Abstract
To search for agents affecting thermal stability of proteins, a test based on the registration of protein aggregation in the regime of heating with a constant rate was used. The initial parts of the dependences of the light scattering intensity (I) on temperature (T) were analyzed using the following empiric equation: I = K agg(T−T 0)2, where K agg is the parameter characterizing the initial rate of aggregation and T 0 is a temperature at which the initial increase in the light scattering intensity is registered. The aggregation data are interpreted in the frame of the model assuming the formation of the start aggregates at the initial stages of the aggregation process. Parameter T 0 corresponds to the moment of the origination of the start aggregates. The applicability of the proposed approach was demonstrated on the examples of thermal aggregation of glycogen phosphorylase b from rabbit skeletal muscles and bovine liver glutamate dehydrogenase studied in the presence of agents of different chemical nature. The elaborated approach to the study of protein aggregation may be used for rapid identification of small molecules that interact with protein targets.
Introduction
Senisterra and coworkers [1], [2] elaborated a high-throughput light-scattering-based method for screening of ligands specifically interacting with protein targets. Thermal protein denaturation is used to characterize the binding of ligands to their target protein. This method is based on the assumption that the proteins under study irreversibly denaturate and form aggregates during thermal denaturation. Light scattering as a measure of protein aggregation is a very sensitive technique. Protein aggregation is studied in the regime of heating with a constant rate. The dependence of the light scattering intensity on temperature has a sigmoid shape. At rather high temperatures the light scattering intensity (I) reaches a limiting value (I lim). To characterize thermostability of a protein, Senisterra and coworkers used the temperature (T agg) corresponding to the middle point of the transition, i.e., a temperature at which I = I lim/2. Parameters I lim and T agg are determined by fitting of the experimental dependence of I on temperature with the following empiric equation, analogous to the Boltzmann equation:
| (1) |
where B is a constant.
According to the idea advanced by Senisterra and coworkers a change in the T agg value in the presence of a ligand characterizes the effect of the latter on protein thermostability. To substantiate this conclusion, the authors additionally used differential scanning calorimetry (DSC), which is a source of direct information on the protein resistance to high-temperature exposure. The authors also constructed a plot demonstrating the existence of a correlation between the T agg value and the position of the maximum on the DSC profiles (T m).
It is evident that the accuracy of determining parameter T agg is connected with the reliability of the estimation of parameter I lim. When trying to estimate parameter I lim we should take into account that the “true” limiting level of the light scattering intensity may not be reached because of precipitation of the large-sized aggregates formed at high temperatures. Such a precipitation results in the decrease in the light scattering intensity, and the real experimental dependence of I on temperature looks like a curve passing through a maximum. The maximum value of the light scattering intensity may be lower than the I lim value calculated from Eq. (1). Besides, the correlation between the increment of the light scattering intensity and the degree of protein denaturation should be controlled not only by checking the correlation between parameters T agg and T m, but by stricter analysis of the relationship between turbidimetric data and calorimetric data, supplying direct information on the degree of protein denaturation.
To avoid the uncertainty in the estimation of parameter I lim, in the present work we proposed new parameters, which characterize the rate of aggregation. To determine these parameters, there is no need for the full dependences of the light scattering intensity on temperature, since the proposed parameters (the initial temperature of aggregation and the parameter characterizing the rate of change in the light scattering intensity with temperature) are calculated from the initial parts of the dependences of I on temperature. The use of these parameters allows us to obtain the quantitative characteristics of the effect of the agents to be tested on the rate of aggregation. Since the initial stage of thermal aggregation of the proteins is the stage of denaturation, the ligands under study involve agents affecting both the stage of denaturation and the stage of aggregation. To demonstrate the applicability of the proposed approach, thermal aggregation of glycogen phosphorylase b (Phb; EC 2.4.1.1) from rabbit skeletal muscles and bovine liver glutamate dehydrogenase (GDH; EC 1.4.1.3) were used as examples.
Mathematical description of the initial parts of the dependences of the light scattering intensity on temperature
As a preliminary step we discuss the kinetics of thermal aggregation of the proteins registered at a fixed temperature. To analyze the initial parts of the dependences of the light scattering intensity (I) on time, the following empiric equation was proposed [3]:
| (2) |
In this equation K
agg is a constant with the dimension of (counts/s)⋅min−2 and t
0 is the duration of the lag period (t
0 is a point in time at which the light scattering intensity begins to increase). The applicability of Eq. (2) was demonstrated for thermal aggregation of Phb
[3]–[5], glyceraldehyde-3-phosphate dehydrogenase (GAPDH; EC 1.2.1.12) [6] and creatine kinase (CK; EC 2.7.3.2) from rabbit skeletal muscles [7]. Parameter K
agg characterizes the initial rate of aggregation, and the construction of a dependence of parameter K
agg on the initial protein concentration [P]0 allows calculating the order of aggregation with respect to the protein (n) (K
agg = const⋅ ). The dependence of parameter K
agg on the Phb concentration constructed on the basis of kinetic data for Phb aggregation at 53°C (pH 6.8) was linear [3], and consequently n = 1. This is true when the stage of unfolding of a protein molecule proceeds with a substantially lower rate than the following stages of aggregation of the unfolded protein molecules [8], [9]. In other words, the rate-limiting stage of the overall process of aggregation is the monomolecular stage of protein unfolding. According to the data presented by Kurganov et al. [10] the kinetic scheme of thermal denaturation of Phb involves a stage of the conformational change of the enzyme dimeric molecule followed by dissociation of the dimer into monomers and denaturation of labile monomeric forms.
