Abstract
Curcumin is a plant-derived dietary spice ascribed various biological activities. Curcumin therapeutic applications have been studied in a variety of conditions, but not on periodontal disease. Periodontal disease is a chronic inflammatory condition initiated by an immune response to microorganisms of the dental biofilm. Experimental periodontal disease was induced in rats by injecting LPS in the gingival tissues on the palatal aspect of upper first molars (30 ug LPS, 3 times/week for 2 weeks). Curcumin was administered to rats daily via oral gavage at 30 and 100 mg/Kg. RT-qPCR and ELISA were used to determine the expression of IL-6, TNF-α and PGE2 synthase on the gingival tissues. The inflammatory status was evaluated by stereometric and descriptive analysis on H&E-stained sections, whereas modulation of p38 MAPK and NK-κB signaling was assessed by Western blot. Curcumin effectively inhibited cytokine gene expression at mRNA and protein levels, but NF-kB was inhibited only with the lower dose of curcumin, whereas p38 MAPK activation was not affected. Curcumin produced a significant reduction on the inflammatory infiltrate and increased collagen content and fibroblastic cell numbers. Curcumin potently inhibits innate immune responses associated with periodontal disease, suggesting a therapeutic potential in this chronic inflammatory condition.
Keywords: Curcumin, inflammation, innate immunity, periodontal disease
Introduction
The role of bacteria in the initiation and progress of periodontal disease is undisputed, and lipopolysaccharide (LPS) is one of the main microbial-associated molecular patterns (MAMPs) that can stimulate the expression and production of matrix metalloproteases (MMPs) and proinflammatory cytokines through activation of TLRs. These cytokines are considered the integral players involved in the recruitment of cells and production of effector molecules, such as MMPs and RANKL, that mediate degradation of soft and hard tissues, which are the hallmarks of destructive periodontal disease. This critical role of LPS for periodontal diseases is demonstrated in the LPS-model of experimentally induced periodontal disease, in which direct injection of LPS into the gingival tissues initiates a local host response that involves recruitment of inflammatory cells, generation of prostanoids and cytokines, elaboration of lytic enzymes and activation of osteoclasts, culminating in the degradation of both soft and mineralized tissues of the periodontium1, 2
Curcumin, an extended pseudosymetric polyphenol extracted from plants and used as a dietary spice, has a variety of biological activities including anti-inflammatory properties, which have been partially attributed to direct interaction with protein targets.3 It has been found to modulate the growth and cellular response of various cell types involved in the immune response including T- and B-lymphocytes, macrophages, neutrophils, NK cells and dendritic cells.4
Modulation of innate immunity is supported by the report that curcumin efficiently blocked LPS-induced expression of IL-12, IL-1β, IL-6 and TNF-α in dendritic cells. The same study showed that treatment of dendritic cells with curcumin before LPS stimulation completely suppressed the MAPK and nuclear translocation of NF-κB.5
Besides influencing the activation of the innate immune response by MAMPs, some studies suggest that anti-inflammatory, chemo-preventive and other beneficial effects of certain dietary phytochemicals may be at least in part mediated through the modulation of TLR activation induced by endogenous molecules or chronic infection.6–8 Thus, the immunomodulatory activity of curcumin can involve direct targeting of TLRs. For example, the cellular response to LPS is initiated by its interaction with the transmembrane complex including TLR4 and myeloid differentiation protein 2 (MD-2/TLR4). According to Gradisar et al.6, curcumin binds with high affinity to MD-2 competing with LPS for the same binding site.
These immunomodulatory effects of curcumin can have important implications for the epidemiology, due to its widespread dietary use in some cultures, and treatment of infectious and non-infectious diseases associated with chronic inflammation.6 The therapeutic possibilities of this phytochemical are especially promising due to the lack of adverse effects associated with its consumption.
In this study we assessed the effect of orally administered curcumin on the expression of pro-inflammatory cytokines IL-6, TNF-α and PGE-2 and on the modulation of the signaling pathways NF-κB and p-38 in a model of LPS-induced periodontal disease in rats.
Methods
Experimental design
All the experimental protocols were approved by the Ethical Committee for Animal Experimentation (CEEA) of the School of Dentistry at Araraquara – UNESP and performed in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA).
Sixty male Holtzman rats (Rattus norvegicus albinus, Holtzman), weighing between 100 and 200 g, were randomly distributed into the following experimental groups, comprising 10 animals each: 1) vehicle control (injected with PBS), 2) vehicle-periodontal disease (PD) (injected with LPS), 3) curcumin 30 mg/Kg control (injected with PBS), 4) curcumin 30 mg/Kg PD (injected with LPS), 5) curcumin 100 mg/Kg control (injected with PBS) and 6) curcumin 100 mg/Kg PD (injected with LPS). The rats were kept in a room with controlled temperature (21 ± 1°C) and humidity (65–70%) and a 12 h light–dark cycle.
