Q, Ning Y, Wang Z, Krebsbach PH, Polverini PJ. Arsenic trioxide enhances the therapeutic efficacy of radiation treatment of oral squamous carcinoma while protecting bone. Mol Cancer Ther. 2008;7:2060–9. doi: 10.1158/1535-7163.MCT-08-0287. [DOI] [PubMed] [Google Scholar]
- 33.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest. 2008;88:740–9. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- 34.Kumar P, Benedict R, Urzua F, Fischbach C, Mooney D, Polverini P. Combination treatment significantly enhances the efficacy of antitumor therapy by preferentially targeting angiogenesis. Lab Invest. 2005;85:756–67. doi: 10.1038/labinvest.3700272. [DOI] [PubMed] [Google Scholar]
- 35.Sheridan MT, O’Dwyer T, Seymour CB, Mothersill CE. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5:180–6. doi: 10.1002/(SICI)1520-6823(1997)5:4<180::AID-ROI3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 37.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105:3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyachi M, Kakazu N, Yagyu S, Katsumi Y, Tsubai-Shimizu S, Kikuchi K, et al. Restoration of p53 pathway by nutlin-3 induces cell cycle arrest and apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res. 2009;15:4077–84. doi: 10.1158/1078-0432.CCR-08-2955. [DOI] [PubMed] [Google Scholar]
- 39.Madhusudan S, Middleton MR. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat Rev. 2005;31:603–17. doi: 10.1016/j.ctrv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. 2009;8:118. doi: 10.1186/1476-4598-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, et al. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res. 2006;69:736–45. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–9. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 43.Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56:846–53. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]