Abstract
The present study characterized the inflammatory response following burn injury and determined whether ethanol (EtOH) intoxication at the time of burn injury influences this response. To accomplish this, male mice were gavaged with EtOH (2.9 g/Kg) 4 hours prior to 12–15% total body surface area sham or burn injury. Mice were sacrificed on day one after injury; blood, small intestine, lung and liver were collected to measure IL-6, IL-10, IL-18 and MCP-1 levels. In addition, neutrophil infiltration, MPO activity and edema formation were also measured in the small intestine, lung and liver. There was no difference in the inflammatory markers in the small intestine, lung and liver in mice receiving either sham or burn injury alone except IL-6 which was increased in all 4 tissue compartments following burn injury alone. However, as compared to EtOH or burn injury alone, EtOH combined with burn injury resulted in a significant increase in cytokines, neutrophil infiltration, MPO activity and edema in the small intestine, liver and lung tissue. Furthermore, a significant increase in IL-6 and MCP-1 was observed in circulation following EtOH and burn injury compared to either EtOH intoxication or burn injury alone, no other cytokines were detected in circulation. These findings suggest that acute EtOH intoxication exacerbates the inflammatory response following burn injury.
Keywords: Thermal injury, ethanol, cytokines, chemokines, leukocytes, neutrophils, tissue damage
Introduction
Alcohol remains the most abused substance in the United States and other parts of the world. An association between alcohol and trauma has long been recognized 1. Nearly, one million burn injuries are reported every year in the United States 2 and half of these are found to be under the influence of alcohol/ethanol (EtOH) intoxication 3–7. Alcohol exposure at the time of injury has also been shown to have adverse affect on the recovery of the injured patients 4–6, 8. The patients who are intoxicated at the time of injury require more surgical procedures, have a longer hospital stay and exhibit a delay in wound healing compared to the patients who did not consume alcohol prior to sustaining the injury 3–8.
Several lines of evidence indicate that burn injury regardless of prior alcohol exposure induces an inflammatory response characterized by uncontrolled production of inflammatory mediators, including cytokines, chemokines and leukocyte infiltration 9–11. Furthermore, experimental data indicate that EtOH intoxication at the time of burn injury exacerbates the suppression of host immune response as characterized by antigen presentation, T cell activation and phagocytic capability 7, 12. Such decrease in host defense may enhance susceptibility to bacterial infection 3, 7, 13. Although, the initial release of cytokines or other inflammatory mediators is a normal host response to injury, if remains uncontrolled, it can lead to multiple organ dysfunction and failure, which is a major cause of death in injured patients. Since alcohol abuse is also associated with tissue damage 13, we attempted in the present study to characterize the inflammatory response in various organs following burn injury and determine whether EtOH intoxication at the time of burn injury has any influence on this.
Materials and methods
Animals and reagents
Male C57BL/6 mice (22–25g) were obtained from Harlan Laboratories. All the animal procedures were carried out in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. These studies were initiated at the University of Alabama at Birmingham (UAB) and were approved by UAB and the authors’ current institution Loyola University Chicago Medical Center Institutional Animal Care and Use Committee. EtOH kit was obtained from Roche (Nutley, NJ). Mouse IL-6, MCP-1 and IL-10 ELISA kits were obtained from BD Biosciences (San Jose, CA) and mouse IL-18 ELISA kit was obtained from Bender MedSystems (Burlingame, CA).
Blood EtOH levels
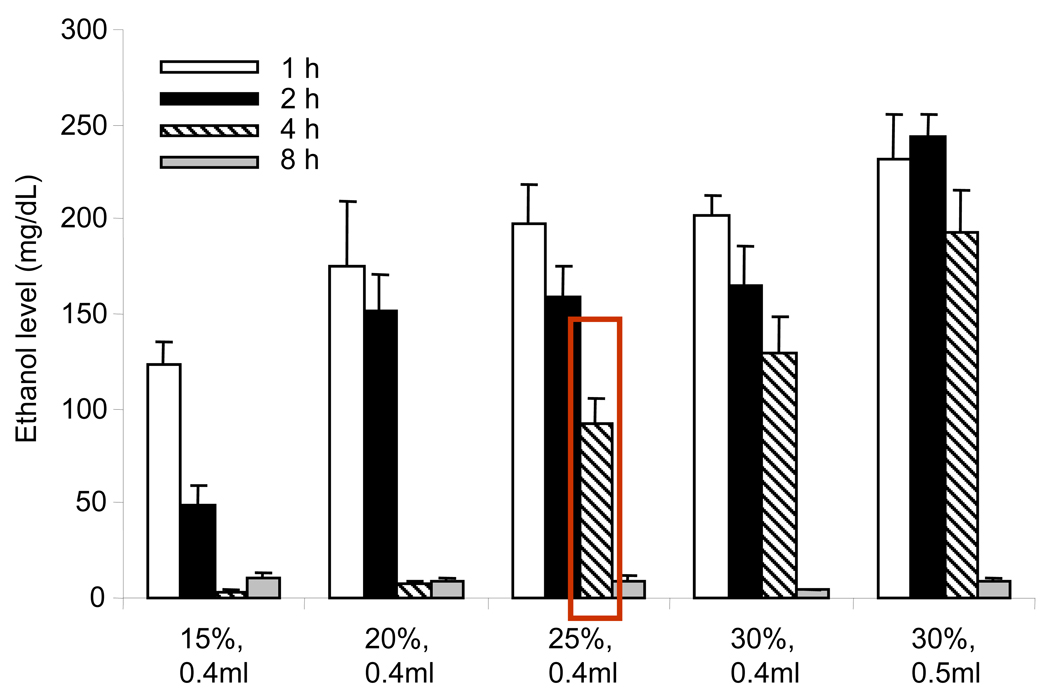
Mice were randomly divided into 5 groups with 3–6 mice in each to receive 0.4 ml of 15%, 20%, 25%, 30% and 0.5 ml of 30% EtOH in water by gavage as previously described in our studies 14. Mice were sacrificed at various time points after EtOH administration. Blood was drawn via cardiac puncture into heparinized tubes and plasma was collected by centrifuging at 8,000 rpm at 4°C for 10 min. EtOH levels in the plasma were determined 14. The results as summarized in Fig. 1 indicate that the blood EtOH levels were dose dependently increased within one hour in mice after its ingestion. The blood EtOH levels were in the range of 122 ± 19.7 mg/dL in mice gavaged with 0.4 ml of 15% EtOH and 231 ± 24 mg/dL in the group receiving 0.5 ml of 30% EtOH at this time point. The blood EtOH levels returned to basal value in mice given 0.4 ml of 15–20% EtOH within 4 hours after the gavage, however, blood EtOH levels remained above 100 mg/dL in mice gavaged with higher EtOH doses. Regardless of the EtOH dose, nearly 90–95% of the circulating EtOH was metabolized within 8 hours of its administration. We elected to use 0.4 ml of 25% EtOH (2.9g/Kg) in all subsequent experiments, since mice gavaged with this dose showed blood EtOH levels in the range of 90–100 mg/dL at 4 hours after EtOH administration. This blood EtOH level is within the range of legal limits and comparable to clinical findings as well as similar to our previous studies performed in the rat model of EtOH intoxication and burn injury 8, 14, 15.