). The dependence of parameter K
agg on the Phb concentration constructed on the basis of kinetic data for Phb aggregation at 53°C (pH 6.8) was linear [3], and consequently n = 1. This is true when the stage of unfolding of a protein molecule proceeds with a substantially lower rate than the following stages of aggregation of the unfolded protein molecules [8], [9]. In other words, the rate-limiting stage of the overall process of aggregation is the monomolecular stage of protein unfolding. According to the data presented by Kurganov et al. [10] the kinetic scheme of thermal denaturation of Phb involves a stage of the conformational change of the enzyme dimeric molecule followed by dissociation of the dimer into monomers and denaturation of labile monomeric forms.
The kinetics of thermal aggregation of GDH at various concentrations of the protein was studied by Sabbaghian et al. [11] (50°C; pH 8.0). The order of aggregation with respect to the protein calculated on the basis of these kinetic data is close to unity: n = 0.8±0.1.
The analysis of the data on thermal aggregation of βL-crystallin from bovine lens at 60°C (pH 6.8) obtained in [12] shows that parameter n is close to 2 (n = 1.99±0.07). It is generally accepted that the initial stage of aggregation is the stage of nucleation [3], [8], [9], [13]–[19]. The fact that the value of parameter n for thermal aggregation of βL-crystallin exceeds unity is indicative of the involvement of several molecules of denatured βL-crystallin in the nucleation process.
Consider a situation when Eq. (2) may be strictly fulfilled. The theoretical analysis carried by Ferrone [16] shows that for the nucleation-dependent aggregation the accumulation of monomers incorporated in the aggregate (Δ) proceeds according to the time-squared law:
| (3) |
where J is the elongation rate of aggregates, J* is the elongation rate of the nucleus and c* is the concentration of the nuclei. Such an early kinetics of aggregation was demonstrated experimentally, for example, for aggregation of polyglutamine peptides [20], aggregation of a slow-folding mutant of a β-clam protein, cellular retinoic acid-binding protein (P39A CRABP I) [21], and aggregation of protein L [22].
When studying the kinetics of thermal aggregation of some proteins by dynamic light scattering (DLS) we have shown that the hydrodynamic radius (R h) of the initially registered aggregates (the start aggregates) amounts to tens of nanometers [23]. This means that a start aggregate contains hundreds of denatured protein molecules. We could not detect intermediate states between the original forms of the proteins and the start aggregates. It should be noted that rather low temperatures were selected for the study of protein aggregation in this investigation (37°C for Phb and 45°C for GAPDH). The nuclei formed at the initial stages of aggregation involve several monomers. Therefore one can assume that there is no accumulation of nuclei in the system, and coalescence of the formed nuclei into larger structures, namely start aggregates, takes place. The initial increase in the light scattering intensity is connected with accumulation of the start aggregates, the size of the latter remaining unchanged (the hydrodynamic radius of the start aggregates was designated by R h,0). It is evident that in the time interval where the R h value is constant (R h = R h,0; see [23]) the light scattering intensity is strictly proportional to the amount of the aggregated protein. Thus, in this case Eq. (2) may be used for the quantitative description of the early kinetics of the aggregation process, and parameter K agg is a true measure of the initial rate of aggregation. When protein aggregation is studied at elevated temperatures, the time interval corresponding to the accumulation of the start aggregates is greatly reduced, and the registered increase in the hydrodynamic radius of the protein aggregates at t>t 0 is due to the attachment of individual denatured protein molecules to the start aggregates or the sticking of the start aggregates. This results in the additional increment in the light scattering intensity apart from the main contribution accounted for the formation of the start aggregates. Eq. (2) should be treated as an empiric equation where parameter K agg is a rough characteristic of the initial aggregation rate.
When studying the dependences of the hydrodynamic radius of protein aggregates on temperature obtained in the experiments with heating of protein solutions with a constant rate, we observed that the character of these dependences is identical to that for the dependences of the hydrodynamic radius of protein aggregates on time obtained at a fixed temperature [24]–[26]. Therefore we propose to use the following equation analogous to Eq. (2) for analysis of the initial parts of the dependences of the light scattering intensity on temperature obtained in the regime of heating with a constant rate:
| (4) |
In this equation T 0 is the initial temperature of aggregation, i.e., the temperature at which the light scattering intensity begins to increase, and K agg is a constant with the dimension of (counts/s)⋅°C−2 Parameter T 0 indicates the moment of origination of the start aggregates, whereas parameter K agg characterizes the rate of aggregation. It was of interest to check the applicability of Eq. (4) for the analysis of protein aggregation in the regime of heating with a constant rate, and to clarify how parameters T 0 and K agg change with variation in the protein concentration.
Materials and Methods
Hepes, glucose 1–phosphate, AMP, trimethylamine N-oxide (TMAO), creatine kinase (CK) from rabbit skeletal muscles, bovine liver GDH, NAD, NADH, ADP, L-glutamate, L-leucine and α-crystallin were purchased from “Sigma” (USA). The concentrations of CK, GDH and α-crystallin were determined from the absorbance at 280 nm using the extinction coefficients  of 8.8 [27], 9.7 [11] and 8.5 [28], respectively. NaCl was purchased from “Reakhim” (Russia), 2-hydroxypropyl-β-cyclodextrin (degree of substitution 3±1) (HP-β-CD) was obtained from CycloLab LTD (Hungary).
of 8.8 [27], 9.7 [11] and 8.5 [28], respectively. NaCl was purchased from “Reakhim” (Russia), 2-hydroxypropyl-β-cyclodextrin (degree of substitution 3±1) (HP-β-CD) was obtained from CycloLab LTD (Hungary).
Isolation of Phb
Phb was isolated from rabbit skeletal muscles as described in [29]. Phb concentration was determined spectrophotometrically at 280 nm using the extinction coefficient  of 13.2 [30].
of 13.2 [30].
