Abstract
PURPOSE
Women who carry a FMR1 premutation are at risk for fragile X-associated primary ovarian insufficiency (FXPOI) and should be counseled for a potentially reduced fertility. Multiple factors can affect the age of onset and severity of FXPOI. Here, we assessed the predictive power of several factors with menopausal age, a surrogate measure of onset of FXPOI.
METHODS
Genetic, environmental and reproductive factors were analysed by Cox proportional hazard models in 1,068 women, 385 of fragile X families ascertained through the Nijmegen study and 683 of fragile X families or general population families ascertained through the Atlanta study.
RESULTS
The highest association with menopausal age among FMR1 premutation carriers was found for risk index by CGG repeat size (HR 1.43) and smoking (HR 1.34). Women from the Nijmegen cohort showed an overall lower age at menopause onset, for which a correction was made. A prediction model based on these two parameters, mean menopausal age of first degree relatives with the same mutation status and the correction for ascertainment site, estimated the probability of becoming postmenopausal for premutation carriers, with a margin of 7-10%.
CONCLUSION
We conclude that this model serves as a first step towards clinical application of FXPOI prediction.
Keywords: FMR1 premutation, menopause, fragile X-associated primary ovarian insufficiency (FXPOI), prediction
INTRODUCTION
In the early 1990s, premature ovarian failure (POF), or cessation of menses prior to the age of 40 years, was noted among heterozygous carriers of the FMR1 premutation.1,2 Additional studies showed that the risk of POF was almost exclusively seen in females carrying a premutation in the FMR1 gene and that approximately 20% of these carriers had POF.3,4 To better denote the complete spectrum of this disorder, including altered menstrual cycles, altered hormone profiles, infertility and intermittent ovarian function, this premutation disorder was renamed as Fragile X-associated Primary Ovarian Insufficiency (FXPOI) 5, defined as amenorrhea for at least 4 months and two serum FSH levels above 40 IU/l.
The FMR1 premutation represents an expansion of a trinucleotide (CGG) repeat in the 5′ untranslated region of the fragile X mental retardation (FMR1) gene.6 Based on the number of repeats present and their instability when passed from one generation to the next, four types of FMR1 alleles can be distinguished: 1) a normal allele with <45 repeats, which is stably inherited; 2) an intermediate mutant allele with 45 to 54 repeats, which may show instability upon maternal or paternal transmission; 3) a premutation allele with 55 to 200 repeats, which can expand to a full mutation within one generation; and 4) a full mutation allele with at least 200 CGG repeats (ACMG practice guidelines; 2007), which results in an intellectual and developmental disability syndrome, the fragile X syndrome.7 Women who carry the premutation should be informed, not only about their risk for having a child with fragile X syndrome, but also about their potential risk for FXPOI, as symptoms include infertility, early cessation of menses and early exposure to estrogen deficiency.
In the general population menopause ranges from ages 40 to 60 years with a mean of 51 years 8 and appears to be modulated by both environmental and genetic factors.9-12 As yet, Allen et al.13 showed that smoking led to an earlier age at menopause among premutation carriers, however the association of menopausal age with other factors, such as menarche and parity is still a matter of debate. Although environmental and reproductive factors both contribute to variance in age at menopause, genetic factors seem to act as superior predictors for age at menopause.12 The size of the FMR1 CGG repeat is a major risk factor for FXPOI. Within the FMR1 premutation range of 55 to 200 repeats, Allen et al.13 and Sullivan et al.14 identified high risk alleles of 80-99 repeats. This non-linear association was subsequently confirmed by others.15,16 The subdivision of the premutation ranges used by Allen et al.13 into low (55-79 repeats), medium (80-99 repeats), and high (100-200 repeats) has, however, been based on the risk to expand to a full mutation17, and any detailed information on high risk alleles for FXPOI is therefore still lacking. Here, we have developed an integrative model that allows the prediction of the risk to develop FXPOI in individual premutation carriers. Such a model would serve as an excellent adjunct to current fertility counseling modalities and the clinical intervention in premutation carriers.
METHODS
Study population
The study cohort is comprised of participants ascertained through two centers, the Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands (Nijmegen cohort) and the Emory University Department of Human Genetics, Atlanta, USA (Atlanta cohort). The Dutch study was approved by the Institutional Review Board of the Radboud University Nijmegen Medical Centre. The Atlanta protocol and consent forms were approved by the Institutional Review Board at Emory University.