Fig. 1. Circulatory EtOH levels in mice.
Mice were gavaged with EtOH and sacrificed at various time points thereafter. Blood was drawn via cardiac puncture and plasma EtOH levels were measured by using EtOH detection kit. Values are mean ± SEM from 3–6 animals in each group
Mouse model of acute alcohol intoxication and burn injury
Briefly, mice were randomly divided into four groups: sham vehicle; sham EtOH; burn vehicle and burn EtOH. In the EtOH-treated group, mice were gavaged with 0.4 ml of 25% EtOH in water. In the vehicle group, mice were gavaged with 0.4 ml of water. At 4 hours after the gavage, mice were anesthetized with a mixture of ketamine and xylazine at a dose of 79.7 mg/Kg and 1.18 mg/Kg, respectively, by intraperitoneal injection and the dorsal surface was shaved. The mice were transferred into a template, which was fabricated to expose 12–15% of the total body surface area (TBSA). TBSA was calculated by using Walker and Mason formula 16, 17. For burn injury, the template with mice was immersed into hot water maintained at 85–90°C for 7–8 sec 17. For sham injury, mice received identical anesthesia and were shaved but were immersed in lukewarm water for 7–8 sec. All animals were dried immediately and resuscitated with 1.0 ml physiological saline by intraperitoneal injection.
Tissue harvesting
Mice were sacrificed on day one after injury. Blood was drawn via cardiac puncture into heparinized tubes and centrifuged (8,000 rpm, 10 min, 4°C). Plasma was collected and stored at −80°C for further analysis. Lung, distal part of small intestine and the liver were removed aseptically and were either stored in liquid nitrogen or fixed in 10% formalin for further analysis. In addition, a portion of the tissues from each organ was used to determine edema formation as described below.
Preparation of tissue homogenates
For the measurement of inflammatory mediators, equal weights of tissues (100 mg) from various groups were sonicated in 1 ml of phosphate buffer saline containing protease inhibitor cocktail (Sigma Chemical Co., St Louis, MO) 18–20. For the measurement of myeloperoxidase (MPO) activity, equal weights of tissues (100 mg) were sonicated in 1 ml buffer containing (0.5% hexadecyltrimethylammonium bromide in 50 mM phosphate buffer, pH 6.0) 18–20. Homogenates were cleared by centrifuging at 10,000 rpm at 4°C for 15 min. The supernatants were stored at −80°C for the analysis of MPO. Tissue MPO activity and cytokine levels were normalized to the total protein in the homogenates, which was determined by using the BioRad assay kit (Hercules, CA).
Measurement of inflammatory cytokines in blood and tissue homogenates
IL-6, IL-10, MCP-1 and IL-18 levels in blood and tissue homogenates were determined by using ELISA kits according to manufacture’s instructions.
Measurement of MPO activity
MPO activity in the tissue homogenates was measured as described previously 18, 19. Briefly, the reaction was carried out in a 96-well plate by adding 290 µl 50 mM potassium phosphate buffer, 3 µl substrate solution (containing 20 mg/ml o-dianisidine hydrochloride), and 3 µl H2O2 (20 mM). Samples (10 µl) were added to the wells to start the reaction. Standard MPO (Sigma Chemical Co.) was used in parallel to determine MPO activity in the sample. The reaction was stopped by adding 3 µl sodium azide (30%). Plates were read at 460 nm. MPO activity was determined by using the curve obtained from the standard MPO and normalized to the protein contents.
Measurement of tissue edema
Tissue edema was measured by determining the tissue water content. In brief, lung, small intestine and liver from various experimental groups were removed, weighed, and dried for 48 hours at 80°C 18, 19. Water content (%) of tissue was calculated as (wet wt–dry wt)/wet wt × 100%.
Histological analysis of neutrophil infiltration in organs
Lung, small intestine and liver tissues were fixed in 10% formalin for 24 hours and were sent to Comparative Pathology Laboratory at the University of Alabama at Birmingham for further processing. Briefly, the tissues were embedded in paraffin. These were then cut (4–5 µm thick) and mounted on glass slides. Tissue sections were stained with hematoxylin-eosin (H&E) and observed under the microscope (Nikon Eclipse TS100) at a magnification of 1000× for morphology and neutrophil accumulation in different organs and photographed using a camera (SPOT, RTcolor, Diagnostic Instrument, Inc, Iowa City, Iowa) attached with the microscope 20, 21.
Statistical analysis
Results are presented as means ± SEM and were analyzed using ANOVA. The significance between the groups was determined using Tukey’s test (GraphPad InStat). A p<0.05 between two groups was considered statistically significant.