General anesthesia was induced with intraperitoneal injections of ketamine and xylazine chlorydrate at 0.08 mL/100g body weight and 0.04 mL/100g body weight, respectively. 30 µg of Eschericia coli LPS (strain 055:B5 - Sigma Chem Co., St. Louis, MO, USA) diluted in PBS was injected into the palatal gingiva (3 µL volume per injection) using a Hamilton micro-syringe (Agilent). Injections were performed between the upper 1st and 2nd molars bilaterally three times a week for 15 days (a total of 6 injections and 180 µg of LPS in each site). Control animals received injections of the same volume of PBS vehicle. Administration of curcumin started the day before beginning of LPS injections for the induction of periodontal disease. Curcumin diluted in corn oil vehicle was administered daily by oral gavage in two different doses: one group received the lower dose of 30 mg/Kg/body weight, and the other group received 100 mg/Kg/body weight. Control animals were given the same volume of the corn oil vehicle.
After 15 days the animals were sacrificed and the maxillae were harvested and hemisected. Some of the block sections including 1st and 2nd molars with their surrounding tissues were submitted to routine histological processing to be used in the stereometry, whereas the soft tissues surrounding the 1st molars were carefully dissected for extraction of RNA and protein. For the stereometric analysis, 3 blocks including the upper molars of the control group (treated with vehicle by oral gavage) were analyzed, whereas 4 blocks were analyzed for each of the experimental groups treated with curcumin. These tissue blocks were immersed directly in 10% buffered formalin fixative solution for 48 h and decalcified in tetrasodium-EDTA aqueous solution (0.5 M, pH 7.4) during 2–3 months, under agitation at room temperature. Each specimen consisted of a section containing the 1st and 2nd molars and the surrounding alveolar process and soft tissues and was included in paraffin blocks. Serial sections of 4 µm thickness were obtained in the bucco-lingual direction and stained with hematoxylin-eosin (H/E).
Stereometry
The analysis was conducted by a single examiner that was blind to the experimental groups using an optical microscope (Diastar Cambridge) set at 200 X magnification. Semi-serial sections of 4 µm were obtained from the tissue blocks on a buccal-lingual orientation. A total of 3 sections, spaced 100 µm from each other, were evaluated per tooth. A 50 × 50 µm grid was overlayed the histological images allowing the analysis of an ‘area of interest’ of 2,500 µm2. Two grids composed of 5 × 5 squares of 10 µm each were used in each histological image: one was positioned with its lower border 25 µm below the top of the alveolar bone crest and perpendicularly to the root surface, and the other was positioned with its upper border at the base of the junctional epithelium, representing the ‘bone crest’ and ‘submarginal’ areas, respectively. In both cases, the lateral border of the grids was always positioned over the most prominent part of the root surface in the area. Each one of the 25 points of the grid projected on each section was counted and the proportion of collagen, fibroblastic cells and inflammatory cells (distinguished by the morphological characteristics) in the area of interest was determined as percentage of the total points counted. A total of three images obtained from equally spaced slides (spanning 900 µm of the buccal-lingual aspect of the molars) were evaluated from each animal. Slides from at least four different animals in each experimental group were used.
Evaluation of cytokine gene expression at the mRNA level (RT-qPCR)
Total RNA was extracted from tissue samples using Trizol reagent (Invitrogen Corp) according to the manufacturer’s instructions and 700 ng of total RNA were reverse-transcribed into cDNA using random hexamers as primers (High Capacity cDNA synthesis kit, Applied Biosystems). The cDNA was used for real-time reverse transcription-PCR (RT-qPCR) using Taqman chemistry and pre-designed sets of primers and probes (TaqMan Gene Expression Assays, Applied Biosystems) on a StepOne Plus Real-Time PCR System (Applied Biosystems). The reactions were carried out in a 96-well plate on a final reaction volume of 30 µL that included Taqman Universal PCR Master Mix (Applied Biosystems), Taqman Gene Expression Assays (Applied Biosystems) for each target gene: TNF-α (tumor necrosis factor alpha), NM_013693; IL-6 (interleukin 6), NM031168; Ptgs-2 (prostaglandin-endoperoxide synthase-2), NM_011198; Gapdh (glyceraldehyde-3-phosphate dehydrogenase), NM_008084; and cDNA template (corresponding to 30 ng of cDNA). Optimized thermal cycling conditions were: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. For each sample, analyses of gene expression were performed in duplicate. The experiments were performed with samples isolated from at least three different animals in each experimental group. To normalize the amount of mRNA present in each reaction, the expression of Gapdh, which was not altered by the experimental conditions, was used as a housekeeping gene. To compare the expression levels among different samples, the relative expression level of the genes was calculated using the comparative ΔCT method using the thermocycler’s software.
Activation of signaling pathways (Western-blot) and determination of cytokine gene expression at the protein level (ELISA)
Total proteins were extracted from gingival tissue samples using a detergent-based extraction buffer (T-PER, Tissue Protein Extraction Reagent – Pierce Biotechnology) containing a protease inhibitor cocktail (Protein Stabilizing Cocktail – Santa Cruz Biotechnology) according to manufacturer’s instructions (Pierce Biotechnology). The proteins were quantified using Lowry method (DC assay, Bio-Rad Laboratories) and 60 µg of total protein were heated-denaturated and loaded on 10% SDS-polyacrylamide gels.