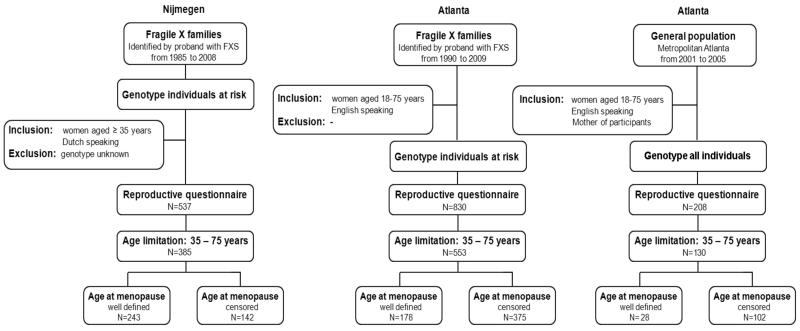
All fragile X families that were diagnosed at the Department of Human Genetics Nijmegen between 1984 and 2008 were ascertained for this study. All female relatives of a proband at age 35 years or older were approached to participate in this study, and asked to complete a general health and reproductive history questionnaire. A detailed description of the study population is depicted in Figure 1. The Atlanta cohort was comprised of women who were recruited through the Emory Study of Adult Learning and Reproduction. Ascertainment protocols have been described in detail by Sullivan et al.14
Figure 1.
Flow chart of the study population
Data collection
Reproductive history questionnaire
For both studies comparable questionnaires on general health and reproduction were administered by phone, in written format, or through an internet survey. Both reproductive questionnaires gathered information on time since last menstrual period, cause for cessation of menstrual periods (for example menopause, hormones, surgery), age at last period, age at start hormone use / hormone replacement therapy (HRT) and oral contraceptives (OC), time period of hormone use, and current hormone use.
Definition of age at menopause
Age at menopause was used as a surrogate for severity for FXPOI and was defined as amenorrhea for more than 1 year due to natural menopause. Ages at menopause for all women with other reasons than natural menopause for cessation of menstrual cycles were “censored”. For women who had their last period due to surgery (e.g. hysterectomy or oophorectomy), chemotherapy, radiation or pregnancy, menopausal age was censored at age at last period. Women who still had menstrual cycles (last period < 1 year) without current hormone use were censored at age at interview. Women with recent periods and current hormone use were censored at age when hormone use was started.
FMR1 CGG repeat size measurement
From the participants included in the Dutch study, DNA was extracted from peripheral blood cells. PCR amplification of the FMR1 alleles was performed using fluorescein (FAM)-labeled reverse primers as described by Fu et al.18 PCR conditions are available upon request. Fragment lengths were measured using an ABI prism 3730 DNA Analyser (Applied Biosystems) and quantified using ABI prism Genemapper Analysis Software v4.0 (Applied Biosystems) up to 1 or 2 repeats accurate until 130 to 140 repeats. From the women participating through Atlanta, DNA was extracted from buccal samples or peripheral blood cells using Qiagen QiAmp DNA Blood Mini Kit. FMR1 CGG repeat sizes were determined by a fluorescent-sequencer method as described by Sullivan et al.14, with the same accuracy as the Dutch method, up to 100 repeats and with an inaccuracy of 5-10 repeats for repeat sizes 100-140.
Statistical analysis
The data were analyzed using age at menopause, a surrogate for FXPOI, as outcome variable in Cox proportional hazards models. To this end, the time origin was set at the age of 25 years and the time from 25 years to start menopause was considered as the time to event. To adjust for the correlation among outcomes within the same family we applied robust estimates each time we made use of a Cox regression mode.19 Analyses were performed separately for FMR1 premutation carriers and non-carriers.
Construction of risk index for earlier menopause based on FMR1 CGG repeat size
Cox regression with robust estimations were used to determine risk estimates for menopause at a younger age based on FMR1 CGG repeat size. A reference region based on most common repeat sizes encountered in the general population was defined to be 28-33 repeats.18,20 For repeat sizes 20-28 and 34 repeats to 100 repeats, a set of moving windows with a width size of 11 repeats was created. In case of overlap between window and reference region, repeats present in the overlap were removed from the window. The menopausal age for subjects within a window was compared to menopausal age of subjects in the reference region, resulting in a window-specific hazard ratio, HR, where the index r refers to the central repeat size in the window. To determine these HRs, we applied Cox regression with robust estimations. Because repeat size determination of 100-130 repeats was less accurate for the Atlanta subset, a wider window (+/− 10 repeats) around the reported repeat size was used. Since sizes above 130 repeats could not be determined with accuracy for either cohort, they were not assessed in this hazard risk analysis. To reduce the noise in the resulting HR graph, we superimposed a smoothed curve that is fitted to the HRs by repeat scatter plot. To this end, we used the LOWESS smoother with smoothing factor = 0.1.21 The resulting variable is referred to as the “Risk Index by Repeat size”: (RIR) and ranged from 1 to 3.6, with 1 indicating similar risk for menopause as women with repeat size 28-33 and 3.6 indicating an odds of 3.6 of becoming postmenopausal per unit time compared to women with repeat sizes 28-33.