Result
EtOH intoxication prior to burn injury enhances IL-6, IL-10 and IL-18 levels
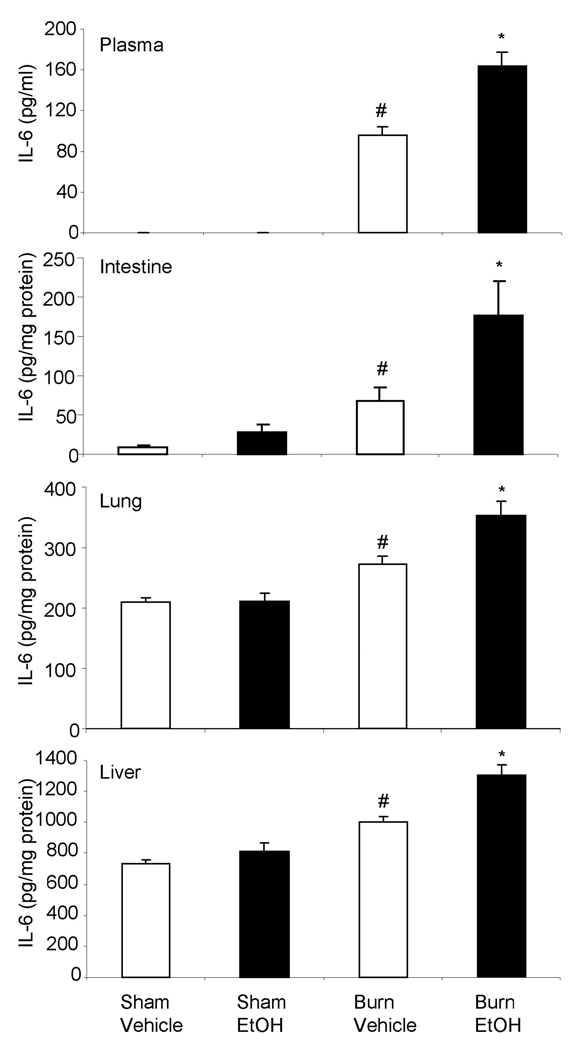
IL-6 was undetectable in circulation of sham animals regardless of EtOH exposure (Fig. 2). Furthermore, no differences in IL-6 levels was found in the small intestine, lung and liver tissue in sham animals following EtOH intoxication compared to shams vehicle group. The levels of IL-6 were significantly elevated in circulation and in other organs following burn injury alone compared to shams. IL-6 levels were several folds higher in circulation in mice receiving ethanol and burn injury compared to shams. An approximately 2-fold increase in IL-6 levels was observed in the intestine, lung and liver in animals receiving a combined insult of EtOH intoxication and burn injury compared to sham injury regardless of EtOH intoxication. Moreover, IL-6 levels were significantly higher in all 4 compartments in mice receiving a combined insult of EtOH intoxication and burn injury compared to those, receiving burn injury alone.
Fig. 2. IL-6 levels in circulation, small intestine, lung and liver after EtOH and burn injury.
On day one after injury, blood, intestine, lung and liver were collected from various experimental groups. Tissues were homogenized and IL-6 levels in the tissue homogenates and plasma were determined by using ELISA kit. Values are mean ± SEM from 4–7 animals per group. *, p <0.05 compared with others. #, p < 0.05 compared with sham vehicle.
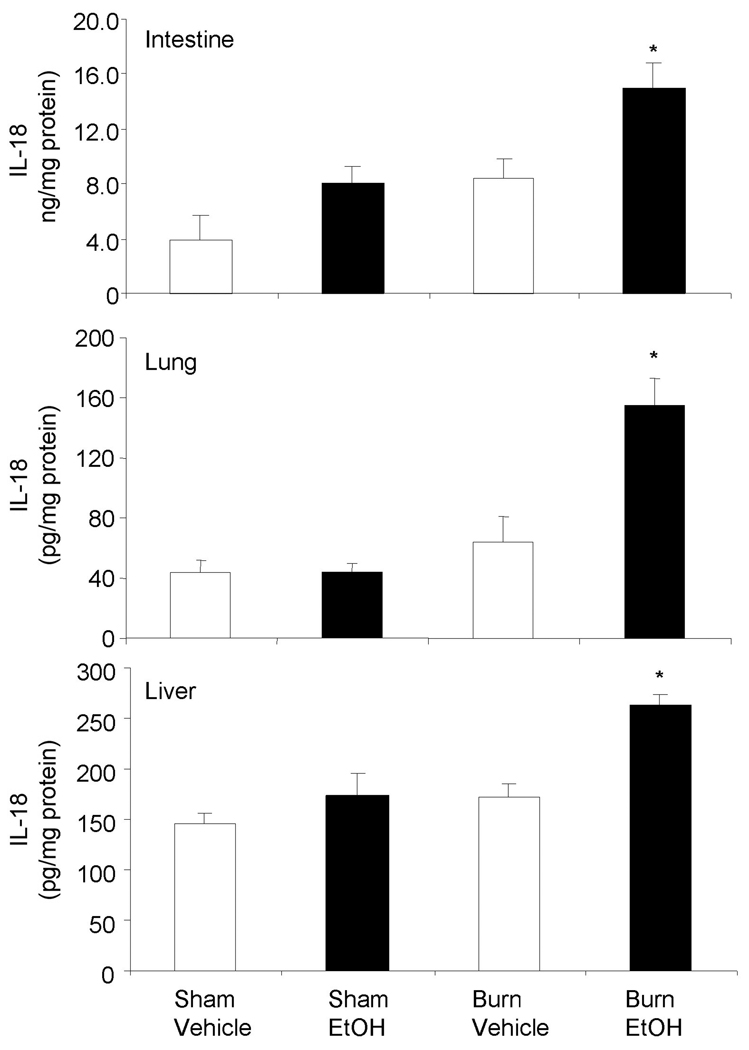
In contrast to IL-6, IL-10 (Fig. 3) and IL-18 (Fig. 4) were not detected in circulation in any of the experimental groups. Additionally, no difference in the IL-10 and IL-18 levels was noted in the small intestine, lung and liver tissues on day one after EtOH intoxication or burn injury alone compared to shams gavaged with vehicle. However, ~2-fold increase in IL-10 levels in the small intestine was observed in mice receiving a combined insult of EtOH intoxication and burn injury compared to mice receiving either insult alone (Fig. 3). Similarly, although a less than 2-fold but a significant increase in IL-10 levels was observed in the lung and liver tissues following a combined insult of EtOH intoxication and burn injury compared to mice receiving either insult alone. The levels of IL-18 on the other hand were found to be nearly 3-fold high in the small intestine and lung, and 2-fold in the liver following EtOH intoxication and burn injury compared to shams (Fig. 4). As compared to burn vehicle group, IL-18 levels remain significantly higher in mice receiving combined insult of EtOH intoxication and burn injury.