After electrophoretic separation, proteins were electro-transferred to 0.2 µm nitrocellulose membranes. These membranes were blocked in Tris-buffered saline containing 5% non-fat dry milk and 0.1% Tween-20 and incubated with the primary antibodies overnight at 4°C (1:100 dilution in PBS – phospho-p65 and phospho-p38 – Cell signaling). Detection of the primary antibodies was done with secondary antibodies conjugated to horseradish peroxidase (1:1000 dilution in the blocking buffer) and a chemiluminescence system (Lumi-Glo, Cell Signaling).
Proteins from these same samples were also used in the ELISA assays to determine the concentration of PGE2, IL-6 and TNF-α. These assays were performed according to the manufacturer’s instructions (R&D Systems) and the results were normalized to the total concentration of protein in the samples. Samples from at least three different animals in each experimental group were used and assayed in duplicate.
Data analysis
The purpose of data analysis was to compare the results from curcumin-treated animals with those of vehicle treated control animals. Considering the two experimental groups treated with different doses of curcumin (30 and 100 mg/kg body weight) as independent variables, we used t-tests for the pairwise comparisons between all the groups (control × 30 mg/Kg of curcumin, control × 100 mg/Kg of curcumin and 30 × 100 mg/Kg of curcumin) with a significance level of 5%.
Results
Curcumin effectively inhibits PGE2-synthase gene expression in LPS-induced periodontal disease
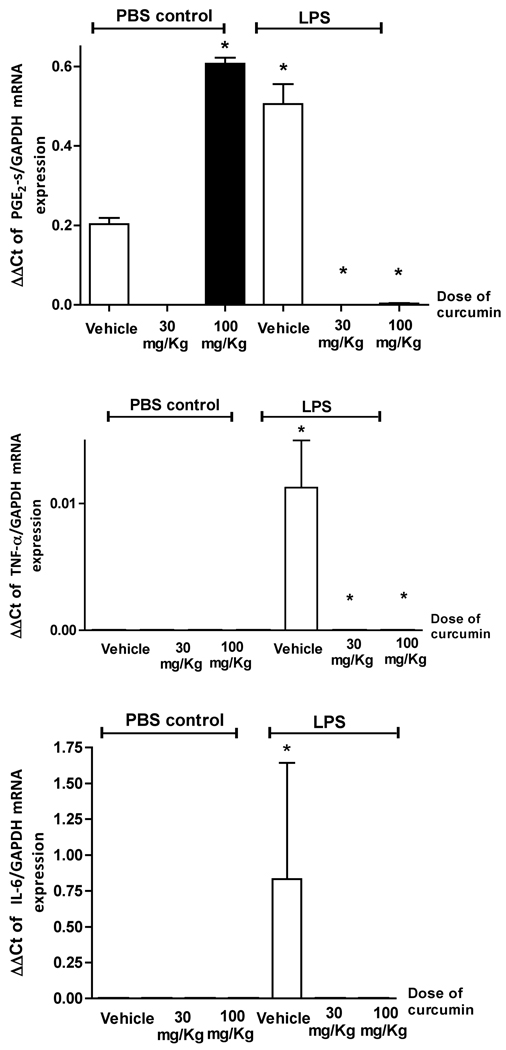
To evaluate the effect of curcumin on LPS-induced inflammatory gene expression the total RNA was extracted from periodontal tissues surrounding the upper first molars of rats treated with curcumin or vehicle both with and without LPS injections. PGE2-synthase (PGE2-s) is the murine homolog of human COX-2 and its expression was determined by RT-qPCR (Fig. 1). Interestingly, the higher dose of curcumin (100 mg/Kg) induced PGE2-s mRNA expression in healthy gingival tissues. On the other hand, administration of curcumin completely abrogated LPS-induced PGE2-s mRNA expression in the gingival tissues. Expression of PGE2 at the protein level could not be detected in our samples (data not shown).
Figure 1.
Curcumin inhibits cytokine gene expression induced by LPS in gingival tissues. Animals were treated with 30 or 100 mg/Kg of curcumin by oral gavage daily for 15 days. Control animals received the same volume of the vehicle by oral gavage. LPS or an equivalent volume of PBS vehicle were injected in palatal aspect of the gingival tissues around the upper first molars three times/week for 2 weeks. Total RNA was isolated from gingival biopsies and used for RT-qPCR. All samples were assayed in duplicate and the results were analyzed by the delta-Ct method and expression of target genes was normalized to GAPDH expression. (*) indicates significant reduction (p< 0.01) in comparison to vehicle control in LPS-injected tissues. Bars indicate means and vertical lines the standard error of mean of at least three animals in each experimental group.