Isolation of GAPDH
GAPDH was isolated from rabbit skeletal muscles as described by Scopes and Stoter [31] with an additional purification step using gel-filtration on Sephadex G-100. GAPDH concentration was determined spectrophotometrically at 280 nm using the extinction coefficient  of 10.6 [32].
of 10.6 [32].
Differential Scanning Calorimetry
Thermal denaturation of Phb was investigated by DSC using the adiabatic scanning microcalorimeter DASM–4M (Institute of Biological Instruments, Russian Academy of Sciences, Pushchino, Russia) with 0.47 ml capillary platinum cells. All measurements were carried out at the rate of heating by 1°C/min using the temperature range from 20 to 85°C and constant pressure of 2.2 atm. The dependences of the heat power on temperature were calculated using the program Origin software (MicroCal, Inc., USA).
The capillary construction of calorimetric cells prevents the artifacts caused by protein precipitation, which are often observed in batch calorimetric cells as exothermic peaks. In the capillary cells large-sized aggregated particles of protein are unable to settle and stir the solution, so no extra thermal production can arise [33].
Light Scattering Intensity Measurements
DLS measurements were performed on a commercial instrument Photocor Complex (Photocor Instruments Inc., USA; www.photocor.com). An He-Ne laser (Coherent, USA, Model 31-2082, 632.8 nm, 10 mW) was used as the light source. The temperature of sample cell was controlled by the proportional integral derivative (PID) temperature controller to within ±0.1°C. To carry on the light scattering intensity measurements in the regime of heating with a constant rate, a fast thermostat with the uniform distributed heater has been developed. The special design of this compact unit allows simply replacing the standard sample cell holder of the main thermostat of the Photocor Complex setup by the fast thermostat. Fast platinum thermometers with time constant of 1 s have been used both for temperature control and for real-time monitoring of temperature directly in the sample cell. The fast thermostat is fully controlled with the existing PID controller through the macro procedure of the Photocor program. Temperature scanning rate can be assigned from 10°C/min to any slower value.
A quasi-cross correlation photon counting system with two photomultiplier tubes was used to increase the accuracy of particle sizing in the range from 1.0 nm to 5.0 µm. DLS data have been accumulated and analyzed with a multifunctional real-time correlator. DynaLS software (Alango, Israel) was used for polydispersity analysis of DLS data. The diffusion coefficient D of the particles is directly related to the decay rate τc of the time-dependent correlation function for the light-scattering intensity fluctuations: D = 1/2τc k 2. In this equation k is the wave number of the scattered light, k = (4πn/λ)sin(θ/2), where n is the refractive index of the solvent, λ is the wavelength of the incident light in vacuum and θ is the scattering angle. The mean hydrodynamic radius of the particles, R h, can then be calculated according to the Stokes-Einstein equation: D = kBT/6πηR h, where k B is Boltzmann's constant, T is the absolute temperature and η is the shear viscosity of the solvent.
All solutions for light scattering experiments were prepared using deionized water obtained with Easy-Pure II RF system (Barnstead). The buffer was placed in a cylindrical cell with a diameter of 6.3 mm and incubated for 5 min at 25°C. Cells with stoppers were used for incubation at high temperature to avoid evaporation. The aggregation process was initiated by the addition of an aliquot of protein to a final volume of 0.5 ml. When studying the kinetics of aggregation of proteins, the scattering light was collected at 90° scattering angle.
Dimensions of the fast thermostat used for heating of the protein solution were the following: 15 mm (diameter)×40 mm (height). Temperature gradients in such a thermostat are less than 0.1 degree. Notice that large temperature gradients in the sample cell would result in a developed convection of fluid. The correlation function of light, scattered on the convective flow, is quite different from the exponential correlation function in case of light scattering on diffusing particles. That is why we can reliably estimate the appearance of temperature gradients by analyzing measured correlation functions.
Calculations
OriginPro 8.0 SR0 software (OriginLab Corporation, USA) and Scientist software (MicroMath, Inc., USA) were used for the calculations. To characterize the degree of agreement between the experimental data and calculated values, we used the coefficient of determination R 2 (without considering the statistical weight of the measurement results) [34]:
 |
(5) |
where  is the average of the experimental data (
is the average of the experimental data ( ), Y
calc is the theoretically calculated value of the function Y and n is the number of measurements.
), Y
calc is the theoretically calculated value of the function Y and n is the number of measurements.
Results and Discussion
Turbidimetric Registration of Phb Aggregation
Thermal aggregation of Phb was studied in the regime wherein temperature was elevated with a constant rate (1°C/min). Fig. 1A shows the dependences of the light scattering intensity on temperature for aggregation of Phb at concentrations of 0.95 and 1.9 mg/ml (curves 1 and 2, respectively). When the temperature is elevated, the light scattering intensity reaches the limiting value. The decrease in the light scattering intensity at rather high temperatures is due to precipitation of the large-sized aggregates. Fig. 1B demonstrates the results of fitting Eq. (1) to the experimental data. The following values of parameters were established: I lim = (0.84±0.01)⋅106 counts/s, T agg = 56.1±0.1, B = 1.57±0.02 (R 2 = 0.999) at the Phb concentration of 0.95 mg/ml and I lim = (1.05±0.01)⋅106 counts/s, T agg = 55.5±0.1, B = 1.34±0.02 (R 2 = 0.999) at the Phb concentration of 1.9 mg/ml.
Figure 1. The dependences of the light scattering intensity at 632.8 nm on temperature for thermal aggregation of Phb registered in the regime of heating with a constant rate (1°C/min).