Fig. 3. IL-10 levels in small intestine, lung and liver after EtOH and burn injury.
One day after injury, intestine, lung and liver were collected and homogenized. IL-10 levels in the tissue homogenates were determined by using ELISA kit. Values are mean ± SEM from 4–7 animals in each group. *, p <0.05 compared with other groups.
Fig. 4. IL-18 levels in small intestine, lung and liver after EtOH and burn injury.
One day after injury, intestine, lung and liver were collected and homogenized. IL-18 levels in the tissue homogenates were determined by using ELISA kit. Values are mean ± SEM from 4–7 animals in each group. *, p <0.05 compared with other groups.
EtOH intoxication prior to burn injury enhances MCP-1 levels
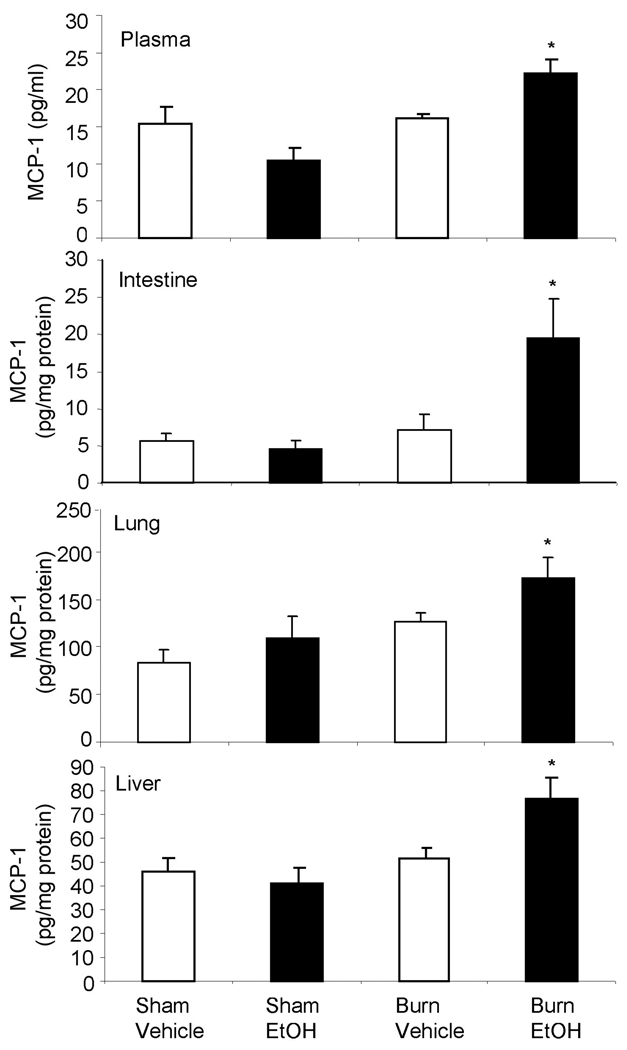
As compared to sham vehicle, there was no change in MCP-1 levels in the circulation, small intestine, lung and liver tissues following EtOH exposure or burn injury alone. However, a significant increase in MCP-1 levels in all four tissue compartments was observed in mice receiving a combined insult of EtOH intoxication and burn injury compared to mice receiving either insult alone (Fig. 5).
Fig. 5. MCP-1 levels in circulation, small intestine, lung and liver after EtOH and burn injury.
One day after injury, blood, intestine, lung and liver were collected. MCP-1 levels in the tissue homogenates and plasma were determined by using ELISA kit. Values are mean ± SEM from 4–7 animals in each group. *, p <0.05 compared with other groups.