Assessment of putative predictors for menopausal age in premutation carriers
Predictive factors for age at menopause were based on previous studies on menopause in general and included smoking, body mass index (BMI), age at menarche, oral contraceptives (OC) use and parity.12,22,23 The abundant evidence of heritability of menopausal age, and the large additive genetic component that Hunter et al.24 showed to be present after correction for the premutation repeat size, required a representation into our putative predictor assessment as well. To this end, for each subject the mean age at menopause of first degree relatives with the same mutation status (premutation if 55- 200 repeats, non-carrier if ≤ 54 repeats) was determined. If a subject was lacking a first degree relative with the same mutation status and well-defined age at menopause, the median age at menopause observed in subjects (well-defined age at menopause) of the same premutation status, 44 years for carriers and 50 years for non-carriers, was employed. Because this study comprises participants from two separate studies from different centers, we also considered ascertainment location as a possible relevant explanatory variable. Putative predictors were assessed as continuous variables, except for smoking and OC use, which were dichotomized for ever smoking or ever OC use. As a first step in the modeling process we assessed various potential predictors for menopausal age using univariate Cox models.
Modeling process and validation
For model building, backward selection was applied, starting with the set of variables that showed significance in the univariate analyses. For each potential predictor the proportional hazard assumption was determined by inspecting plots of weighted Schoenfeld residuals against age at menopause.25 The appropriate functional form of continuous predictors was checked using martingale residuals as described by.26 To assess the fit of each resulting model, the subjects were divided in three tertile groups based on their values of the linear part of the Cox regression model. For each tertile the Kaplan-Meier curve and the mean of the predicted Cox survival curves were computed. A perfect fit should give comparable curves for each tertile. Both resulting Cox models were validated using Efron’s optimism bootstrap to estimate the optimism in R2 and the concordance index c, to quantify the over-fitting and to assess the calibration needed.27
RESULTS
Delineation of the study populations
In total 1,575 women were ascertained in the original studies, 537 from the Nijmegen cohort and 1,038 from the Atlanta cohort. Approximately 90% of them were Caucasian. Since the number of women from other ethnicities was too small to denote an effect, all non-Caucasians were excluded from the initial cohort. To correct for age differences in recruitment schemes (Nijmegen recruited 35 years and older, Atlanta recruited 18 to 75 years), we selected only women aged 35 -75 years from both cohorts, resulting in a final study cohort of 1,068 women. The characteristics of the final study cohort are shown in Table 1. Women included from Nijmegen were older at interview and, thus, had more often reached menopause (72%) compared to women included from Atlanta, of whom 34% had a well-defined age at menopause. In addition, women from the Nijmegen cohort exhibited an overall significant earlier menopausal age. When comparing the distribution of FMR1 CGG repeat sizes of the Nijmegen and Atlanta cohorts, no difference was seen (p=0.58, Mann-Whitney U).
Table 1.
Characteristics of the study population by mutation status
| Premutation | Non-carriers | Total | |||
|---|---|---|---|---|---|
| Nijmegen | Atlanta | Nijmegen | Atlanta | ||
| Age at ascertainment (yrs) | 56.4 | 49.6 | 54.6 | 52.7 | 53.0 |
| Age at menopause (yrs) | |||||
| - Well defined | 42.5 | 43.5 | 48.3 | 49.9 | 45.9 |
| (%) | (72%) | (34%) | (58%) | (25%) | (42%) |
| - Censored | 41.7 | 39.0 | 42.9 | 42.2 | 41.0 |
| (%) | (28%) | (66%) | (42%) | (75%) | (58%) |
| Menarche (yrs) | 13.2 | 12.4 | 13.1 | 12.5 | 12.7 |
| Parity | 2.2 | 2.1 | 2.0 | 2.1 | 2.1 |
| Ever used hormones (%) | 79% | 80% | 74% | 85% | 80% |
| Ever smoking (%) | 41% | 36% | 35% | 40% | 38% |
| BMI | 24.6 | 27.0 | 25.8 | 27.2 | 26.5 |
| Ascertainment (N) | |||||
| - Fragile X family, USA | 370 | 183 | 51.7% | ||
| - General population, USA | 3 | 127 | 12.2% | ||
| - Fragile X family, NL | 144 | 241 | 36.1% | ||
Definition of putative predictors
Risk index for earlier menopause based on FMR1 CGG repeat size
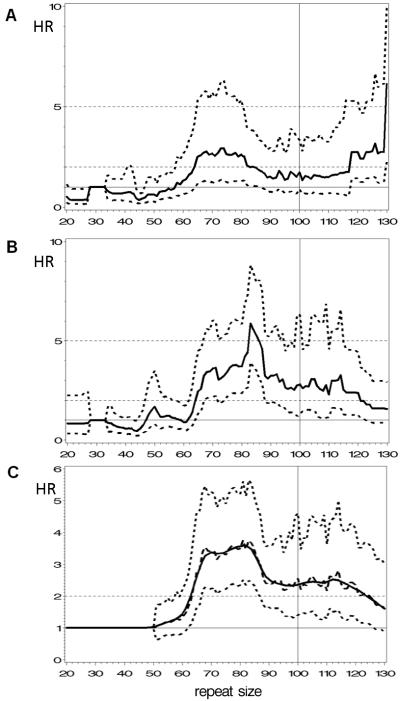
To incorporate the non-linear association of repeat size and risk for FXPOI, a risk index based on repeat size was developed. In order to explore possible differences between the two geographic ascertainment sites, unsmoothed results are presented separately. By doing so, both the Nijmegen and Atlanta cohorts exhibited no risk for earlier menopause for repeat sizes in the normal or intermediate range, as depicted by the 95% confidence interval that includes HR=1. In addition, both analyses showed that the risk for earlier menopause starts at 64 CGG repeats, at which the lower confidence interval rises above a hazard ratio of 1 (Figure 2A and 2B). Also, some differences were seen between the two ascertainment sites, i.e., the hazard ratios for 64 repeats or more were lower for the Nijmegen study cohort as for that from Atlanta. Also, within the Nijmegen cohort the hazard ratios were significantly elevated until repeat sizes of 90, while for the Atlanta cohort the significantly elevated hazard ratio’s reached 100 repeats. Though differences in hazard ratios were seen, the shape of the curves was found to be similar, thus allowing pooling of both datasets. For the total study population, a significant high risk (lower confidence band > 1) started above 62 repeats, then varied from 1.5 to 3.8 and, finally, became non-significant at 120 repeats (Figure 2C). Repeat sizes of 131-200 (n=21) had a HR of 2.1 (95% CI: 1.0-4.3), with a significant isolated HR-peak at 160 repeats (data not shown). The value of the smoothed Lowess curve was used to assess a predictor to which we will refer to as the risk index by repeat size (RIR).