Curcumin abrogates LPS-induced IL-6 and TNF-alpha mRNA expression. Inhibition of IL-6 and TNF-alpha protein is associated with different doses of curcumin
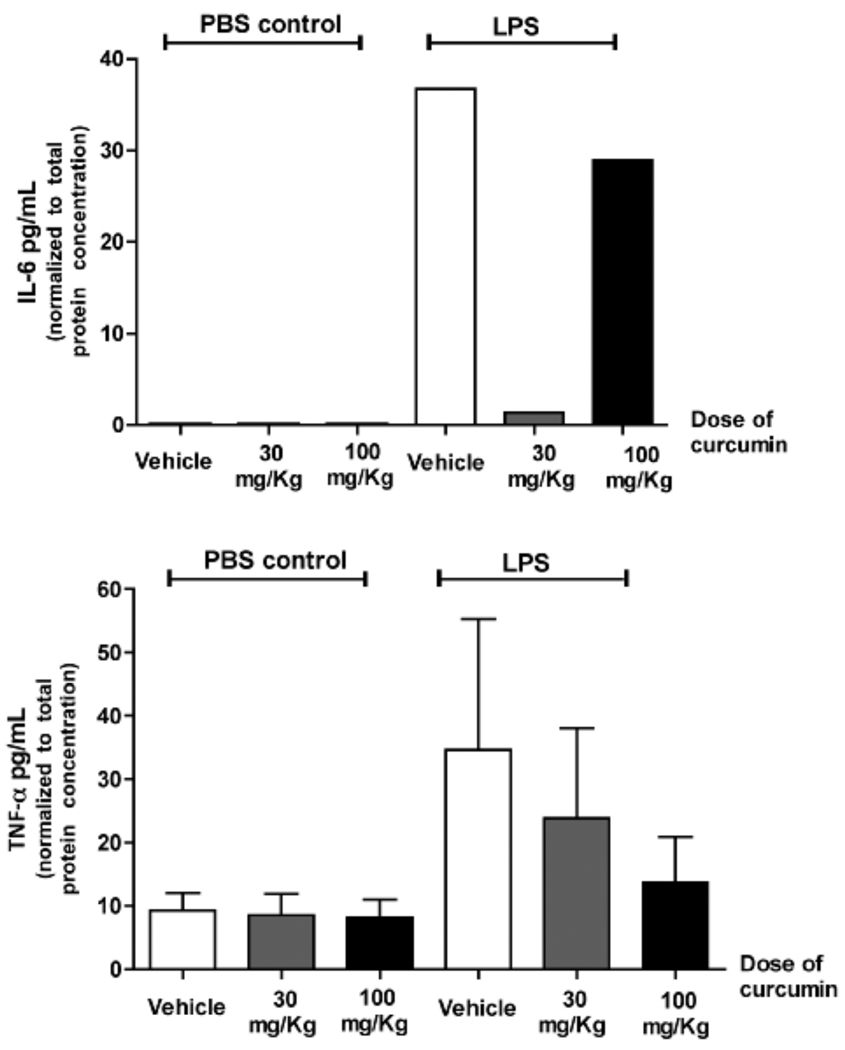
Healthy and LPS-injected gingival tissues surrounding the upper first molars from animals treated with curcumin or vehicle were harvested and total RNA and protein were isolated and used for RT-qPCR and ELISA experiments, respectively. Similarly to the results for PGE2-s, administration of curcumin inhibited completely LPS-induced TNF-alpha and IL-6 mRNA expression in the gingival tissues (Fig. 1). In fact, IL-6 expression was only detected in LPS-injected tissues, whereas TNF-alpha expression was not affected by curcumin in healthy gingival tissues. Inhibition of IL-6 protein was observed only in animals treated with the lower dose of curcumin (30 mg/Kg), whereas TNF-alpha was inhibited only in animals treated with the higher dose of curcumin (100 mg/Kg) (Fig. 2).
Figure 2.
Effect of curcumin administration on IL-6 and TNF-α protein production in gingival tissues with and without LPS injections. Total protein was extracted from gingival biopsies obtained 15 days after the start of LPS injections and used in ELISA tests. These tests were performed according to the manufacturer’s instructions and the results for each sample were normalized to the concentration of total proteins determined by a Lowry-based microassay (DC assay, Bio-Rad). For TNF-alpha production, bars indicate means and vertical lines standard error of mean of at least three animals in each experimental group. IL-6 concentration was determined in pooled samples from three animals in each experimental group due to the low level of expression, and the bar indicate the average of the triplicate measurement from these pooled samples.