EtOH intoxication prior to burn injury enhances MPO activity and neutrophil infiltration
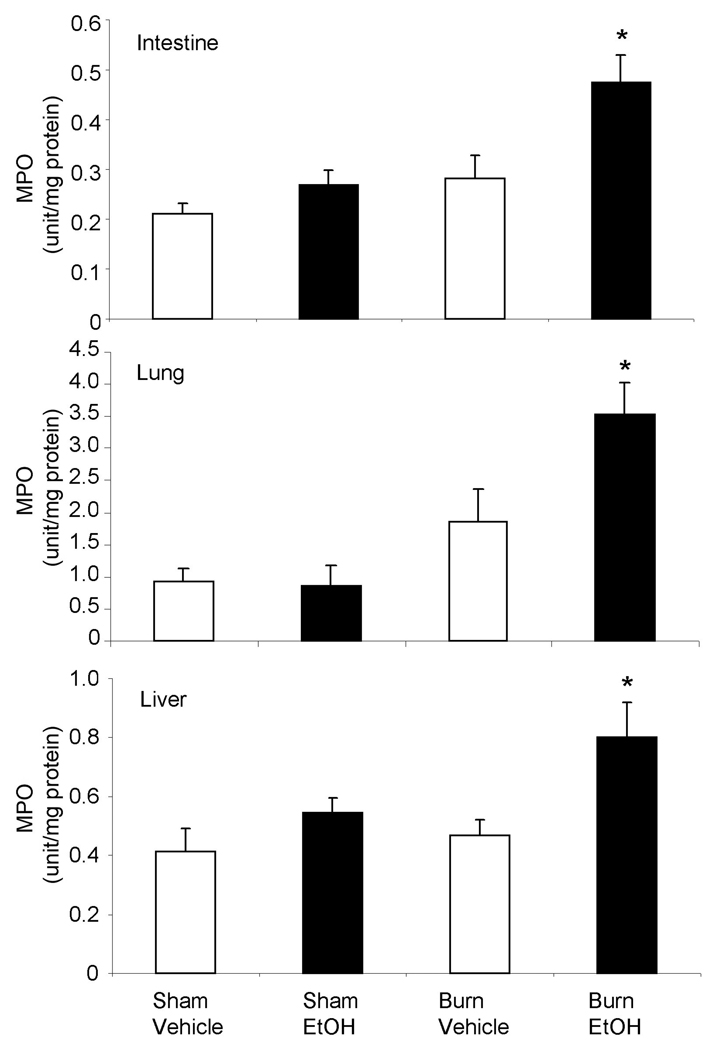
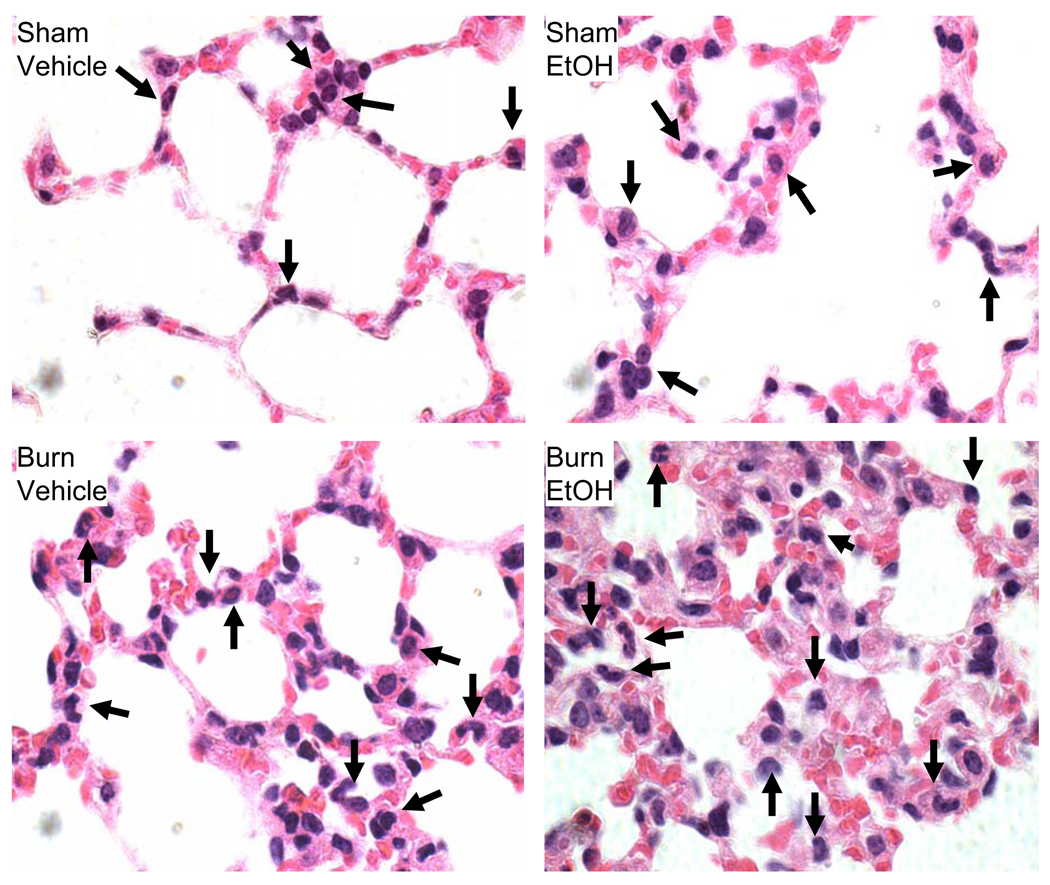
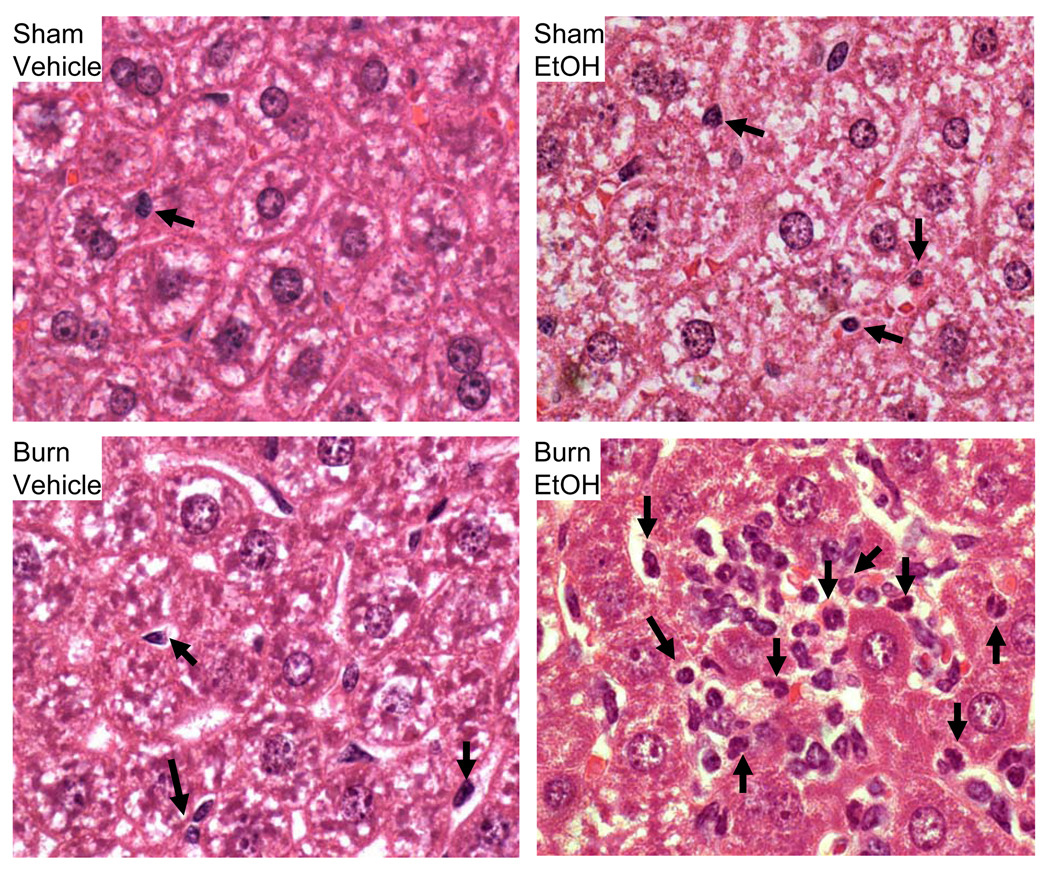
As shown in Fig. 6, no difference in MPO activity was observed in the small intestine, lung and liver following EtOH exposure or burn injury alone compared to sham vehicle. A significant increase in MPO activity in the intestine, lung and liver homogenates was observed following a combined insult of EtOH intoxication and burn injury compared to either insult alone. In addition to MPO activity, the presence of neutrophil infiltration in the intestine, lung and liver tissue was further confirmed by H&E staining. H&E stained sections were examined at ×1000 magnification and neutrophils were identified by their distinct morphological feature such as small, pale granular cytoplasm with a multi-lobed nucleus. Photomicrographs shown in Fig. 7 clearly demonstrate the presence of neutrophils in most of the groups in the small intestine (Fig. 7A), lung (Fig. 7B) and liver (Fig, 7C). However, the number of neutrophil was much higher in mice receiving a combined insult of EtOH intoxication and burn injury compared to either insult alone (Table 1).