Figure 2.
Hazard ratios (HRs) for earlier menopause by repeat size of the largest allele compared to the referent category of 29-32 repeats. Panel A depicts hazard ratios (solid line) for the Nijmegen study sample with 95% confidence interval (dotted lines). Panel B depicts hazard ratios (solid line) for the Atlanta study sample with 95% confidence interval (dotted lines); Panel C depicts the hazard ratios for the total study sample (black dashed line) with 95% confidence interval (dotted lines) and superimposed a smoothed curve (solid line), using LOWESS smoother with smoothing factor 0.1. The horizontal solid line depicts HR=1 and the 2 dashed lines depict HR=2 and HR=5.
Value of putative predictors
In order to estimate the value of all putative predictors, they were assessed for their association with age at menopause in univariate and multivariate Cox analyses with robust estimations in the subgroups of premutation carriers and non-carriers separately. For all potential predictors no relevant violation of the proportional hazards model assumption was found and all continuous variables were of the correct functional form. For premutation carriers, the RIR was highly associated with age at menopause, with an HR of 1.26 for univariate analysis and 1.43 for multivariate analysis (Table 2). By univariate analysis, two other potential predictors showed a significant association, i.e., mean menopausal age of first degree relatives with a premutation (HR=0.97) and smoking (HR=1.30). Among non-carriers, except for RIR, the same putative predictors were univariate significantly associated with menopausal age: HR=0.90 for the effect of mean menopausal age of first degree relatives and HR=1.44 for the effect of smoking. The HR<1 for mean age at menopause among first degree relatives suggests a protective factor, or one that helps to increase the age at menopause. The HR>1 for smoking indicates a risk factor, or one that reduces the age at menopause. Two possible confounders, age at interview and ascertainment site, were significantly associated with menopausal age in both groups, though only ascertainment site held significance at multivariate levels.
Table 2.
Analyses of association of possible risk factors with age at menopause by Cox proportional hazards model with robust estimate for family relations
| Premutation | Non-carrier | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Univariate | Multivariate | Final model | Univariate | Multivariate | Final model | ||||||
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value | |
| Genetic | ||||||||||||
| FMR1: Risk index by repeat | 1.26 | <0.001 * | 1.43 | <0.001 * | 1.63 | <0.001 * | - | - | - | - | 1 | NA |
| Mean menopausal age 1st degree relatives |
0.97 | 0.02 * | 0.98 | 0.24 | 0.90 | 0.007 * | 0.91 | 0.01 * | 0.91 | 0.01 * | ||
| Environmental | ||||||||||||
| Smoking (ever) | 1.30 a | 0.048 * | 1.34 | 0.02 * | 1.35a | 0.02 * | 1.44a | 0.03 * | 1.46 | 0.03 * | 1.46 | 0.03 * |
| BMI | 0.98 a | 0.056 | 1.00a | 0.87 | ||||||||
| Reproductive | ||||||||||||
| Menarche | 1.04 a | 0.41 | 1.05a | 0.3054 | ||||||||
| Parity | 0.85 a | 0.25 | 0.92a | 0.5673 | ||||||||
| Hormone use (ever) | 1.14 a | 0.34 | 0.99a | 0.9272 | ||||||||
| Confounders | ||||||||||||
| Age at interview | 1.02 | 0.003 * | 1.02 | 0.01 * | ||||||||
| Ascertainment site | 1.52 | 0.004 * | 1.92 | <0.001 * | 1.77 | <0.001 * | 1.93 | <0.001 * | 1.99 | <0.001 * | 1.99 | <0.001 * |
HR: Hazard ratio; FMR1: Fragile X mental retardation 1;
significant at level α=0.05;
all environmental and reproductive factors were adjusted for the FMR1 risk group at univariate analysis; Multivariate, only significantly associated factors are shown; Final model shows all variables selected for our model based on significant association for either premutation model or non-carrier model to improve comparability. HR>1 indicate increased odds for an earlier menopause (risk factor), HR <1 indicate a longer time to menopause (protective factor).