Conditions: 0.08 M Hepes-buffer, pH 6.8, containing 0.1 M NaCl. (A) The original experimental dependences. The Phb concentrations were as follows: (1) 0.95 and (2) 1.9 mg/ml. (B) The results of fitting the experimental curves to Eq. (1). Points are the experimental data. The solid curves are calculated from Eq. (1). The dotted horizontal lines correspond to the limiting values of the light scattering intensity (I lim).
Denaturation of Phb Registered by DSC and Comparison of the Turbidimetric and Calorimetric Data
When studying thermal aggregation of Phb, we should take into account that the initial stage of the general process of aggregation is the stage of protein denaturation. To characterize Phb denaturation, we used DSC. Fig. 2 shows DSC profiles obtained at the Phb concentrations of 0.95 and 1.9 mg/ml (curves 1 and 2, respectively). The heating rate was 1°C/min. The increase in the Phb concentration from 0.95 to 1.9 mg/ml results in the displacement of the maximum on the DSC profiles (T m) from 53.7±0.1 to 54.2±0.1°C. This change in the shape of the DSC profile with variation of the Phb concentration agrees with the dissociative mechanism of thermal denaturation of Phb [10].
Figure 2. Thermal denaturation of Phb (0.08 M Hepes-buffer, pH 6.8, containing 0.1 M NaCl).
The dependences of the excess heat capacity ( ) on temperature, obtained at the following concentrations of Phb: (1) 0.95 and (2) 1.9 mg/ml.
) on temperature, obtained at the following concentrations of Phb: (1) 0.95 and (2) 1.9 mg/ml.  was calculated per dimer of Phb with the molecular mass of 194.8 kDa. The heating rate was 1°C/min.
was calculated per dimer of Phb with the molecular mass of 194.8 kDa. The heating rate was 1°C/min.
When a definite temperature has been achieved, the portion of the protein denatured by this temperature may be calculated from the area under the part of the DSC profile limited at this temperature (Q). If we know the area under the whole  versus temperature profile (Q
total), the portion of denatured protein (γden) is equal to the Q/Q
total ratio. Fig. 3 shows the relationships between the light scattering intensity registered in the course of Phb aggregation and the γden value at two concentrations of Phb. It can be seen that the increase in the light scattering intensity (I) is not a linear function of γden. Thus, in the case of thermal aggregation of Phb the increment of the light scattering intensity cannot be considered as a direct measure of the degree of protein denaturation. It should be noted that in some cases a linear proportionality between the increase in the light scattering intensity and the degree of protein denaturation is really fulfilled. This, for example, was demonstrated by us for thermal aggregation of GAPDH [35].
versus temperature profile (Q
total), the portion of denatured protein (γden) is equal to the Q/Q
total ratio. Fig. 3 shows the relationships between the light scattering intensity registered in the course of Phb aggregation and the γden value at two concentrations of Phb. It can be seen that the increase in the light scattering intensity (I) is not a linear function of γden. Thus, in the case of thermal aggregation of Phb the increment of the light scattering intensity cannot be considered as a direct measure of the degree of protein denaturation. It should be noted that in some cases a linear proportionality between the increase in the light scattering intensity and the degree of protein denaturation is really fulfilled. This, for example, was demonstrated by us for thermal aggregation of GAPDH [35].
Figure 3. The relationships between the increment of the light scattering intensity accompanying Phb aggregation and the portion of the denatured Phb (γden) calculated from the DSC data.
Phb concentrations were as follows: (1) 0.95 and (2) 1.9 mg/ml. The inset shows the initial parts of the curves. Points are the experimental data. The solid curves are calculated from Eq. (6).
The analysis of the relationship between the increment of the light scattering intensity (I) and the portion of the denatured Phb presented in Fig. 3 showed that at γden<0.5 the I value was a power function of γden:
| (6) |
The following values of parameters K and N were found: K = (4.0±0.1)⋅105 counts/s and N = 1.32±0.02 (R 2 = 0.999) at the Phb concentration of 0.95 mg/ml and K = (6.6±0.1)⋅105 counts/s and N = 1.34±0.02 (R 2 = 0.999) at the Phb concentration of 1.9 mg/ml.
When comparing calorimetric and turbidimetric data obtained in the regime of heating with a constant rate, we should take into account the following features of the temperature dependences of excess heat capacity and the light scattering intensity. There is no threshold temperature on the DSC profile. The dependence of excess heat capacity on temperature asymptotically approaches zero level with decreasing temperature. The lower the temperature, the lower is the amount of the denatured protein accumulated to the moment when the given temperature has been achieved. On the contrary, an initial increase in the light scattering intensity is registered at definite temperature designated as T 0. According to the mechanism of thermal aggregation of proteins proposed in our works [12], [23], [35]–[37], the initial increase in the light scattering intensity corresponds to the moment of the origination of the start aggregates. The formation of the start aggregates proceeds on the all-or-none principle. The intermediate states between native enzyme forms (or denatured protein molecules) and start aggregates have not been detected.
