Summary
In cancer treatment, apoptosis is a well-recognized cell death mechanism through which cytotoxic agents kill tumor cells. Here we report that dying tumor cells use the apoptotic process to generate potent growth-stimulating signals to stimulate the repopulation of tumors undergoing radiotherapy. Surprisingly, activated caspase 3, a key executioner of apoptosis, plays key roles in the growth stimulation. One downstream effector that caspase 3 regulates is prostaglandin E2, which can potently stimulates growth of surviving tumor cells. Deficiency of caspase 3 either in tumor cells or in tumor stroma caused significant tumor sensitivity to radiotherapy in xenograft or mouse tumors. In human cancer patients, higher levels of activated caspase 3 in tumor tissues are correlated with significantly increased rate of recurrence and deaths. We propose the existence of a “Phoenix Rising” pathway of cell death-induced tumor repopulation in which caspase 3 plays key roles.
Introduction
There is a massive amount of cell death during cytotoxic cancer therapy such as radiation therapy1,2. It is normally assumed that dying or dead cells get absorbed by “scavenger” cells such as macrophages or other surviving cells in the vicinity. The small number of surviving tumor cells, if any, would then gradually and slowly proliferate and re-establish the tumor. However, studies that originated more than 40 years ago 3,4 have indicated that tumors respond to radiotherapy by initiating a process called “accelerated repopulation”. In this process, the few surviving cells that escaped death after exposure to radiotherapy or chemotherapy can rapidly repopulate the badly damaged tumor by proliferating at an significantly accelerated pace. This phenomenon, for which little is understood at the molecular level, has played an important role in modern radiotherapy and chemotherapy5.
There have been past efforts to understand the molecular mechanism of tumor repopulation after cytotoxic therapy. For example, radiation-induced up-regulation of angiogenic activities in tumors has been linked with activation of upstream transcriptional factors such as HIF-16. Recent studies have also indicated the importance of macrophages in facilitating tumor recovery after radiation 7,8. Furthermore, the integrity of endothelial cells has been implicated in tumor response to radiotherapy9. These reports, while revealing interesting mechanisms of tumor re-growth, fall short in describing the initial driving events responsible for tumor repopulation after radiotherapy.
In this study, we examined the hypothesis that dying cells in the tumor mass may provide the initial signals to promote tumor repopulation. Specifically, we hypothesized that dying cells could release growth-promoting signals to stimulate the proliferation of surviving cells. Our data demonstrated a crucial role for intratumoral dying cells in promoting the rapid repopulation of tumors from a small number of live tumor cells. In addition, we made the surprising but important discovery that caspase 3, a cysteine protease involved in the “execution” phase of cellular apoptosis, is a key regulator of growth-promoting signals generated from the dying cells. We believe this newly discovered caspase-mediated, cell-death stimulated tumor repopulation mechanism, which we named the “Phoenix Rising” pathway, has profound implications for cancer biology and cancer therapy.
Results
Tumor cell repopulation stimulated by cell death in vitro
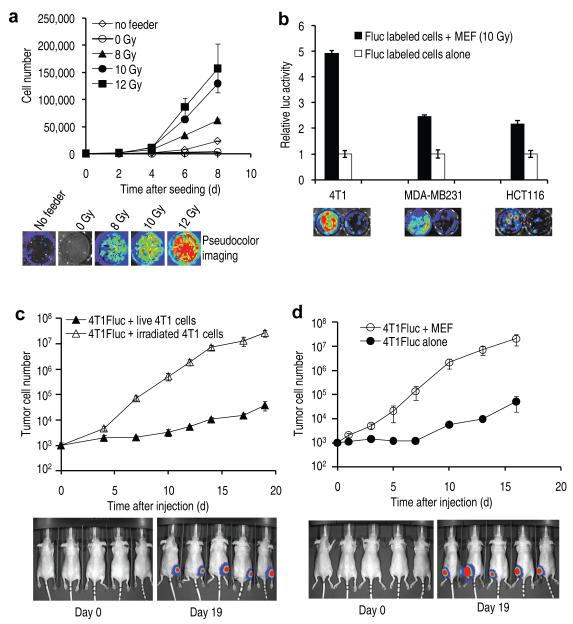
To test our hypothesis, we carried out experiments to examine if dying tumor cells could indeed stimulate the growth of living tumor cells. In order to simulate in vivo scenarios where the vast majority of tumor cells are killed by radiation or chemotherapy, we seeded a small number (about 500) of firefly luciferase (Fluc)-labeled murine breast cancer 4T1 cells onto a bed of a much larger number (2.5×105) of unlabeled “feeder” 4T1 tumor cells that were irradiated with x-rays at different doses. Growth of the small number of labeled living cells was then monitored through non-invasive bioluminescence imaging10 (see Supplementary Fig. 1 for data validating bioluminescence quantification of tumor cells). Our results indicated that 4T1Fluc cells grew significantly faster when seeded onto dying cells than when seeded alone (Fig. 1a). In addition, there was a dose-dependent response from the feeder cells, with non-irradiated feeder cells exhibited no supportive roles and those irradiated with higher radiation doses exhibiting higher growth-enhancing ability (Fig. 1a). Additional supporting evidence came from combinations of other dying vs living cell types, which also showed growth-stimulating properties (Supplementary Figs. 2 and 3).