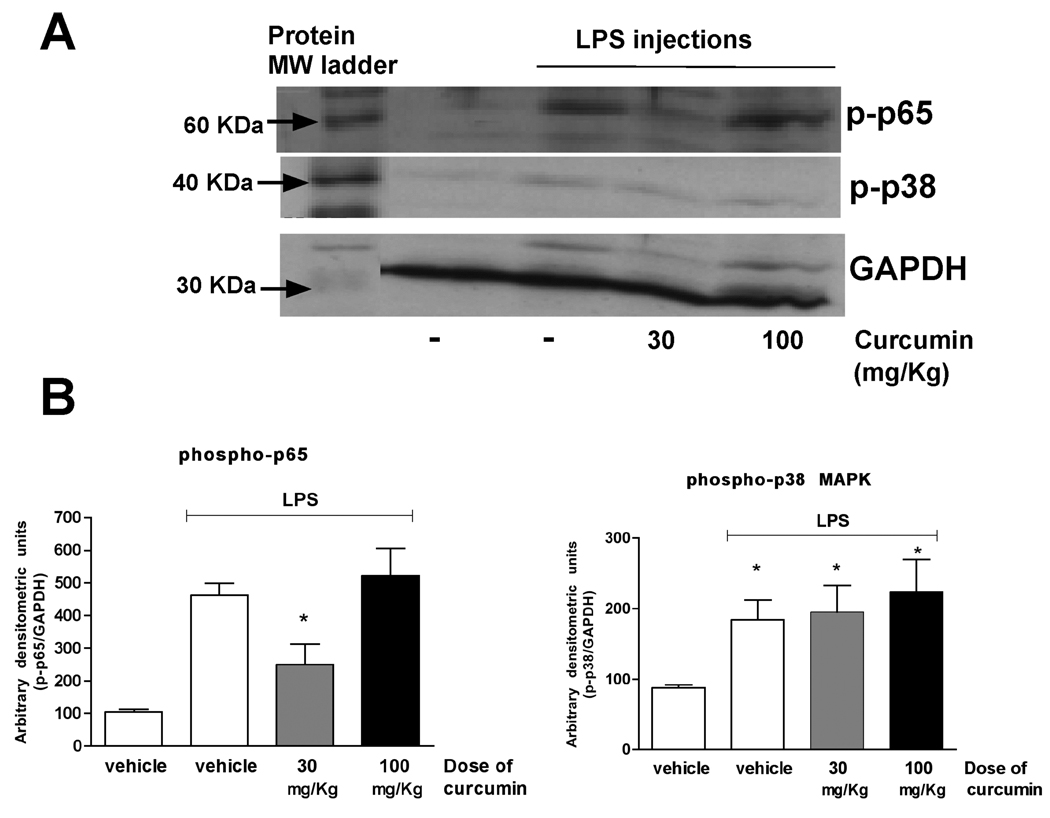
Only the lower dose of curcumin inhibited LPS-induced activation of NF-kB. Activation of p38 MAPK was not affected by curcumin
Considering the prominent role of NF-kB and p38 MAPK on the expression of inflammatory genes downstream of TLR activation, we evaluated the effect of curcumin on the activation status of these two signaling pathways. LPS injections increased activation of NF-kB in the gingival tissues, which was markedly inhibited in the animals treated with the lower dose of curcumin (30 mg/Kg). The higher dose of curcumin (100 mg/Kg) did not change NF-kB activation status in the gingival tissues and neither the lower nor the higher dose had any effect on the activation of p38 MAPK (Fig. 3).
Figure 3.
Curcumin inhibits activation of NF-kB, but not of p38 MAPK, in the LPS model of periodontal disease. Total protein was isolated from gingival biopsies obtained 15 days after the start of LPS injections with a detergent-containing buffer (T-Per, Pierce) supplemented with protease and phosphatase inhibitor cocktails (Complete and Phos-Stop, Roche). Protein concentration was determined with a Lowry-based microassay (DC assay, Bio-Rad) and 60 µg of each sample were used for the Western blot. Activation of the signaling pathways was assessed by the detection of phosphorylated forms of p65 (NF-kB) and p38 MAPKinase. Expression levels of constitutive housekeeping GAPDH are shown to confirm equal protein loading (A). In (B) the bars represent means and vertical lines the standard error of mean of the densitometric quantification of three western blots performed with three different pools of protein samples. The densitometry of target proteins was normalized to that of GAPDH for the same samples. (*) indicates a significant (p<0.05) difference in comparison with vehicle-treated and PBS-injected control. The images in (A) are representative of samples from three different animals in each experimental group.
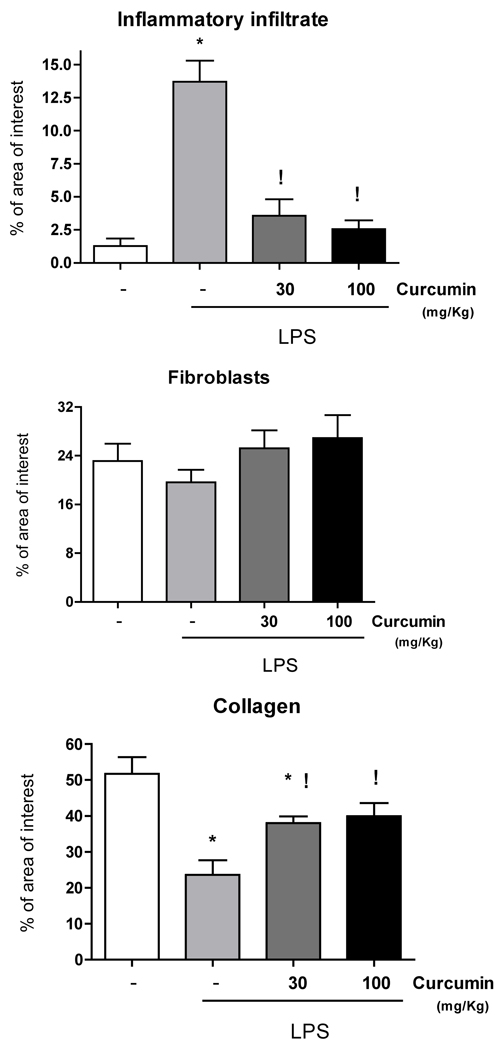
Administration of curcumin reduced the number of inflammatory cells and increased the collagen content in the gingival tissues
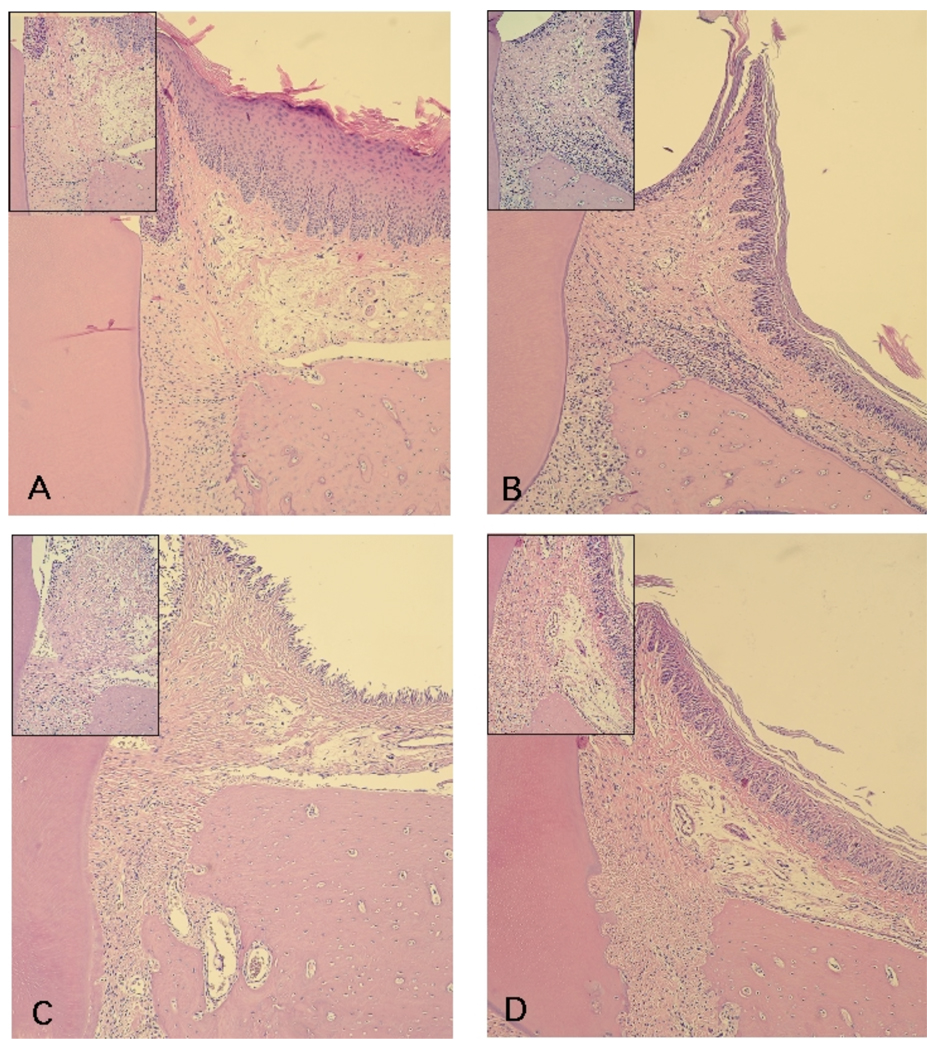
Based on the evidence of anti-inflammatory properties of curcumin in other in vivo models and also in our own data demonstrating inhibition of PGE2-s, IL-6 and TNF-alpha, we then verified the changes on LPS-induced host response in the gingival tissues associated with curcumin administration. In agreement with the modulation of inflammatory gene expression, administration of curcumin makedly inhibited LPS-induced host response in the gingival tissues (Fig. 5). Stereometric analysis demonstrates that both doses of curcumin produced a significant reduction on the inflammatory infiltrate and also inhibited tissue destruction, as indicated by the increased collagen content in the gingival tissues of curcumin-treated animals (Fig. 4).