Analysis of the Initial Parts of the Dependences of the Light Scattering Intensity on Temperature
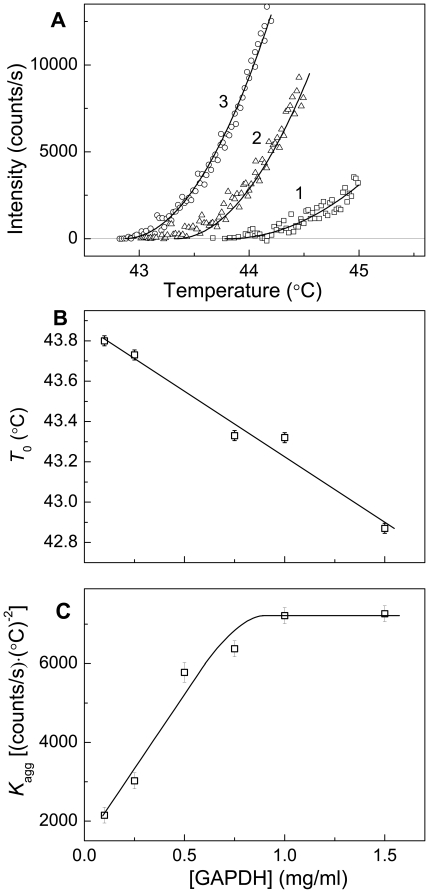
In the present work the focus is concentrated on the initial parts of the dependences of the light scattering intensity on temperature. Fig. 4A shows the influence of the protein concentration on the initial parts of the kinetic curves of aggregation of Phb heated with a constant rate of 1°C/min. The Phb concentration was varied in the interval from 0.1 to 1.5 mg/ml. The experimental curves are satisfactorily described by the Eq. (4). The R 2 values lie in the range from 0.995 at [Phb] = 0.1 mg/ml to 0.990 at [Phb] = 1.5 mg/ml. The calculated values of parameters T 0 and K agg are represented in Fig. 4B and C as a function of the Phb concentration. Parameter T 0, characterizing the temperature at which the light scattering intensity begins to increase, decreases monotonously with increasing the concentration of Phb (Fig. 4B).
Figure 4. Analysis of the kinetics of Phb aggregation registered in the regime of heating at the rate of 1°C/min.
(A) The initial parts of the dependences of the light scattering intensity on temperature obtained at the following concentrations of Phb: (1) 0.1, (2) 0.25, (3) 0.5, (4) 0.75, (5) 1.0 and (6) 1.5 mg/ml. Points are the experimental data. The solid curves were calculated from Eq. (4). (B and C) The dependences of parameters T 0 and K agg calculated from Eq. (4) on the Phb concentration, respectively.
When interpreting the dependence of parameter K agg characterizing the rate of aggregation on the Phb concentration, we should be aware that this dependence can not be used for estimation of the order of aggregation with respect to the protein because the K agg values on the K agg versus the Phb concentration plot correspond, in fact, to different temperatures. It is of interest that at the Phb concentration higher than 0.5 mg/ml parameter K agg is a constant value, which is independent of the protein concentration (Fig. 4C). When the Phb concentration is lower than 0.5 mg/ml, decreasing the protein concentration results in the decrease in the K agg value.
It was of interest to investigate whether the character of the dependences of parameters T 0 and K agg on the protein concentration observed for Phb remains the same for other proteins. Fig. 5 shows the analysis of the initial parts of the dependences of the light scattering intensity on temperature for thermal aggregation of GAPDH. The protein concentration was varied in the interval from 0.1 to 1.5 mg/ml. As an example, the kinetics of GAPDH aggregation at the protein concentrations of 0.1, 0.75 and 1.5 mg/ml and the results of fitting the experimental data to Eq. (4) are represented in Fig. 5A. The R 2 values for approximation of the curves shown in Fig. 5A by Eq. (4) were found to be 0.881, 0.974 and 0.990 at the GAPDH concentrations of 0.1, 0.75 and 1.5 mg/ml, respectively. The calculated values of parameters T 0 and K agg are represented in Fig. 5B and C as a function of the GAPDH concentration. As in the case of Phb, parameter T 0 decreases monotonously with increasing the GAPDH concentration and parameter K agg reaches a constant value at rather high protein concentrations.
Figure 5. Analysis of the kinetics of GAPDH aggregation registered in the regime of heating at the rate of 1°C/min (10 mM Na-phosphate buffer, pH 7.5, containing 0.1 M NaCl).
(A) The initial parts of the dependences of the light scattering intensity on temperature obtained at the following concentrations of GAPDH: (1) 0.1, (2) 0.75 and (3) 1.5 mg/ml. Points are the experimental data. The solid curves were calculated from Eq. (4). (B and C) The dependences of parameters T 0 and K agg calculated from Eq. (4) on the GAPDH concentration, respectively.
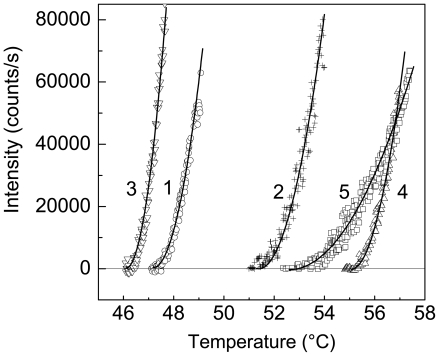
Fig. 6A demonstrates the applicability of Eq. (4) for the description of the initial parts of the dependences of the light scattering intensity on temperature obtained for aggregation of CK at various concentrations of the protein. The R 2 values for approximation of these dependences by Eq. (4) lie in the range from 0.846 at [CK] = 0.25 mg/ml to 0.985 at [CK] = 1.5 mg/ml. When the concentration of CK increases from 0.1 to 1.5 mg/ml, the T 0 value decreases monotonously from 45.2 to 40.7°C (Fig. 6B). The value of parameter K agg increases with increasing the CK concentration (Fig. 6C). There is no tendency for K agg to level off at high concentrations of the protein.
Figure 6. Analysis of the kinetics of CK aggregation registered in the regime of heating at the rate of 1°C/min (30 mM Hepes–NaOH buffer, pH 8.0, containing 0.1 M NaCl).