Figure 1.
In vitro and in vivo evidence for the generation of strong growth-stimulating signals in dying cells.
(a) Stimulation of 4T1Fluc cellular growth in vitro by irradiated 4T1 cells. Top panel: growth of 4T1Fluc cells as observed by luciferase activities. The difference between each of the higher dose irradiated groups (8, 10, and 12 Gys) and controls (0 Gy and no feeder) is statistically significant (P<0.001, t-test). Error bars: SEM, n=4. Middle panel: representative images from bioluminescence imaging. Lower panel: selected photographs of cellular growth after crystal violet staining.
(b) Stimulation of Fluc-labeled cellular growth in vitro by irradiated mouse embryonic fibroblast (MEF) cells. Top panel, relative growth of MEF-supported tumor cells vs tumor cells seeded alone. Error bars: SEM, n=3. Lower panel, representative bioluminescence images. In all three cases, the difference in cellular growth between those with and those without MEF feeders are statistically significant (P<0.001, t test).
(c) Effect of tumor cell death on 4T1Fluc tumor cellular growth in vivo. Top panel: growth of 4T1Fluc cells as followed by luminescence signals. The difference between the two groups were highly significant (P<0.001 from day 4, one way ANOVA test). Error bars: SEM, n=5. Lower panel, representative images of mice at early and late stages of tumor growth.
(d) Effect of dying MEF cells on 4T1Fluc tumor cellular growth in vivo. Top panel, growth of 4T1Fluc cells. The difference between the two groups were again highly significant (P<0.001 from day 1, one way ANOVA test). Error bars: SEM, n=5. Lower panel, representative images of mice at early and late stages of tumor growth.
Because in solid tumors stromal cells play important roles in modulating tumor growth, we also evaluated whether dying fibroblast cells could promote tumor cell growth. Lethally irradiated mouse embryonic fibroblast cells stimulated the growth of different Fluc-labeled tumor cells significantly in vitro (Fig. 1b).
Tumor cell repopulation stimulated by cell death in vivo
We next examined whether cell death-stimulated tumor cell proliferation could be observed in vivo. A mix of untreated, Fluc-labeled and lethally irradiated, unlabeled tumor cells (at a ratio of 1:250, or 1000 live 4T1Fluc cells mixed with 2.5×105 unlabeled, lethally irradiated 4T1 cells) were injected subcutaneously into the hind legs of nude mice. Subsequently the growth of the Fluc-labeled tumor cells was followed non-invasively over time through bioluminescence imaging. As controls, an equal number of Fluc-labeled 4T1 tumor cells mixed with live, unlabeled 4T1 tumor cells were injected into contra-lateral hind legs. Our results show that the presence of lethally irradiated tumor cells significantly increased the growth of Fluc-labeled tumor cells when compared with Fluc-labeled tumor cells injected together with live tumor cells. In fact the difference in the intensities of luciferase signals between the two groups grew exponentially larger and reached as great as 700 fold at the end of the experiment (Fig. 1c).
Similar in vivo tumor growth-promoting properties were also observed for mouse embryonic fibroblasts (MEF) that were irradiated (Fig. 1d). Fluc-labeled 4T1 co-injected with irradiated fibroblast cells grew to signal intensities 400 fold more than those from 4T1-Fluc cells injected alone in contra-lateral hind legs.
Caspase 3 regulates tumor cell repopulation in vitro
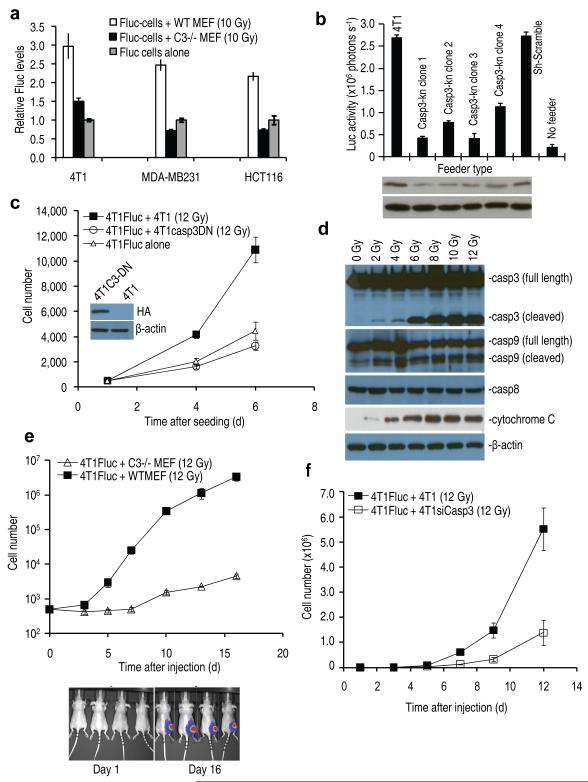
What is the molecular mechanism for the observed cell death-stimulated proliferation of surviving cells? We reasoned that among the many cellular processes activated/deactivated in dying cells, factors/processes directly responsible for cell death are most likely to play key roles in regulating the growth-promoting properties of dying cells. Therefore, we hypothesized that caspases, the proteases that were involved in both the initiation and the execution of programmed cell death11, might be involved in regulating the growth-promoting properties of dying cells. To examine this hypothesis, we obtained mouse embryonic fibroblast (MEF) cells with genetic deletion of their Caspase 3 gene12,13 and evaluated the ability of these cells to support the growth of a small number of Fluc-labeled tumor cells. Our results (Fig. 2a) indicate clearly that deficiencies in Casp3 significantly compromised the ability of lethally irradiated MEF cells to stimulate the growth of Fluc-labeled murine (4T1) and human (MDA-MB231 and HCT116) tumor cells. The proliferation of Fluc-labeled tumor cells among the irradiated Caspase 3 deficient (Casp3−/−) cells was close to Fluc-labeled tumor cells seeded alone, suggesting that caspase 3 was largely responsible for growth stimulation by dying cells.