Fig. 6. MPO activity in small intestine, lung and liver after EtOH and burn injury.
One day after injury, intestine, lung and liver were collected and homogenized. MPO levels in the tissue homogenates were determined and normalized to the protein. Values are mean ± SEM from 4–7 animals in each group. *, p <0.05 compared with other groups.
Fig. 7. Histological analysis of neutrophil infiltration in small intestine, lung and liver after EtOH and burn injury.
One day after injury, intestine, lung and liver were collected and processed for hematoxylin-eosin staining. Sections were examined at a magnification of × 1000 and photographed. Representative photomicrographs of intestine (A), lung (B) and liver(C) from four groups shows for the presence of neutrophils. Neutrophils identified by their specific multilobed nucleus are shown by the arrow. Each slide was examined for 3 microscopic fields and each group contained 3 animals.
Table 1.
The number of neutrophil in small intestine, lung and liver after EtOH intoxication and burn injury$
| Sham vehicle | Sham EtOH | Burn vehicle | Burn EtOH | |
|---|---|---|---|---|
| Intestine | 1.56 ± 0.24 | 1.78 ± 0.28 | 2.67 ± 0.37 | 4.11 ± 0.46* |
| Lung | 8.11 ± 0.89 | 9.11 ± 0.86 | 11.78 ± 0.81# | 19.11 ± 1.09* |
| Liver | 2.11 ± 0.45 | 2.22 ± 0.36 | 3.11 ± 0.39 | 8.67 ± 1.00* |
Small intestine, lung and liver tissue sections were stained with hematoxylin-eosin (H&E) and examined under the microscope at 1000× magnification. Neutrophils were identified by their distinct morphological feature such as small, pale granular cytoplasm with a multi-lobed nucleus. Each section was scanned for 3 microscopic fields, and the data thus obtained from 3 animals in each group were pooled and presented as mean ± SEM per single high powered microscopic field.
p <0.05 compared with other groups.
p<0.05 compared with sham vehicle.
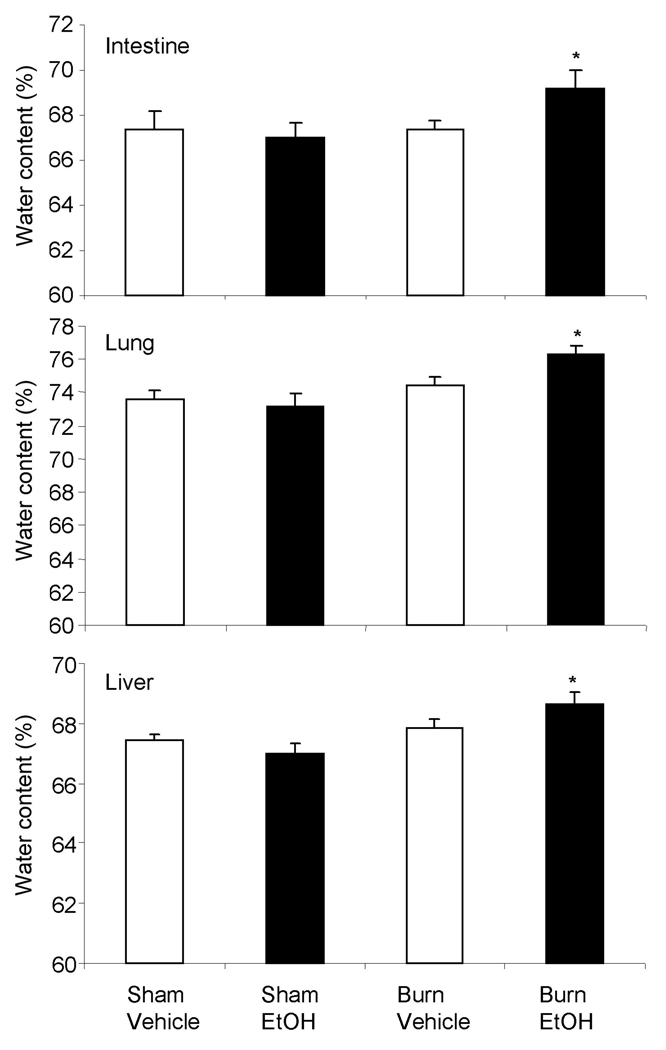
EtOH intoxication prior to burn injury enhances tissue edema
As compared to sham vehicle group, no change in water content in all the three organs was observed following either EtOH intoxication or burn injury alone. However, EtOH intoxication combined with burn injury resulted in a significant increase in edema in the small intestine, lung and liver compared to either shams or burn injury alone (Fig. 8).
Fig. 8. Tissue edema in small intestine, lung and liver after EtOH and burn injury.
One day one after injury, edema formation in the intestine, lung and liver was determined by assessing the tissue water content. Values are mean ± SEM from 4–7 animals in each group. *, p <0.05 compared with other groups.