(A) The initial parts of the dependences of the light scattering intensity on temperature obtained at the following CK concentrations: (1) 0.1, (2) 0.25, (3) 0.5, (4) 0.75 and (5) 1.5 mg/ml. Points are the experimental data. The solid curves were calculated from Eq. (4). (B and C) The dependences of parameters T 0 and K agg calculated from Eq. (4) on the CK concentration, respectively.
Sabbaghian et al. [11] showed that interaction of small molecules (coenzymes, allosteric effectors) with the bovine liver GDH resulted in profound changes in the extent of its thermal aggregation. It was of interest to study thermal aggregation of GDH in the regime of elevating the temperature with a constant rate. The initial parts of the dependences of the light scattering intensity on temperature obtained at various concentrations of GDH were analyzed using Eq. (4) (Fig. 7A), and the corresponding values of parameters T 0 and K agg were calculated. The R 2 values for approximation of the curves presented in Fig. 7A by Eq. (4) were found to be 0.990. The value of parameter T 0 remains practically constant with variation of the GDH concentration (Fig. 7B). The average value of T 0 is equal to 34.8±0.2°C. As it can be seen from Fig. 7C, parameter K agg increases monotonously with increasing the protein concentration.
Figure 7. Analysis of the kinetics of GDH aggregation registered in the regime of heating at the rate of 1°C/min (0.1 M Na-phosphate buffer, pH 7.6).
(A) The initial parts of the dependences of the light scattering intensity on temperature obtained at the following concentrations of GDH: (1) 0.1, (2) 0.12, (3) 0.2, (4) 0.3 and (5) 0.4 mg/ml. Points are the experimental data. The solid curves were calculated from Eq. (4). (B and C) The dependences of parameters T 0 and K agg calculated from Eq. (4) on the GDH concentration, respectively.
We have also analyzed the dependences of the light scattering intensity on temperature for aggregation of human interleukin 1-β presented by Chrunyk et al. [38]. These dependences were obtained at various protein concentrations. The heating rate was 0.5°C/min. The following values of parameters of Eq. (4) were found: T 0 = 50.0±0.1, 48.4±0.1 and 47.5±0.1°C, K agg = 317±15, 1130±50 and 2200±100 (°C)−2 for the interleukin 1-β concentration equal to 1, 2 and 4 mg/ml, respectively. The R 2 values for approximation of the experimental curves by Eq. (4) were equal to 0.965, 0.913 and 0.846 at the interleukin 1-β concentration of 1, 2 and 4 mg/ml, respectively. Thus, the enhancement of the interleukin 1-β concentration results in the diminishing of the value of parameter T 0 and the increase in the K agg value. It is worthy of mention that to characterize the temperature aggregation Chrunyk et al. [39] proposed to use a temperature corresponding to the length cut off on the temperature axis by the straight line, which is a continuation of the linear part of the dependence of the light scattering intensity on temperature reachable at the inflexion point.
In the work of Raibekas [40] the dependences of the light scattering intensity on temperature for aggregation of interleukin-1 receptor antagonist (IL-1ra) are represented in the coordinates {I/I lim; T}. The heating rate was 1°C/min. The dependences I/I lim on temperature were obtained at various concentrations of IL-1ra and characterized by parameter T agg corresponding to the level I/I lim = 0.5. In the interval concentrations of IL-1ra from 1 to 10 mg/ml parameter T agg decreases by 5.3°C, namely from 56.4 to 51.1°C (ΔT agg = 5.3°C). We have analyzed the dependences of I/I lim on temperature using an equation, which is a modification of Eq. (4):
| (7) |
In this equation  is a constant. The change in the T
0 value characterizes the displacement of the dependence of I/I
lim on temperature along the abscissa axis, whereas parameter
is a constant. The change in the T
0 value characterizes the displacement of the dependence of I/I
lim on temperature along the abscissa axis, whereas parameter  characterizes the steepness of the initial part of the dependence of I/I
lim on T. The following values of parameters of Eq. (7) were found: T
0 = 53.7±0.1°C and
characterizes the steepness of the initial part of the dependence of I/I
lim on T. The following values of parameters of Eq. (7) were found: T
0 = 53.7±0.1°C and  = 0.080±0.002 (°C)−2 (R
2 = 0.994) at [IL-1ra] = 1 mg/ml and T
0 = 47.3±0.1°C and
= 0.080±0.002 (°C)−2 (R
2 = 0.994) at [IL-1ra] = 1 mg/ml and T
0 = 47.3±0.1°C and  = 0.044±0.001 (°C)−2 (R
2 = 0.997) at [IL-1ra] = 10 mg/ml. If one assumes that the I
lim value is strictly proportional to the protein concentration, the calculations show that K
agg should increase by a factor of 5.5 when the IL-1ra concentration increases from 1 to 10 mg/ml. The displacement of the dependence of I/I
lim on temperature in this concentration interval was characterized by the change in parameter T
0, and ΔT
0 was equal to 6.4°C. This value is less than ΔT
agg (5.3°C). The discrepancy between the values of ΔT
0 and ΔT
agg is explained by the lesser steepness of the initial part of the dependence of I/I
lim on temperature and, consequently, the lesser slope of this dependence in the inflexion point at [IL-1ra] = 10 mg/ml.
= 0.044±0.001 (°C)−2 (R
2 = 0.997) at [IL-1ra] = 10 mg/ml. If one assumes that the I
lim value is strictly proportional to the protein concentration, the calculations show that K
agg should increase by a factor of 5.5 when the IL-1ra concentration increases from 1 to 10 mg/ml. The displacement of the dependence of I/I
lim on temperature in this concentration interval was characterized by the change in parameter T
0, and ΔT
0 was equal to 6.4°C. This value is less than ΔT
agg (5.3°C). The discrepancy between the values of ΔT
0 and ΔT
agg is explained by the lesser steepness of the initial part of the dependence of I/I
lim on temperature and, consequently, the lesser slope of this dependence in the inflexion point at [IL-1ra] = 10 mg/ml.