Figure 5.
Histological aspect of the gingival tissues according to the experimental group (PBS vs LPS injections) and administration of curcumin (vehicle, 30 mg/Kg and 100 mg/Kg). Semi-serial sections with 4 µm thickness were routinely processed and stained with hematoxylin and eosin. A total of three sections spaced 100 µm were evaluated for each first molar in a minimum of four animals in each experimental group. A thick keratinized epithelium layer corresponding to the palatal gingival with an underlying dense connective tissue with reduced number of cellular infiltrate and a smooth bone crest surface characterize the gingival tissues of control (PBS-injected) animals (A). LPS injections (B) produced a decrease on the thickness of the epithelium layer and an intense cellular infiltrate and irregular bone crest surface with the presence of multinucleated osteoclast-like cells; whereas in animals treated with 30 mg/Kg (C) and 100 mg/Kg (D) of curcumin, the cellular infiltrate was markedly reduced and the collagen content was increased in comparison with the LPS-injected sites (B). Images were obtained at 100 X magnification and the insert depicts the alveolar bone crest area at 200 X magnification. These images are representative of the histological aspect observed.
Figure 4.
Stereometry analysis of gingival tissues injected with LPS, according to the experimental group: vehicle control, 30 or 100 mg/Kg of curcumin administered for 15 days by oral gavage. Negative control groups (PBS injections and vehicle administration by oral gavage) were analyzed for comparative purposes. A total of three images obtained from equally spaced slides were evaluated from each animal. Slides from at least three different animals in each experimental group were used. Both doses of curcumin markedly reduced the inflammatory infiltrate in LPS-injected gingival tissues. There was a significant increase in collagen content in curcumin-treated animals associated with a non-statistically significant trend of increasing numbers of fibroblastic cells. (*) indicates significant reduction (p< 0.01) in comparison to healthy controls and (!) indicates significant difference (p<0.01) in comparison to vehicle-treated and LPS-injected tissues. Bars indicate means and vertical lines standard error of mean of at least four animals in each experimental group.