Discussion
We found that EtOH intoxication combined with burn injury increases tissue edema in the intestine, liver and the lung compared to either EtOH intoxication or burn injury alone. This was accompanied with an increase in cytokines, chemokines and neutrophil infiltration. Several lines of evidence indicate that trauma, burn and sepsis initiate a cascade of events which is characterized by uncontrolled production of inflammatory mediators often referred to as systemic inflammatory response syndrome (SIRS) 22–24. This release of cytokines or inflammatory mediators is known to precede organ dysfunction and failure. Although numerous advances in intensive care units and patient management have improved short-term survival, sepsis and subsequent multiple organ failure continue to be the major cause of morbidity and mortality in patients who survive the initial injury.
Previous studies have suggested that IL-6 is a multifunctional cytokine 25–27. In the early phase of infection, IL-6 induces B cell proliferation, T cell activity and neutrophil infiltration and thus becomes an important player in mediating the immune responses initiated by infection and injury 25–27. However, high level of IL-6 has been widely used as a marker of the severity of inflammatory response in many clinical conditions 28–30. We observed that IL-6 was undetectable in circulation in sham animals. However, a significant increase in IL-6 was observed in circulation, lung, small intestine and liver tissue following burn injury with or without prior EtOH intoxication compared with shams; but the levels were higher in the group of mice receiving a combined insult of EtOH intoxication and burn injury. Consistent with this observation, other studies also demonstrate that the combination of acute ethanol and burn injury lead to a substantial increase in IL-6 levels in the serum and other organs 25, 31, 32. Similarly, IL-18 also possesses broad immunomodulatory properties, including a role in tissue damage. IL-18 is often expressed in inflammatory lesions and is found to be elevated in the blood of patients with a variety of diseases including Crohn’s disease, Sjogren syndrome, graft-versus-host disease and rheumatoid arthritis 33. Furthermore, a significant increase in IL-18 levels in circulation was also observed in patients with lethal sepsis compared with sepsis survivors at all time-points studied 34. These studies suggested that inappropriate IL-18 release contributes to the pathogenesis of these diseases and may influence the clinical outcome in these patients 34–36.
An increase in IL-6 and IL-18 in the small intestine has been correlated with decreased villus height in the small intestine following EtOH intoxication and burn injury 20, 31. Furthermore, both IL-6 and IL-18 are also implicated in lung edema following EtOH intoxication and burn injury 21, 37. However, the mechanism by which increased IL-6 and IL-18 in mice given EtOH and burn injury causes tissue damage in 3 different organs remains largely unknown. One potential common denominator to these cytokines is the recruitment of neutrophils to different organs observed in these mice. Earlier studies in the past have shown that the depletion of neutrophil prior to injury protects organ function following burn and trauma-hemorrhage 38, 39. Consistent with these findings we also observed that depletion of neutrophil prevent the increase in intestine tissue damage following EtOH and burn injury 40. We showed recently that IL-18 plays a critical role in neutrophil superoxide anion production and their recruitment to lung and intestine following EtOH and burn injury 20, 21, 40. A similar role for IL-18 was demonstrated in experimental models of sepsis 41. In another study, intratracheal administration of IL-18 resulted in increased neutrophil infiltration and lung vascular permeability 35. Thus, it is likely that these cytokines directly or via up-regulation of chemokines such MCP-1 may cause an increase in the neutrophil recruitment. MCP-1 is known to recruit monocytes 42; however, there is evidence that MCP-1 enhances intercellular adhesion molecule-1 (ICAM-1) expression 43, a step critical to the recruitment of neutrophils. Thus MCP-1 may facilitate the neutrophil recruitment via ICAM-1. Similarly IL-6 and IL-18 is shown to up-regulate the expression of ICAM-1 and other chemokines critical to neutrophil recruitment.
Regardless the mechanisms, once the neutrophils are recruited, they may contribute to the tissue damage (i.e., edema formation) observed in mice given EtOH and burn injury. Neutrophils are among the first line of defense against invading pathogens and thus they recruit to the site of inflammation within a short period of time after injury 21, 38, 40, 44. After destroying the invading pathogens, neutrophils undergo apoptosis and are cleared by macrophages. In a recent study, we observed that EtOH combined with burn injury prolonged their presence in intestine 40, 44. Whether this increase in neutrophil presence is due to a delay in their apoptosis or an increase in their flux remains to be established. Nevertheless, their prolong presence in the tissue may result in increased O2− and proteases accumulation. Although such an increase in O2− and proteases should be helpful in killing bacteria and other invading pathogens, their prolonged presence and accumulation in excess are considered to be the major determinants in tissue damage in multiple organs in various inflammatory conditions 45–47.
In this study, we used a small area burn injury (12–15%) which by itself only increases IL-6 levels in blood, small intestine, lung and liver tissue, but did not influence other parameters on day one after injury. However, EtOH intoxication combined with burn resulted in significant increase in cytokines and chemokines, neutrophil infiltration and tissue edema. A previous study has shown that burn injury size is a critical factor in postburn pathogenesis 9 and while a small burn itself may not cause a significant change, other existing factors, such as age, gender and preclinical conditions may influence the outcome following small burn injury. Thus, whereas a smaller burn injury by itself may not have an adverse effect on host defense, when combined with EtOH intoxication it may become detrimental. We also recognize that in contrast to a previous study31, we found an increase in intestinal IL-10 levels following a combined insult of EtOH intoxication and burn injury. The definitive cause for these differences remains to be established. However, these differences could result from the different models employed in the two studies. In the present study, EtOH was administered by gavage and the burn injury was induced at 4 hours after its administration. The previous study on the other hand utilized a model in which EtOH was administered intraperitoneally and burn injury was induced 30 minutes after EtOH administration.
In summary, our findings suggest that acute EtOH intoxication prior to burn injury lead to an increase in inflammatory cytokines and chemokines such as IL-6, IL-10, MCP-1 and IL-18. This was accompanied with an increase in neutrophil infiltration and edema formation in more than one organ. These findings form the basis for future studies investigating the mechanism by which EtOH combined with burn injury causes tissue damage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was supported by NIH through R01AA015731 and R21AA015979.