Thus, the quantitative analysis of the initial parts of the dependences of the light scattering intensity on temperature using Eq. (4) allows us to estimate the threshold temperature T 0 and parameter K agg, which may be considered as a measure of the rate of aggregation. Parameter T 0 has a clear physical sense. When the measurement of the light scattering intensity is used for registration of protein aggregation, the initial increment of the light scattering intensity corresponds to the instant of the formation of the start aggregates containing hundreds of denatured protein molecules [25], [26]. The value of parameter T 0 is a function of the protein concentration and, as a rule, decreases with increasing the protein concentration. As it could be expected, the value of parameter K agg characterizing the aggregation rate tends to grow as the protein concentration increases. In some cases the leveling off for the K agg value is observed at rather high concentrations of the protein, as with Phb and GAPDH. Such unexpected decrease in the growth of the aggregation rate with increasing protein concentration may have a simple explanation. When the protein concentration increases, the diminishing of the initial aggregation temperature (T 0) occurs, and, consequently, the initial rate of aggregation is measured in the region of lower temperatures.
Two parameters we proposed to characterize the temperature dependence of the light scattering intensity (T
0 and K
agg) provide more detailed information on the character of protein aggregation than parameter T
agg. Really, the value of parameter T
agg is determined by two factors, namely the threshold temperature T
0 and the steepness of the initial part of the dependence of the light scattering intensity on temperature expressed by parameter K
agg (or parameter  ).
).
Apart from the protein concentration there is another factor affecting the shape on the light scattering intensity versus temperature plot, namely the heating rate. One could expect that an increase in the heating rate would result in the displacement of the dependence of the light scattering intensity on temperature towards higher temperatures. Actually, such a picture was observed for aggregation of α-amylase from Bacillus amyloliquefaciens (BAA). Duy and Fitter [41] studied aggregation of BAA at various values of the heating rate (v). The protein concentration was 50 µg/ml. The experimental data were represented by the coordinates {I/I
lim; T}. An analysis of the dependences of I/I
lim on temperature based on Eq. (7) gives the following values of parameters: T
0 = 74.0±0.1°C and  = 0.0052±0.0002 (°C)−2 (R
2 = 0.964) at v = 0.1°C/min and T
0 = 79.8±0.1°C and
= 0.0052±0.0002 (°C)−2 (R
2 = 0.964) at v = 0.1°C/min and T
0 = 79.8±0.1°C and  = 0.0070±0.0005 (°C)−2 (R
2 = 0.949) at v = 1.0°C/min. Thus the enhancement of the heating rate from 0.1 to 1.0°C/min results in the displacement of the dependence of the light scattering intensity on temperature along the abscissa axis by 5.8°C towards higher temperatures. The change in the steepness of the initial part of the dependence of the light scattering intensity on temperature is not marked. Parameter
= 0.0070±0.0005 (°C)−2 (R
2 = 0.949) at v = 1.0°C/min. Thus the enhancement of the heating rate from 0.1 to 1.0°C/min results in the displacement of the dependence of the light scattering intensity on temperature along the abscissa axis by 5.8°C towards higher temperatures. The change in the steepness of the initial part of the dependence of the light scattering intensity on temperature is not marked. Parameter 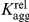 increases by a factor of 1.35, when the heating rate is changed from 0.1 to 1.0°C/min. It should be noted that the change in the value of parameter T
agg corresponding to the level I/I
lim = 0.5 in this interval of the heating rates was equal to 3.3°C. Consequently, ΔT
agg is less than ΔT
0. Apart from the light scattering measurements the authors studied thermal unfolding of BAA using intrinsic fluorescence spectroscopy. Unfolding transitions were analyzed in terms of wavelength shifts of the emission peak and by calculating the peak ratios of intensities as measured at 330 nm and 350 nm (excitation wavelengths of 280 and 295 nm were applied). It was shown that for both heating rates (0.1 and 1.0°C/min) aggregation that was characterized by the normalized change in the light scattering intensity appeared rather concurrent with respect to the unfolding transition as obtained from tryptophan fluorescence.
increases by a factor of 1.35, when the heating rate is changed from 0.1 to 1.0°C/min. It should be noted that the change in the value of parameter T
agg corresponding to the level I/I
lim = 0.5 in this interval of the heating rates was equal to 3.3°C. Consequently, ΔT
agg is less than ΔT
0. Apart from the light scattering measurements the authors studied thermal unfolding of BAA using intrinsic fluorescence spectroscopy. Unfolding transitions were analyzed in terms of wavelength shifts of the emission peak and by calculating the peak ratios of intensities as measured at 330 nm and 350 nm (excitation wavelengths of 280 and 295 nm were applied). It was shown that for both heating rates (0.1 and 1.0°C/min) aggregation that was characterized by the normalized change in the light scattering intensity appeared rather concurrent with respect to the unfolding transition as obtained from tryptophan fluorescence.