Figure 2.
The role of caspase 3 in cell death-induced tumor cell proliferation in vitro and in vivo.
(a) The effect of caspase 3 deficiency on dying cells stimulated tumor growth in vitro. In all three groups, the difference between wild type and casp3−/− MEF cells are statistically significant (P<0.01, t-test). Error bars: SEM, n=3.
(b) The effect of caspase 3 knockdown(Casp3-kn) in 4T1 cells used a feeder cells. The difference between the control groups and the casp3kn groups are statistically significant (P<0.01 between each of the control and each of the caspase 3 knock down clones, n=3, t-test). Error bars: SEM, n=3. Lower panel shows western analysis of caspase 3 levels.
(c) The effect of a dominant negative caspase 3 in dying, unlabeled 4T1 cells on 4T1Fluc tumor cell growth. Inset, western blot showing dominant negative caspase 3 expression. (d) Western blot analyses of key proteins involved in apoptosis.
(e) The effect of lethally irradiated wild type and Casp3−/− MEF cells on growth 4T1Fluc cells in mice. Error bars: SEM, n=4. The difference between two groups were highly significant statistically (P<0.001 from day 3 on, one-way ANOVA test). Lower panel shows representative bioluminescent images.
(f) The effect of caspase 3 knockdown in lethally irradiated 4T1 cells on growth of 4T1Fluc cells in vivo. The difference between wild type 4T1 and 4T1-siCasp3 groups is statistically significant from day 5 (P<0.05, n=5, one-way ANOVA test).
The importance of caspase 3 was also confirmed in lethally irradiated 4T1 cells through shRNA-mediated knockdown of Casp3 expression in feeder cells (Fig. 2b). It was similarly confirmed in the human breast cancer cell line MCF7, which is deficient in casp3 expression. Exogenous expression of caspase 3 significantly increased the ability of lethally irradiated MCF-7 cells to promote co-seeded MCF-7Fluc cells (Supplementary Fig. 4).
Because Casp3−/− and wild type MEF cells showed similar clonogenic survival after irradiation (Supplementary Fig. 5), we reasoned the observed defect for Casp3−/− MEF cells was not due to the lack of cell death in these cells after radiation. We obtained further data indicating how caspase 3 status will affect the modes of cell death in 4T1 (Supplementary Figure 6), MEF (Supplementary Figure 7), and MCF-7 (Supplementary Figure 8) after radiation. Please refer to Supplementary Notes section for additional information on these data.
To examine whether the proteolytic activity of caspase 3 is required, we transduced a dominant-negative version of caspase 3 (C163A)14 to inhibit caspase 3 cleavage activity in 4T1 cells. Our data indicated that 4T1 cells transduced with a dominant-negative Casp3 (C163A)14 gene completely lost its ability to support the growth of 4T1Fluc cells (Fig. 2c). We also obtained similar results by use of a chemical inhibitor of caspase 3 z-VAD-fmk (Supplementary Fig. 9).
To confirm that caspase 3 was activated in irradiated cells, we carried out comprehensive immunoblot analyses of various proteins in the apoptotic pathway in irradiated 4T1 (Fig. 2d, see Supplementary Table 1 for antibody information), and MEF (Supplementary Fig. 10) cells. Our data indicate caspase 3&9 and downstream cytochrome c were activated in both 4T1 and MEF cells in a dose-dependent manner while caspase 8 was not activated.
Caspase 3 regulation of tumor cell repopulation in vivo
To examine the importance of caspase 3 in cell-death stimulated tumor repopulation in vivo, lethally irradiated MEF cells with different capase 3 status were mixed together with a small number (500) of Fluc-labeled 4T1 cells and injected subcutaneously into nude mice (Fig. 2e). In contrast to potent stimulation of 4T1Fluc tumor cellular growth by lethally irradiated wild type MEF cells, significantly attenuated growth stimulation was observed for lethally irradiated caspase 3-deficient MEF cells. The difference between the two groups was as great as 1000 fold at later stages of observation (Fig. 2e). In fact, in separate experiments, 4T1-Fluc cells injected together with lethally irradiated caspase 3-deficient MEF cells grew at a similar rate as those 4T1Fluc cells injected alone in the contra-lateral legs, indicating a lack of growth stimulation from Casp3−/− cells (Supplementary Fig. 11).