Reference List
- 1.Maier RV. Ethanol abuse and the trauma patient. Surg Infect (Larchmt) 2001;2:133–141. doi: 10.1089/109629601750469456. [DOI] [PubMed] [Google Scholar]
- 2.American Burn Association. Burn incidence and treatment in the US: 2000 fact sheet. 2000 [Google Scholar]
- 3.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil. 1996;17:532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Haum A, Perbix W, Hack HJ, Stark GB, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns. 1995;21:194–199. doi: 10.1016/0305-4179(95)80008-c. [DOI] [PubMed] [Google Scholar]
- 6.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 8.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 10.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 11.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 12.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 13.Hoek J, Thiele GM, Klassen LW, Mandrekar P, Zakhari S, Cook RT, Ray NB, Happel KI, Kolls JK, Kovacs EJ, Szabo G. RSA 2004: combined basic research satellite symposium-mechanisms of alcohol-mediated organ and tissue damage: inflammation and immunity and alcohol and mitochondrial metabolism: at the crossroads of life and death session one: alcohol, cellular and organ damage. Alcohol Clin Exp Res. 2005;29:1735–1743. doi: 10.1097/01.alc.0000179313.64522.56. [DOI] [PubMed] [Google Scholar]
- 14.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 15.Albright JM, Kovacs EJ, Gamelli RL, Schermer CR. Implications of formal alcohol screening in burn patients. J Burn Care Res. 2009;30:62–69. doi: 10.1097/BCR.0b013e3181921f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ. Neutrophil chemokine production in the skin following scald injury. Burns. 1999;25:403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- 18.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Rana SN, Schwacha MG, Chaudry IH, Choudhry MA. A novel role for IL-18 in corticosterone-mediated intestinal damage in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;80:367–375. doi: 10.1189/jlb.1205745. [DOI] [PubMed] [Google Scholar]
- 20.Akhtar S, Li X, Chaudry IH, Choudhry MA. Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O2- production and intestinal edema following alcohol intoxication and burn injury. Am J Physiol Gastrointest Liver Physiol. 2009;297:G340–G347. doi: 10.1152/ajpgi.00044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin 18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]
- 22.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leukoc Biol. 2008;84:607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 25.Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–140. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 27.Van SJ. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 28.Kowal-Vern A, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Gamelli RL. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. J Am Coll Surg. 1994;178:357–362. [PubMed] [Google Scholar]
- 29.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Scalfani MT, Chan DM, Murdoch EL, Kovacs EJ, White FA. Acute ethanol exposure combined with burn injury enhances IL-6 levels in the murine ileum. Alcohol Clin Exp Res. 2007;31:1731–1737. doi: 10.1111/j.1530-0277.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 32.Emanuele MA, Emanuele NV, Gamelli RL, Kovacs EJ, LaPaglia N. Effects of insulin on hepatic inflammation induced by ethanol and burn injury in a murine model of critical illness. J Burn Care Res. 2007;28:490–499. doi: 10.1097/BCR.0B013E318053DAED. [DOI] [PubMed] [Google Scholar]
- 33.Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J Immunother. 2002;25 Suppl 1:S4–S11. doi: 10.1097/00002371-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 34.Emmanuilidis K, Weighardt H, Matevossian E, Heidecke CD, Ulm K, Bartels H, Siewert JR, Holzmann B. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: high serum IL-18 as an early predictive indicator of lethal outcome. Shock. 2002;18:301–305. doi: 10.1097/00024382-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol. 2001;167:7060–7068. doi: 10.4049/jimmunol.167.12.7060. [DOI] [PubMed] [Google Scholar]
- 36.Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002;70:6896–6903. doi: 10.1128/IAI.70.12.6896-6903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bird MD, Zahs A, Deburghgraeve C, Ramirez L, Choudhry MA, Kovacs EJ. Decreased Pulmonary Inflammation Following Ethanol and Burn Injury in Mice Deficient in TLR4 but not TLR2 Signaling. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sir O, Fazal N, Choudhry MA, Gamelli RL, Sayeed MM. Neutrophil depletion prevents intestinal mucosal permeability alterations in burn-injured rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1224–R1231. doi: 10.1152/ajpregu.2000.278.5.R1224. [DOI] [PubMed] [Google Scholar]
- 39.Deitch EA, Bridges W, Berg R, Specian RD, Granger DN. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute Alcohol intoxication potentiates neutrophil-mediated intestine tissue damage following burn injury. Shock. 2008;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJ, Pulido EJ, McIntyre RC, Jr., Joosten LA, Van der Meer JW, Dinarello CA. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164:2644–2649. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 42.Turler A, Schwarz NT, Turler E, Kalff JC, Bauer AJ. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G145–G155. doi: 10.1152/ajpgi.00263.2001. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi Y, Matsumura F, Takeya M, Ichiguchi O, Kuratsu JI, Horiuchi T, Akizuki E, Matsuda T, Okabe K, Ohshiro H, Liang J, Mori K, Yamada S, Takahashi K, Ogawa M. Monocyte chemoattractant protein-1 enhances expression of intercellular adhesion molecule-1 following ischemia-reperfusion of the liver in rats. Hepatology. 1998;27:727–734. doi: 10.1002/hep.510270314. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2- production in a two-hit model of alcohol intoxication and burn injury. J Immunol. 2008;180:6933–6940. doi: 10.4049/jimmunol.180.10.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukushima R, Alexander JW, Gianotti L, Pyles T, Ogle CK. Bacterial translocation-related mortality may be associated with neutrophil-mediated organ damage. Shock. 1995;3:323–328. [PubMed] [Google Scholar]
- 46.Partrick DA, Moore EE, Offner PJ, Meldrum DR, Tamura DY, Johnson JL, Silliman CC. Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch Surg. 2000;135:219–225. doi: 10.1001/archsurg.135.2.219. [DOI] [PubMed] [Google Scholar]
- 47.Sayeed MM. Exuberant Ca(2+) Signaling in Neutrophils: A Cause for Concern. News Physiol Sci. 2000;15:130–136. [PubMed] [Google Scholar]