A FXPOI prediction model
A model to generate risk estimates for FXPOI, or menopausal age before age 40 years, for premutation carriers was built upon the significant predictors based on multivariate analysis by backward selection of the predictors with significant association for univariate analysis, i.e., RIR and smoking as a predictor for menopausal age in premutation carriers and ascertainment site (Table 2). For comparison, a model for non-carriers was developed in the same way and included mean menopausal age of first degree relatives, smoking and ascertainment site. We decided to also include mean age at menopause of first degree relatives into the prediction model for the premutation carriers because of our findings on heritability (description of our heritability analyses and results are as Supplement available online) and because we saw an additive genetic effect in our previous study.24 These models predicted the probability of becoming postmenopausal before 40 years of age with an accuracy of +/− 0.10 among premutation carriers and +/−0.07 for non-carriers.
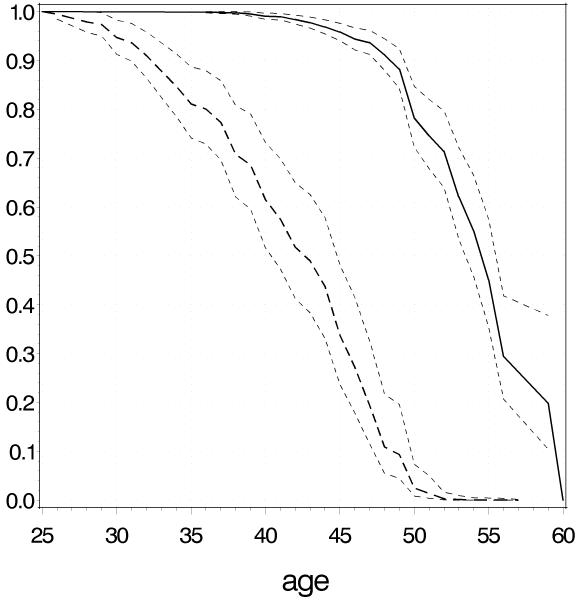
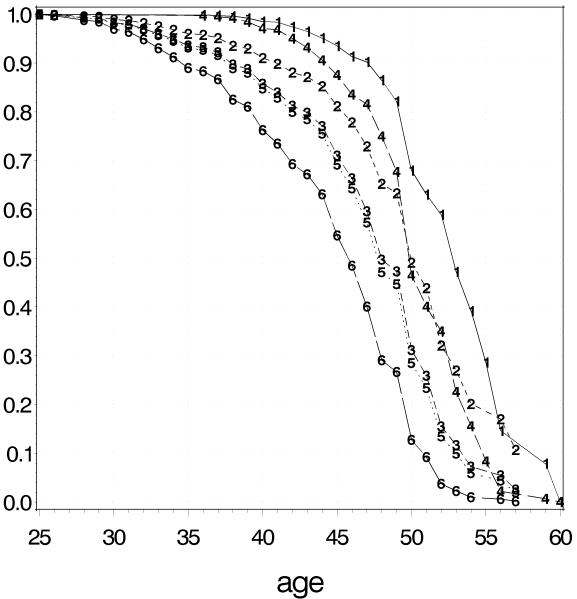
The high risk profile for premutation carriers consisted of a repeat size window centered on 80 repeats, smoker, ascertainment site Nijmegen and young mean menopausal age of first degree family members, while the low risk profile was based on the non-carrier model and consisted of a repeat size 50, never smoked, ascertainment site Atlanta, late mean menopausal age of first degree relatives, respectively. According to this model, women with this high risk profile have 38% (95% CI 29-47) chance of being menopausal before the age of 40 years and, thus, are at high risk for FXPOI (Figure 3). The effect of ascertainment location is a 3 to 4 years earlier menopause for the Nijmegen cohort as shown for different repeat sizes in Figure 4. On average, the fit of the premutation carrier and the non-carrier model was good, as shown by inspecting the tertile plots (Figure 5). Only for subjects in the tertiles with the latest predicted menopause, the observed Kaplan Meier curves suggested a somewhat later start of menopause as predicted by the Cox model, but this difference was only a few months. Internal validation of the premutation model showed that the optimism in R2Nagelkerke, original value 0.09 (95%CI 0.04-0.15), was 0.01. The optimism in the concordance index c, original value 0.66 (95%CI 0.61 – 0.70), was 0.008. The linear shrinkage factor appeared to be 0.95. For the non-carriers comparable values were seen: R2Nagelkerke = 0.09 ( 95%CI: 0.06 - 0.18), optimism = 0.02; c = 0.69 (95%CI 0.62-0.75), optimism = 0.005 and shrinkage factor = 0.90.