The importance of caspase 3 in regulating growth-promoting properties of dying cells in vivo was also confirmed by co-injecting 4T1-Fluc cells with lethally irradiated 4T1 transduced with an shRNA minigene targeted against caspase 3 (Fig. 2f). A significant reduction in the ability of lethally irradiated 4T1 cells to stimulate the growth of 4T1-Fluc cells were observed, consistent with the results obtained with Casp3−/− MEF cells.
Caspase 3 activation and growth of injected tumor cells in established tumors
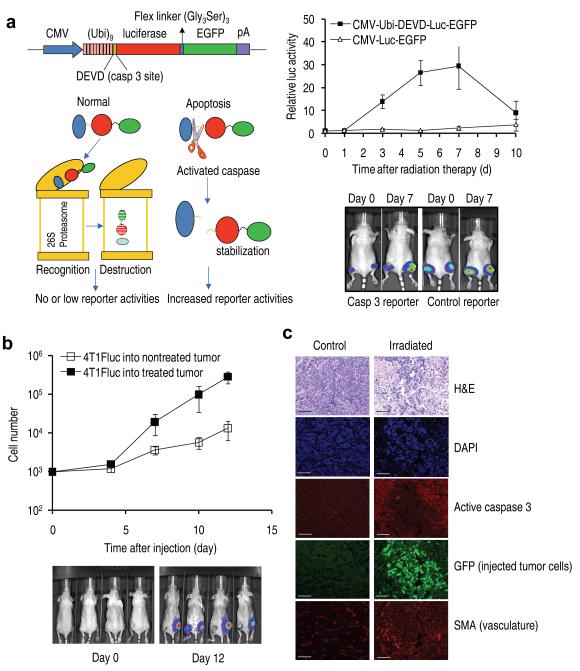
To show that caspase 3 activation is indeed activated in solid tumors during radiotherapy, we established murine 4T1 tumor cells transduced with a novel luciferase/GFP and proteasome-based reporter (Fig. 3a, left panel). Supplementary Fig. 12 shows data supporting the functionality of our caspase 3 reporter. Tumors were irradiated with x-rays (6 Gy) when they reach 5-7 mm in diameter and observed for bioluminescence signals periodically afterwards. It is clear that caspase 3 was significantly activated (as much as 30 fold at its peak) at days 3, 5, and 7 after radiotherapy (Fig. 3a, right panels).
Figure 3.
Relationship between caspase activation and growth of externally injected tumor cells in the irradiated tumor microenvironment.
(a) Caspase 3 activation in 4T1 tumors as detected by a caspase 3 reporter. Left panels depict the structure of a proteasome-based caspase 3 reporter (top left) and its principle of action (lower left). Right panels showed caspase 3 activities in 4T1 tumors transduced with the control as well as caspase reporter genes. The difference between the control and caspase 3 reporter groups are significant at days 3, 5, and 7 (P<0.01, n=5, t-test). Error bars: SEM.
(b) Growth of the 4T1Fluc cells injected into irradiated and non-irradiated established tumors. The difference between the two groups were statistically significant (P<0.05 from day 7, t test). Error bars: SEM, n=4. Lower panel shows representative images of tumor-bearing mice at early and late stages of observation.
(c) Imnnofluorescence analysis of growth of intratumorally injected GFP-labeled cells and key protein expression surrounding the injected cells. SMA, smooth muscle actin, a marker for blood vessels. The size bars represent 100μm.
In another experiment, a small number of Fluc-labeled 4T1 tumor cells (about 1000) were injected into irradiated and non-irradiated tumors in the same group of mice and observed for growth. Our results (Fig. 3b) demonstrated that cells injected into irradiated tumors grew at a significantly faster rate than those injected into the non-treated tumors, consistent with earlier results.
In still another experiment, 4T1 cells stably transduced with green fluorescence protein (GFP) was injected into irradiated tumors and allowed to grow for 5-8 days. The mice were then sacrificed and tumors excised and examined for expression of various proteins (Figure 3c). Our data show a clear relationship between activated caspase 3 and the proliferation of injected, GFP-labeled tumor cells in irradiated tumor cells, as demonstrated by the complementary pattern of caspase 3 staining and injected tumor cell staining. In contrast, very little injected tumor cell growth and caspase activation was observed in non-irradiate tumors. In addition to caspase activation, irradiated tumors also showed increased, localized neovaculature.