Discussion
In this study we investigated the effect of curcumin on the expression of proinflammatory mediators and host response in an LPS model of periodontal disease. We found that administration of curcumin by oral gavage completely blocked PGE2 expression and produced a dose-dependent inhibition of IL-6 and TNF-alpha levels in the gingival tissues. The suppression of proinflammatory cytokines by curcumin was accompanied by a marked reduction of the inflammatory process verified by the stereometric analysis. These changes may be, at least partially, due to the dose-independent inhibition of NF-kB activation in the gingival tissues of curcumin-treated animals. Interestingly, administration of curcumin had no effect on LPS-induced p38 MAPK activation, which agrees with our observations in vitro using LPS-stimulated murine macrophages (data not shown - Guimaraes et al., submitted).
IL-6 and TNF-alpha play relevant roles in various chronic inflammatory conditions where curcumin may be an effective therapeutic agent. Intragastric administration of curcumin (100 mg/Kg) significantly reduced IL-6 and TNF-alpha serum levels in a sodium taurocholate infusion model of acute pancreatitis.9 Similarly to our observations in the LPS-injected gingival tissues, treatment with curcumin improved pancreatic histology in two models of experimental pancreatitis in rats.10 In this study, IL-6, TNF-alpha and iNOS expression were all reduced in curcumin-treated animals through a mechanism involving inhibition of NF-κB and AP-1—transcription factors.
In another study, curcumin administered by daily oral gavage decreased TNF-alpha and IL-6 levels in the liver of rats in a carbon tetrachloride (CCL4)-induced model of liver injury.11 The doses (200 and 400 mg/Kg) and duration of treatment (8 weeks) were both greater than those used in the present study (30 and 100 mg/Kg for 2 weeks) indicating that the doses used in the present study would not cause adverse effects, which is supported by lack of changes in weight or behavior of the experimental animals. We selected 30 and 100 mg/Kg due to the solubility of curcumin in the corn oil vehicle used, since pilot studies using ethanol or DMSO to dilute higher doses of curcumin proved to be toxic for the animals (data not shown). Two doses were used to obtain insight into possible dose-dependent effects.
Numerous studies demonstrated that certain phytochemicals including polyphenols possessing anti-inflammatory effects inhibit NF-κB and MAP Kinase signaling downstream of various receptors, including TNF-alpha receptor and TLR4.12 In a rabbit model of sepsis, curcumin reduced LPS-induced fever, which was associated with decrease of IL-1β, IL-6 and TNF-alpha concentrations in the serum. This was attributed by the authors to the suppression of NF-κB activation by curcumin.13
Activation of NF-kB is known to be pivotal for the expression of inflammatory cytokines involved in the pathogenesis of various inflammatory diseases,14, 15 which turns it into a major target for host modulation therapeutic strategies. Activation of NF-kB was also observed in oral epithelial cells exposed to the periodontopathogens Fusobacterium nucleatum and Porphyromonas gingivalis,16 indicating the crucial role of NF-kB on innate immunity in the oral cavity. Indeed, induction of gene expression in human monocytic cell line by Porphyromonas gingivalis LPS was abolished by inactivation of IKK/NF-kB.17 In another study, inhibition of NF-kB activation by endocannabinoids, an emerging class of lipid mediators with immunosuppressive and anti-inflammatory effects, derived from arachidonic acids and found in several tissues, reduced the production of proinflammatory mediators (IL-6, IL-8 and MCP-1) induced by Porphyromonas gingivalis LPS in human gingival fibroblasts,18 another resident cell type with an important role in innate immune response in periodontal diseases. Taken together, these studies indicate a role of NF-kB on the expression of fundamental mediators in periodontal disease.19 There is plenty of evidence indicating that NF-kB is one of the main targets of curcumin, and in this study we show that the potential effects of curcumin on periodontal disease may be, at least in part, due to the modulation of NF-kB activation downstream of TLR4 activation by LPS.
Inhibition of LPS signaling by curcumin may have important implications for treatment and epidemiology of chronic inflammatory diseases caused by bacterial infections.6 Moreover, it is now recognized that inflammation plays a fundamental role in many chronic diseases including cancer, atherosclerosis and rheumatoid arthritis. Some studies suggest that anti-inflammatory, chemopreventive and other beneficial effects of certain dietary phytochemicals may to be associated with the modulation of inflammation by interfering with TLR activation by MAMPs and endogenous molecules.6, 8
In our study, LPS-induced activation of NF-kB in the gingival tissues was inhibited only with the lower dose of curcumin. However, both low and high doses of curcumin produced a marked decrease on inflammation and a significant decrease of the expression of the proinflammatory mediators PGE2, TNF-alpha and IL-6. This suggests that curcumin may modulate other signaling pathways downstream of TLR activation that are also relevant for the expression of these cytokines. One signaling pathway that is relevant for the expression of inflammatory mediators and could be a candidate for a ‘NF-kB alternative’ is p38 MAPK; however, in our experimental model, p38 activation was not modulated by curcumin.