Figure 3.
Predicted survival curves for reaching postmenopausal state for high risk premutation women (with repeat size 80, smoking, mean menopausal age of first degree relatives 38 years and ascertainment location Nijmegen; dashed curve) and low risk, based on the non-carriers model (repeat size 50, never smoked, mean menopausal age of first degree relatives 55 and location Atlanta; solid curve) with 95% confidence bands.
Figure 4.
In order to illustrate the effect of ascertainment location, Kaplan-Meier estimates of probability of being postmenopausal are provided for each ascertainment location varying repeat size and mean age of menopause for first degree relatives and fixing smoking. For the Atlanta location: repeat size 50, smoking, mean menopausal age of first degree non-carrier relatives (curve 1); repeat size 70, smoking, mean menopausal age of first degree relatives carrying a premutation (curve 2); repeat size 80, smoking, mean menopausal age of first degree relatives carrying a premutation (curve 3). For the Nijmegen location: repeat size 50, smoking, mean menopausal age of first degree non-carrier relatives (curve 4); repeat size 70, smoking, mean menopausal age of first degree relatives carrying a premutation (curve 5); repeat size 80, smoking, mean menopausal age of first degree relatives carrying a premutation (curve 6). The curves 1 and 4 are based on the non-carrier model and the curves 2, 3, 5 and 6 on the premutation model.
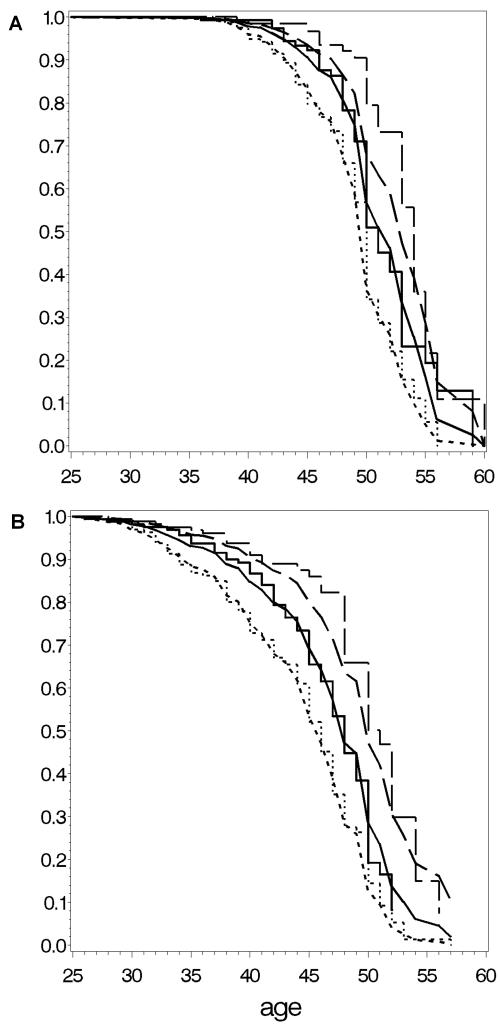
Figure 5.
Fit of our prediction models: panel A shows Kaplan-Meier estimates of observed menopausal age and the predicted menopause by our model for non-carriers for tertiles based on observed menopausal age. Panel B shows Kaplan-Meier estimates of observed menopausal age and the predicted menopause by our model for premutation carriers for tertiles. Smoothed curves represent the observed data and stepped curves the predicted data.
DISCUSSION
By using a selected cohort of 1,068 women, we built a FXPOI prediction model based on FMR1 CGG repeat size, menopausal age of first degree relatives, and the environmental factor smoking, together with a correction for ascertainment location. We found that this model can predict the risk for menopause at a certain age, as indicated by the acceptable fit, the small confidence intervals and the good results of the internal validation procedure (i.e., small amounts of optimism in R2 and concordance index c and a shrinkage factor close to 1). To our knowledge, this is the first model developed to help predict menopausal age in FMR1 premutation carriers. Other reproductive factors such as menarche and hormone use were not significantly associated with menopausal age in the premutation carriers, and thus not included in the prediction model. Van Noord et al.12 reported that the impact of environmental factors on menopausal age is limited, which seems to be true for premutation carriers as well. A prominent association with smoking (hazard ratio of 1.34) and menopausal age in premutation carriers was found, in accordance with data previously reported by Allen et al.13 The toxic effect of smoking could give rise to destruction of the primordial oocytes at the level of the ovary 28 and could, therefore, accelerate the onset of FXPOI.
Many studies dealing with relationship between various factors, such as menarche, OC use, BMI and parity, and early menopause (including POI and EM) have turned out to be controversial.12,29-32 In our set of premutation carriers no significant association with menopausal age was seen for these factors.