Caspase 3 activates iPLA2 to stimulate tumor repopulation
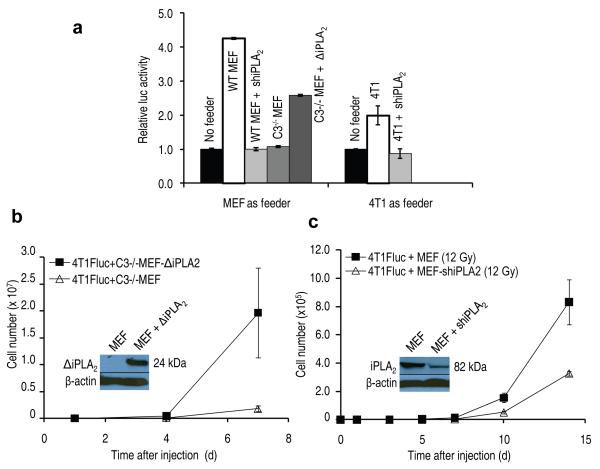
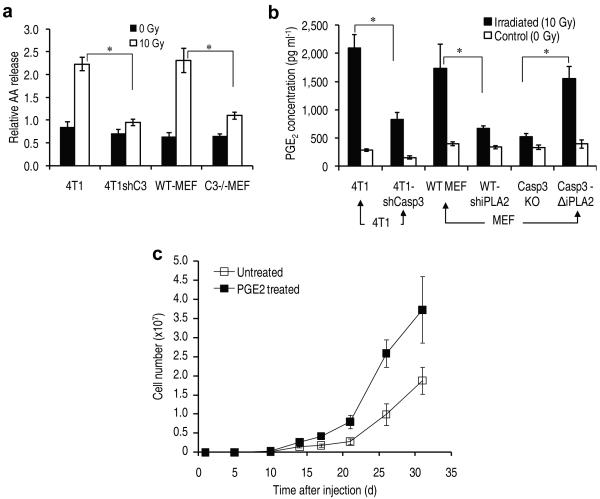
We next attempted to identify the downstream factors of caspase 3 that were involved in generating growth-promoting factors from the dying cells. We focused on calcium independent phospholipase A2 (iPLA2) because it had been reported that its phosopholipase activity was activated by caspase 3 cleavage15-17. Importantly, the activation was shown to increase production of arachidonic acid (AA)15, whose downstream eicosanoid derivatives (i.e. prostaglandin E2), had been implicated in stimulating tumor growth18 and stem cell proliferation19. To evaluate the potential involvement of caspase-activated iPLA2-AA-PGE2 axis in cell death-induced tumor cell proliferation, we transduced shRNA against the iPla2 gene into 4T1 tumor cells or wild type MEF cells and examined whether these cells, when lethally irradiated, could still support Fluc-labeled tumor cell growth as much as their wild-type counterpart. Our results (Fig. 4a) indicated in both MEF and 4T1 cells, expression of shiPLA2 significantly reduced ability of these cells to stimulate Fluc-labeled 4T1 cellular proliferation. We also have western blot data to indicate that iPLA2 is indeed activated in a capase 3-dependent manner (Supplementary Fig. 13) in both 4T1 and MEF cells. These results are consistent with multiple earlier reports. 15-17
Figure 4.
An important role for caspase 3-activated iPLA2 in facilitating cell death stimulated tumor cell repopulation.
(a) The effect of iPla2 levels in dying cells on the growth of 4T1Fluc cells in vitro. The differences between wild type feeder cells (MEF or 4T1) and those with iPla2 knockdown are significant, so is the difference between Casp3−/− MEF cells and those transduced with a truncated, constitutively active iPla2 (P<0.01, n=4, t-test).
b). The effect of a constitutively active iPLA2 in Casp3−/− MEF cells in vivo. The differences between the luciferase signals were statistically significant (P<0.01 on days 7, n=5, t-test). Error bars: SEM. The inset shows expression of truncated iPla2.
c). The in vivo effect of iPLA2 knockdown in wild type MEF cells. The difference between the two groups was statistically significant from day 3 (P<0.02, from day 7, n=5, one-way ANOVA). The inset shows western blot analysis of shRNA knockdown of iPla2.
The importance of caspase 3-mediated activation of iPLA2 was further confirmed when a truncated version of the iPla2 gene (ΔiPla2), which encoded an iPla2 fragment (containing the catalytic domain) that was a predicted product of caspase 3 cleavage of iPla2, was transduced into caspase 3-deficient MEF cells. After ΔiPla2 transduction, lethally irradiated Casp3−/− cells had significantly increased ability to stimulate the growth of Fluc-labeled 4T1-Fluc cells both in vitro (Fig. 4a) and in vivo (Fig. 4b and Supplementary Fig. 14) when compared with parental Casp3−/− cells.
We also showed that knocking down iPla2 gene expression significantly reduced the ability of lethally irradiated MEF cells to stimulate the growth of 4T1-Fluc cells in vivo (Fig. 4c and Supplementary Fig. 15). Furthermore, we show that by use of established wild type or iPla2 knockdown 4T1 tumors, growth of subsequently transplanted 4T1 cells was significantly slowed down (Supplementary Fig. 16).