Considering this as an initial study on the potential of curcumin to modulate the immune response associated with periodontal diseases, it is important to acknowledge its methodological limitations: probably due to the high collagen content and limited dimensions of the PBS- and LPS-injected areas, we had to pool samples from different animals to detect expression of target genes for some experiments, which precluded us to perform appropriate statistical analyses for these experiments. It should also be noted that we used commercially-available LPS purified from Eschericia coli as a TLR4 agonist, instead of LPS from microbial species associated with periodontal diseases, which would also produce a significant activation of TLR2. Since we began administration of curcumin the day before the start of LPS injections, it will be interesting to determine the effect of curcumin on established periodontal disease. Optimization of dose and delivery route will depend on determination of pharmacodynamic properties of curcumin, which were also not assessed in this study. Since we introduced the curcumin suspension directly into the esophagus of the animals we assume that topical exposure of the oral mucosa and gingivae to the curcumin suspension was minimal; however we cannot rule out the possibility of a topical effect. In fact, topical application is another interesting aspect to be evaluated, since it would reduce the overall dose of curcumin and provide maximum effect in the areas of interest.
In summary, our results demonstrate a potent anti-inflammatory activity of orally administered curcumin in a model of LPS-induced periodontal disease and suggest a potential therapeutic role for curcumin in the treatment of this condition. Future studies will aim to further characterize the spectrum of the biological effects of curcumin in this in vivo model and also in vitro, in relevant cells of the immune response associated with periodontal disease by using a focused qPCR array approach.
Acknowledgements
This work was supported by the Brazilian Federal Government through the National Council for Scientific and Technological Development (CNPq) and Coordination for Improvement of Higher Education Personnel (CAPES) (grant number 4638-05), and by the National Institutes of Health – National Institute of Dental and Craniofacial Research (NIH/NIDCR, grant numbers 1R01DE018290, P20RR017696).
Contributor Information
Morgana R. Guimarães, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Sabrina Garcia de Aquino, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Leila S. Coimbra, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Luis C. Spolidorio, Department of Physiology and Pathology, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
Keith L. Kirkwood, Department of Craniofacial Biology and Center for Oral Health Research, College of Dental Medicine – Medical University of South Carolina (MUSC), Charleston, SC, USA.
Carlos Rossa, Jr, Department of Diagnosis and Surgery, Faculdade de Odontologia de Araraquara-Univ Estadual Paulista (UNESP), Araraquara, SP, Brazil.
References
- 1.de Aquino SG, Guimaraes MR, Stach-Machado DR, da Silva JA, Spolidorio LC, Rossa C., Jr Differential regulation of MMP-13 expression in two models of experimentally induced periodontal disease in rats. Arch Oral Biol. 2009;54:609–617. doi: 10.1016/j.archoralbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Rogers JE, Li F, Coatney DD, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007;78:550–558. doi: 10.1902/jop.2007.060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Jagetia GC, Aggarwal BB. "Spicing up" of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 6.Gradisar H, Keber MM, Pristovsek P, Jerala R. MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol. 2007;82:968–974. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- 7.Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–135. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 8.Chearwae W, Bright JJ. 15-deoxy-Delta(12,14)-prostaglandin J(2) and curcumin modulate the expression of toll-like receptors 4 and 9 in autoimmune T lymphocyte. J Clin Immunol. 2008;28:558–570. doi: 10.1007/s10875-008-9202-7. [DOI] [PubMed] [Google Scholar]
- 9.Gulcubuk A, Altunatmaz K, Sonmez K, et al. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49–54. doi: 10.1111/j.1439-0442.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 10.Gukovsky I, Reyes CN, Vaquero EC, Gukovskaya AS, Pandol SJ. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G85–G95. doi: 10.1152/ajpgi.00138.2002. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 12.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 13.Huang WT, Niu KC, Chang CK, Lin MT, Chang CP. Curcumin inhibits the increase of glutamate, hydroxyl radicals and PGE2 in the hypothalamus and reduces fever during LPS-induced systemic inflammation in rabbits. Eur J Pharmacol. 2008;593:105–111. doi: 10.1016/j.ejphar.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 14.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 15.Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 16.Milward MR, Chapple IL, Wright HJ, Millard JL, Matthews JB, Cooper PR. Differential activation of NF-kappaB and gene expression in oral epithelial cells by periodontal pathogens. Clin Exp Immunol. 2007;148:307–324. doi: 10.1111/j.1365-2249.2007.03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carayol N, Chen J, Yang F, et al. A dominant function of IKK/NF-kappaB signaling in global lipopolysaccharide-induced gene expression. J Biol Chem. 2006;281:31142–31151. doi: 10.1074/jbc.M603417200. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima Y, Furuichi Y, Biswas KK, et al. Endocannabinoid, anandamide in gingival tissue regulates the periodontal inflammation through NF-kappaB pathway inhibition. FEBS Lett. 2006;580:613–619. doi: 10.1016/j.febslet.2005.12.079. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Wu HF, Ang ES, et al. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009;20:7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]