In the final model the FMR1 CGG repeat size, represented by ‘risk index by repeat size (RIR)’, is the most influential predictor. This was expected since the repeat size has been strongly associated with FXPOI before.13-16 To accommodate the non-linear association of repeat size and risk for FXPOI and to avoid the somewhat arbitrary classification of repeat groups, we used an HR-based approach and incorporated these risks relative to a reference repeat group into the prediction model. In this way the fast rise in risk after 60 CGG repeats and the slower decline in risk after 100 CGG repeats can be modelled. The HR pattern for repeat size indicates that a significant high risk for FXPOI starts earlier and continues longer than what was previously reported.13,14 Due to the low HRs from 55-65 repeats, the higher risk at the end of the previously defined 55-79 premutation range might not have been evident before. The high risk around 105-110 repeats has never been described before and may be attributed to small numbers (three women with a repeat size of 110 and one with a repeat size of 105 and a menopause at a relatively young age). An isolated high risk for FXPOI based on a few repeats is considered unlikely. Despite a small sample size, a significant risk was also seen for 160 repeats. Thus, an elevated risk above 130 repeats could not be excluded and requires further assessment. Discrepancies exist about risk for ovarian insufficiency among intermediate CGG repeat size carriers. Our analysis confirms that intermediate sized FMR1 CGG repeats should not be considered a high risk factor for ovarian insufficiency, as recently reported by Bennett et al.33 In addition, we did not observe a higher risk for menopause at an early age among intermediate repeat alleles. We examined this association using the defined genotypes by Gleicher and his colleagues, where they defined a normal allele as those with 26-34 repeats and abnormal alleles as those outside of this range.34,35 None of the women in our study who were either heterozygous or homozygous abnormal had menopausal age before the age of 40. Most had menopausal age above 45 years. Additional studies need to be conducted to further evaluate the association of these genotypes on phenotypes associated with ovarian function.36-39
We observed a significant effect of ascertainment location of our study population. Several possible explanations for this effect have been explored. We excluded a difference in distribution of CGG repeat sizes and the proportion of premutation carriers in both groups as a basis for the observed ascertainment location effect. Also, a difference in genetic background of the Dutch and American populations seems unlikely because of the inclusion of Caucasians only. Most Caucasian Americans are from European descent. One explanation could be the manner in which the interviews were taken. In the Atlanta study, the reproductive questionnaire was part of a large assessment, often including neuropsychological, medical and neurological assessments as well. The Nijmegen study was set up for reproductive evaluation and the study information included a description of FXPOI, which could result in participants reporting menopause at an earlier age than when it actually occurred.
A limitation of all cross-sectional studies is the use of time-dependent variables such as smoking, BMI, parity, and hormone use. Such variables are preferably analyzed by an extended Cox proportional hazards model. However, exact information of these variables per subject at different time points between 25 years of age and the start of menopause were lacking. Therefore, these variables were applied as if they were fixed at the age of 25 years. Even though this is a raw approximation of the reality, results based on this approach are at least indicative for the overall effect of these variables on menopausal age. In addition, age at menopause is a transitory state and subject to reporting and recall bias. A previous study on recall of menopausal age showed a bias towards the mean as women deviated from the final menstrual period.40 In our case, recall and memory bias might have led to an earlier menopausal age because of awareness for FXPOI.
Furthermore, we have used age at menopause as a surrogate for severity of FXPOI, but in general POI should not be considered a natural menopause, because of intermittent ovarian function and a 5-10% chance of pregnancy after diagnosis.41 We used the definition of amenorrhea for at least 1 year due to menopause as a uniform criterion that applies to older and younger women.
Taken together, our data strongly indicate that a prediction model for FXPOI is feasible, when taking ascertainment site into account. This effect clearly confirms the importance of data collection and ascertainment protocols, as they could bias results. Nevertheless, we consider our model as a first step in developing clinically applicable risk estimates for FXPOI, which could facilitate counseling. To further develop and individualize risk estimates for FXPOI, a predictor based on endocrine markers, such as AMH, could be included. AMH is an early marker of ovarian reserve and a very promising predictor for menopause and POI in general, and for premutation carriers in particular.42 The decline in ovarian reserve with time in premutation carriers with FXPOI, however, is not fully comprehended yet. Development of reference values of AMH by age for premutation carriers might allow early identification of women at high risk for FXPOI. In addition, examination of different ethnic/racial groups might be important in these prediction models. Studies have shown that the frequency distribution of FMR1 alleles differs among groups,43,44 however, no studies have examined whether the affect of the FMR1 alleles differs by ethnic/racial group. Using our current model based on RIR, mean menopausal age of first degree relatives with the same mutation status and smoking, premutation carriers with a high risk for FXPOI might benefit from monitoring of ovarian reserve regularly and consider vitrification, i.e., cryopreservation of oocytes.