Caspase 3 and iPLA2 stimulate AA and PGE2 release
We next conducted experiments to confirm the role of caspase 3 in regulating the activity of the iPLA2 by examining its enzymatic activities. Because arachidonic acid is one of two main catalytic products of activated iPLA2 (the other being lysophosphatidic choline, or LPC), we measured radiation-induced release of arachidonic acid into the extracellular milieu to examine potential relationship between caspase 3 and arachidonic acid release. Our results indicate that radiation stimulated the release of arachidonic acid into the supernatant significantly in wild type MEF cells (Fig. 5a). However, the release was significantly reduced in Casp3−/−MEF cells, indicating a crucial role for caspase 3. In addition, arachidonic acid (AA) release was also significantly reduced in 4T1 cells with effective knockdown of the Casp3 gene expression (Fig. 5a).
Figure 5. Regulation of radiation-induced arachidonic acid release and PGE2 production by caspase 3.
(a) The role of caspase 3 in radiation-induced arachidonic acid release. Error bars: SEM (n=3). *P<0.02 (t-test).
(b) Regulation of radiation-induced PGE2 secretion by caspase 3. Error bars: SEM (n=3). *P<0.05 (t-test).
(c) PGE2 stimulated tumor growth from 1000 4T1Fluc tumor cells injected subcutaneously into nude mice. Error bars: SEM. The difference between the two groups are statistical significant from day 17 (P<0.05, n=5, one-way ANOVA).
Because prostaglandin E2 (PGE2), a key regulator of tumor growth, is a downstream product of AA, we measured PGE2 production induced by ionizing radiation in the supernatants of cells. Our results (Fig. 5b) indicated that exposure to ionizing radiation significantly induced the production of PGE2 in wild type MEF cells as well as in 4T1 tumor cells. However, in Casp3−/− MEF cells and in 4T1 cells transduced with a shRNA against caspase 3, the production of PGE2 in irradiated cells was significantly reduced. In contrast, transduction of a constitutively active, truncated iPla2 (which is equivalent to a caspase-cleaved version of iPLA2), significantly restored PGE2 production in caspase 3 deficient (Casp3−/−) MEF cells.
In an additional experiments (Supplementary Fig. 17), we showed that cyclooxygenase 2 (Cox 2) and its upstream transcription factor NF-kB, which have crucial roles in PGE2 production, were activated in a caspase 3-independent manner. Consistently, indomethacin (a Cox 1&2 inhibitor) administration effectively suppressed growth of intratumorally injected 4T1 and HCT116 cells (Supplementary Fig. 18).
The importance of PGE2 was demonstrated by the fact that treatment of a small number (1000) of tumor cells with PGE2 gave the treated cells a significant head start, allowing them to grow at a faster pace than non-treated cells when injected into mice (Fig. 5c). On the other hand, shRNA mediated down-regulation of EP2, a receptor for PGE2, in 4T1Fluc cells significantly attenuated the proliferation of the latter when seeded together with lethally irradiated 4T1 cells (Supplementary Fig. 19).
Caspase 3 and radiosensitivities of tumors in mice and man
To examine the intriguing and exciting prospect of enhancing cancer radiotherapy through inhibition of caspase 3, we carried out two experiments in mouse tumor models.
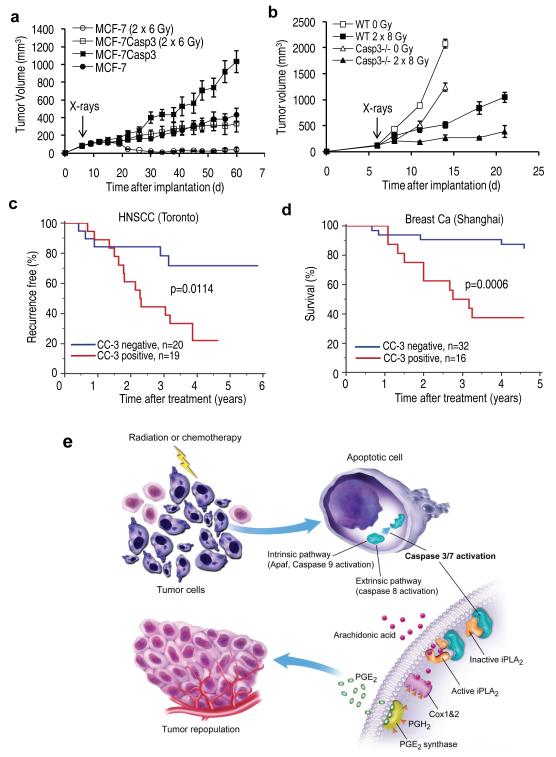
In the first experiment, we used MCF-7 cells, which are naturally deficient in caspase 3 expression. We established tumor xenograft in female nude mice using both parental MCF-7 cells as well as a modified MCF-7 cell line with an exogenously transduced copy of caspase 3 gene. The transduction of CASP3 into parental MCF-7 cells rendered them significantly more susceptible to apoptosis (Supplementary Fig. 8). Surprisingly, caspase 3 transduction also enabled MCF-7 cells to form tumors at a faster pace (Fig. 6a). Mostly importantly, the presence of caspase 3 makes MCF-7CASP3 tumors significantly more resistant to radiotherapy than parental MCF-7 tumors, which disappeared completely after 2×6 Gy or x-rays and did not re-grow during the entire course of observation (Fig. 6a). In contrast, MCF-7CASP3 kept growing after irradiation despite being slowed down. These surprising results indicated that deficiency of caspase 3 activity in tumor cells will render the whole tumor significantly more susceptible to radiotherapy.