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the volunteers and their families whose participation made the work possible. This work was supported in part by National Institutes of Health grants NIH RO1 HD29909 and NIH PO1 HD35576 (SLS, EGA).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38:269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- (2).Schwartz CE, Dean J, Howard-Peebles PN, et al. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- (3).Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study-preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- (4).Murray A, Webb J, Grimley S, Conway G, Jacobs P. Studies of FRAXA and FRAXE in women with premature ovarian failure. J Med Genet. 1998;35:637–640. doi: 10.1136/jmg.35.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- (6).Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- (7).Kronquist KE, Sherman SL, Spector EB. Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genet Med. 2008;10:845–847. doi: 10.1097/GIM.0b013e31818b0c8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- (9).Cramer DW, Xu H, Harlow BL. Family history as a predictor of early menopause. Fertil Steril. 1995;64:740–745. doi: 10.1016/s0015-0282(16)57849-2. [DOI] [PubMed] [Google Scholar]
- (10).de Bruin JP, Bovenhuis H, van Noord PA, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16:2014–2018. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- (11).Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet. 1998;352:1084–1085. doi: 10.1016/S0140-6736(05)79753-1. [DOI] [PubMed] [Google Scholar]
- (12).van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde ER. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- (13).Allen EG, Sullivan AK, Marcus M, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- (14).Sullivan AK, Marcus M, Epstein MP, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- (15).Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Tejada MI, Garcia-Alegria E, Bilbao A, et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause. 2008;15:945–949. doi: 10.1097/gme.0b013e3181647762. [DOI] [PubMed] [Google Scholar]
- (17).Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72:454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- (19).Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- (20).Brown WT, Houck GE, Jr., Jeziorowska A, et al. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993;270:1569–1575. [PubMed] [Google Scholar]
- (21).Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- (22).Do KA, Treloar SA, Pandeya N, et al. Predictive factors of age at menopause in a large Australian twin study. Hum Biol. 1998;70:1073–1091. [PubMed] [Google Scholar]
- (23).Torgerson DJ, Avenell A, Russell IT, Reid DM. Factors associated with onset of menopause in women aged 45-49. Maturitas. 1994;19:83–92. doi: 10.1016/0378-5122(94)90057-4. [DOI] [PubMed] [Google Scholar]
- (24).Hunter JE, Epstein MP, Tinker SW, Charen KH, Sherman SL. Fragile X-associated primary ovarian insufficiency: evidence for additional genetic contributions to severity. Genet Epidemiol. 2008;32:553–559. doi: 10.1002/gepi.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- (26).Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990;77:147–160. [Google Scholar]
- (27).Efron B, Tibshirani R. An introduction to the bootstrap. Chapman and Hall; New York: 1993. [Google Scholar]
- (28).Neal MS, Zhu J, Foster WG. Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod Toxicol. 2008;25:100–106. doi: 10.1016/j.reprotox.2007.10.012. [DOI] [PubMed] [Google Scholar]
- (29).Progetto Menopausa Italia Study Group Premature ovarian failure: frequency and risk factors among women attending a network of menopause clinics in Italy. BJOG. 2003;110:59–63. doi: 10.1016/s1470-0328(02)02929-4. [DOI] [PubMed] [Google Scholar]
- (30).Cramer DW, Xu H, Harlow BL. Does “incessant” ovulation increase risk for early menopause? Am J Obstet Gynecol. 1995;172:568–573. doi: 10.1016/0002-9378(95)90574-x. [DOI] [PubMed] [Google Scholar]
- (31).de Vries E, den Tonkelaar I, van Noord PA, van der Schouw YT, te Velde ER, Peeters PH. Oral contraceptive use in relation to age at menopause in the DOM cohort. Hum Reprod. 2001;16:1657–1662. doi: 10.1093/humrep/16.8.1657. [DOI] [PubMed] [Google Scholar]
- (32).Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40:995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- (33).Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod. 2010;25:1335–1338. doi: 10.1093/humrep/deq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online. 2010;20:768–775. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- (35).Gleicher N, Barad DH. The FMR1 gene as regulator of ovarian recruitment and ovarian reserve. Obstet Gynecol Surv. 2010;65:523–530. doi: 10.1097/OGX.0b013e3181f8bdda. [DOI] [PubMed] [Google Scholar]
- (36).Bodega B, Bione S, Dalpra L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21:952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- (37).Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- (38).Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Mullerian hormone. Fertil Steril. 2009;91:1700–1706. doi: 10.1016/j.fertnstert.2008.01.098. [DOI] [PubMed] [Google Scholar]
- (39).Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19:385–390. doi: 10.1016/s1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- (40).Rockhill B, Colditz GA, Rosner B. Bias in breast cancer analyses due to error in age at menopause. Am J Epidemiol. 2000;151:404–408. doi: 10.1093/oxfordjournals.aje.a010220. [DOI] [PubMed] [Google Scholar]
- (41).Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril. 2005;83:1327–1332. doi: 10.1016/j.fertnstert.2004.11.059. [DOI] [PubMed] [Google Scholar]
- (42).Rohr J, Allen EG, Charen K, et al. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- (43).Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gleicher N, Weghofer A, Barad DH. Effects of race/ethnicity on triple CGG counts in the FMR1 gene in infertile women and egg donors. Reprod Biomed Online. 2010;20:485–491. doi: 10.1016/j.rbmo.2009.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.