Figure 6.
Deficiency in caspase 3 correlated with tumor response to therapy in mice as well as in human patients.
(a) Result of radiation therapy in tumors established from wild type caspase 3-deficient MCF-7 breast cancer cells and MFC-7CASP3 with a exogenous copy of caspase 3 Error bars: SEM. The differences between the two groups are highly significant after radiotherapy (P<10−6, n=10, one – way ANOVA).
(b) Results of radiation therapy B16F10 murine melanoma tumors grown in wild type C57BL6 and Casp3−/− mice. Error bars: SEM. The difference between the two groups are significant after radiotherapy from day 11 (P<0.04, n=5, one-way ANOVA).
(c) Kaplan-Meier analysis of cancer recurrence in a cohort of head and neck squamous cell carcinoma (HNSCC) patients treated at Princess Margaret Hospital in Toronto, Canada. Log-rank test (p-value= 0.0114, HR=3.44, 95%CI: 1.35-8.75) was used for analysis of statistical significance.
(d). Kaplan-Meier analysis of survival in a cohort of advanced breast cancer patients treated at Shanghai No. 1 People’s Hospital. Log-rank test (Log-rank p-value = 0.0006, HR=5.29, 95%CI: 1.70-16.46) was used to analyze statistical significance of the difference in survival between the two groups.
(e) A schematic representation of the “Phoenix Rising” pathway for cell death-mediated tumor cell repopulation.
In the second experiment, we attempted to examine the potential importance of stroma-derived caspase 3 activities in tumor response to radiotherapy because stroma accounted for a significant percentage of tumor mass. We established tumors in wild type as well as Casp3−/− mice with B16F10 cells, which is a metastatic and radioresistant tumor line syngeneic with the C57BL/6 mice. We carried out radiotherapy (2×8 Gy) when tumor reached 5-7 mm in diameter. Our results indicate that B16F10 tumor grown in Casp3−/− mice exhibit significant sensitivity to radiotherapy when compared with those in wild type C57BL/6 mice (Fig. 6b). Our results therefore confirmed the importance of caspase 3 in both tumor cells and tumor stroma in stimulating tumor response to radiotherapy.
In order to determine the relevance of our newly discovered pathway for caspase-mediated tumor repopulation in human cancer treatment, we evaluated caspase 3 status in two cohorts of human cancer patients. In the first cohort, 39 head and neck cancer patients treated with radiotherapy, or chemo-radiotherapy at the Princess Margaret Hospital in Toronto, Canada (see Supplementary Table 2 for details of patient characteristics) were examined for activated caspase 3 through immunohistochemical analysis. Consistent with our results in mice (Fig. 6a, b), patients with high cleaved (and thus activated) caspase 3 levels in their tumor samples showed significantly (p=0.011) higher rate of tumor recurrence (Fig. 6c and Supplementary Fig. 20). In the second cohort, 48 advanced stage breast cancer patients treated at Shanghai No. 1 People’s hospital (see Supplementary Table 3 for details on patient characteristics) were analyzed for activated caspase 3 expression through IHC analysis. Results indicated that patients with high cleaved caspase 3 staining were at a significant (p=0.0002) disadvantage in terms of survival (Fig. 6d and Supplementary Fig. 21). A further piece of supporting evidence came from our own analysis of public domain microarray data from a previously published study20. In 249 breast cancer patients from Sweden and Singapore, elevated caspase 3 mRNA levels correlated with significantly elevated risk (p=0.0001) of relapse (Supplementary Fig. 22). Taken together, we conclude that elevated tumor caspase 3 levels predict for worse treatment outcomes in human cancer patients.
Discussion
For tumor cells exposed to severe stress such as cytotoxic cancer therapeutics, life and death are the two polar opposites of cellular fates. In this study, we have provided strong evidence that these two fates are inextricably associated, manifested by the surprising observation that apoptotic tumor cells stimulate the repopulation of tumors from a small number of surviving cells. Even more surprising is the revelation that caspase 3, the master “executioner” during apoptotic cell death, serves as a direct link between cell death and tumor repopulation. Despite the paradoxical nature of these results at first glance, however, we believe the observed link between cellular death and proliferation may be one of the key mechanisms of metazoan tissue homeostasis exploited by tumors to preserve themselves when damaged by cytoxic treatments. Indeed, the phenomenon of apoptosis-stimulated tissue regeneration has been observed by other investigators in lower organisms such as Drosophila and hydra systems21-23. Under those circum stances, it has been proposed that apoptotic cells stimulate so-called “compensatory proliferation” for tissue regenration. The mechanisms involved in those circumstances are not entirely clear. However, it was reported that β-catenin/wnt signaling was involved in some instances of compensatory proliferation23. In this context, it is of interest to note that PGE2 has been shown to activate the β-catenin/wnt signaling pathway in colon cancer18. Therefore the caspase-iPLA2 pathway that we unveiled here may be part of the evolutionarily conserved mechanism for tissue repair.
Although a direct role for caspase 3 in stimulating tumor repopulation has not been described before, there have been many published studies on non-apoptotic roles of caspases in mammalian biology. Examples of these include caspase-mediated stimulation of differentiation24-26, de-differentiation27, and T-cell activation28. In addition, because caspase status affect how cells die, they can in theory affect the tumor microenvironment in significant way, which can also affect the outcome of radiotherapy. Our results in Fig. 6b clearly demonstrate the effect of caspase 3 on tumor stroma. Further, we also have data to indicate that there is significant influx of macrophages (Supplementary Fig. 23). Other possible effects include how the host immune system will be mobilized against cancer, which will be heavily influenced by how cells die (for example, necrosis vs apoptosis).
One of the most important practical implications of this study is a novel and counter-intuitive approach to enhance cancer radiotherapy through caspase 3 inhibition. Surprisingly positive results shown in Fig. 6a,b point to the potential efficacy of adjuvant use of caspase inhibitors in radiotherapy. Another important implication of our study is the potential use of activated caspase 3 as a biomaker to predict tumor response to treatment, as supported by data from IHC analyses of human tumor samples (Fig. 6c,d).
Finally, because the newly discovered mechanism in this study enables dying cells to stimulate the proliferation of surviving tumor cells and subsequent repopulation of tumors, we propose to name the mechanism the “Phoenix Rising” pathway (Fig. 6e). This mechanism not only has profound implications in our understanding of cancer biology and treatment, it also provides new insights into how metazoan organisms repair tissue damage in general29.
Supplementary Material
Acknowledgements
We thank R. Flavell of Yale University for providing us with MEF cells with caspase 3 or 7 deficiencies. We thank B. Liu of University of Colorado School of Medicine for providing us with the MCF-7 and MCF-7CASP3 cells. We also thank Dr. P. Kabos for insightful discussions. This study was supported in part by grants CA131408 and CA136748 from the US National Cancer Institute (to C-Y Li), and grant NNX09AH19G (to C-Y Li) from US National Aeronautics and Space Administration Ground-based Space Radiation Biology Research Program. It was also supported by a subcontract (to C-Y Li) of grant DEFG0207ER64350 (to JS Bedford) from the US Department of Energy Low Dose Radiation Research Program. Qian Huang was supported by Grant 2010CB529900 from the National Basic Research Project of China and Outstanding Young Scientist grants 30325043 & 30428015 from China National Natural Science Foundation. None of the authors have any financial interest in the work that is presented in this study.
Footnotes
Competing Financial Interest The authors declare no competing financial interest.
Online Methods Please see Supplementary Method section for details of our experimental methods.
For animal experiments, all procedures were approved by the University of Colorado Denver Institutional Animal Care and Use Committee (IACUC).
Clinical tumor samples from Princess Margaret Hospital and Shanghai No. 1 People’s Hospital were acquired with informed consent following protocols approved by institutional review boards at the two hospitals.
Supplementary Information Supplementary Information contains 23 supplementary figures, supplementary notes, supplementary methods, and 3 supplementary tables.
References
- 1.Steel GG. Cell survival as a determinant of tumor response. In: Steel GG, editor. Basic Clinical Radiobiology. Hodder Arnold; London: 2002. pp. 52–63. [Google Scholar]
- 2.Gilewski T, Norton L. Cytokinetics. In: Kufe D, et al., editors. Cancer Medicine (7) BC Decker Inc; Hamilton, London: 2006. pp. 570–589. [Google Scholar]
- 3.Hermens AF, Barendsen GW. Changes of cell proliferation characteristics in a rat rhabdomyosarcoma before and after x-irradiation. Eur J Cancer. 1969;5:173–189. doi: 10.1016/0014-2964(69)90065-6. [DOI] [PubMed] [Google Scholar]
- 4.Stephens TC, Currie GA, Peacock JH. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer. 1978;38:573–582. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall E, Giaccia A. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 6.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 7.Li F, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 10.Contag CH, Jenkins D, Contag PR, Negrin RS. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia. 2000;2:41–52. doi: 10.1038/sj.neo.7900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 12.Kuida K, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 13.Lakhani SA, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 15.Atsumi G, et al. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- 16.Lauber K, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, et al. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J Biol Chem. 2006;281:29357–29368. doi: 10.1074/jbc.M513105200. [DOI] [PubMed] [Google Scholar]
- 18.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 19.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivshina AV, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 21.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chera S, et al. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kang TB, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 25.Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. Active caspase-3 is required for osteoclast differentiation. J Cell Physiol. 2006;209:836–844. doi: 10.1002/jcp.20770. [DOI] [PubMed] [Google Scholar]
- 26.Fujita J, et al. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, et al. Apoptotic caspases regulate induction of iPSCs from human fibroblasts. Cell Stem Cell. 2010;7:508–520. doi: 10.1016/j.stem.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. Cover story for Feb.23, 2010 issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.