Estimation of the Size of Protein Aggregates
Consider what additional information on the aggregation process studied in the regime of heating with a constant rate may be obtained from the measurements of the size of the protein aggregates. Fig. 8A shows the initial parts of the dependences of the hydrodynamic radius (R h) of the protein aggregates on temperature obtained for GAPDH aggregation at various concentrations of the protein. These dependences are linear, and the following equation was used for their analysis [24], [25]:
| (8) |
In this equation R h,0 is the hydrodynamic radius of the start aggregates, T 0 is the temperature at which the start aggregates appear and ΔT 2R is the temperature interval over which the hydrodynamic radius of the protein aggregates increases from R h,0 to 2R h,0. The reciprocal value of parameter ΔT 2R is a measure of the aggregation rate. The higher the 1/ΔT 2R value, the higher is the aggregation rate. Taking T 0 in this equation to be identical to parameter T 0 in Eq. (4), we can calculate other parameters, namely R h,0 and ΔT 2R. As it can be seen from Fig. 8B, the marked decrease in the R h,0 value occurs with increasing GAPDH concentration (from 64±3 nm at [GAPDH] = 0.1 mg/ml to 14±3 nm at [GAPDH] = 1.5 mg/ml). Such a decrease in the R h,0 value is accompanied by the increase in the rate of aggregation expressed by the reciprocal value of parameter ΔT 2R (Fig. 8C).
Figure 8. Estimation of the size of the protein aggregates formed in the course of GAPDH aggregation registered in the regime of heating at the rate of 1°C/min (10 mM Na-phosphate buffer, pH 7.5, containing 0.1 M NaCl).
(A) The initial parts of the dependences of the hydrodynamic radius (R h) of the protein aggregates on temperature obtained at the following GAPDH concentrations: (1) 0.1, (2) 0.25, (3) 0.5 and (4) 1.5 mg/ml. (B and C) The dependences of parameter R h,0 and the reciprocal value of parameter ΔT 2R calculated from Eq. (8) on the GAPDH concentration, respectively.
Fig. 9A shows the initial parts of the dependences of the hydrodynamic radius of protein aggregates on temperature obtained for CK aggregation at various concentrations of the protein. These dependences are exponential, and the following equation was used for their analysis [24]–[26]:
| (9) |
In this equation ΔT 2R is the temperature interval over which the hydrodynamic radius is doubled. When the concentration of the protein increases, a slight diminishing of the R h,0 value takes place (from 44±2 nm at [CK] = 0.1 mg/ml to 34±2 nm at [CK] = 1.5 mg/ml) with simultaneous increase in the 1/ΔT 2R value (Fig. 9B and C).
Figure 9. Estimation of the size of the protein aggregates formed in the course of CK aggregation registered in the regime of heating at the rate of 1°C/min (30 mM Hepes–NaOH buffer, pH 8.0, containing 0.1 M NaCl).
(A) The initial parts of the dependences of the hydrodynamic radius (R h) of the protein aggregates on temperature obtained at the following CK concentrations: (1) 0.1, (2) 0.75 and (3) 1.5 mg/ml. (B and C) The dependences of parameter R h,0 and the reciprocal value of parameter ΔT 2R calculated from Eq. (9) on the GAPDH concentration, respectively.
As is with CK, the initial parts of the dependences of the hydrodynamic radius of protein aggregates on temperature obtained for GDH aggregation at various concentrations of the protein are exponential (Fig. 10A). The values of parameters R h,0 and 1/ΔT 2R as a function of the GDH concentration are represented in Fig. 10B and C. The increase in the GDH concentration results in the diminishing of the R h,0 value with simultaneous increase in the 1/ΔT 2R value.
Figure 10. Estimation of the size of the protein aggregates formed in the course of GDH aggregation registered in the regime of heating at the rate of 1°C/min (0.1 M Na-phosphate buffer, pH 7.6).
(A) The initial parts of the dependences of the hydrodynamic radius (Rh) of the protein aggregates on temperature obtained at the following GDH concentrations: (1) 0.1, (2) 0.2, (3) 0.3 and (4) 0.4 mg/ml. (B and C) The dependences of parameter R h,0 and the reciprocal value of parameter ΔT 2R calculated from Eq. (9) on the GDH concentration, respectively.
The following explanation can be proposed for the decrease in parameter T 0 with increasing the protein concentration (Fig. 4– 6). The increase in the protein concentration favors nucleation and further formation of start aggregates. Therefore one may expect that the temperature corresponding to the moment of origination of the start aggregates will decrease with increasing the protein concentration. This picture resembles the well-known phenomenon of the decrease in the duration of the lag period on the kinetic curves of aggregation registered at a fixed temperature when the concentration of the model protein increases (see, for example [17], [26], [35], [36]).
We can interpret the linear character of the dependence of the hydrodynamic radius of protein aggregates on temperature observed for GAPDH as an indication of the fulfillment of the diffusion-limited aggregation regime, wherein the rate of aggregation is limited by diffusion of the colliding particles (the so-called regime of diffusion-limited cluster–cluster aggregation) [24], [25]. In other words, the sticking probability for the colliding particles for this regime of aggregation is equal to unity. We interpret the exponential character of the dependence of the hydrodynamic radius of the protein aggregates on temperature observed for CK and GDH as an indication for the fulfillment of the regime of reaction-limited cluster-cluster aggregation wherein the sticking probability for the colliding particles is lower than unity [24]–[26].
Consider the differences between the model of protein aggregation used in the present work and models proposed by other investigators. A general model of protein aggregation was recently elaborated by Li and Roberts [42]. This model takes into account the conformational transition of monomers between folded and unfolded states, nucleation of the smallest aggregate species, growth of aggregates via chain polymerization and aggregate growth due to aggregate-aggregate association. When studying the kinetics of thermal aggregation of proteins by dynamic light scattering, we observed that at the moment of time when the initial increment in the light scattering intensity is observed, protein aggregates containing hundreds of denatured protein molecules are registered. These primary aggregates were called the start aggregates. We could not detect intermediate states between the non-aggregated form of the protein and the start aggregates. Thus we infer that the formation of the start aggregates proceeds on the all-or-none principle. This pattern was demonstrated for thermal aggregation of βL-crystallin from bovine lens [12], [37], GAPDH from rabbit skeletal muscle [6], [23], [35], [37], [43], yeast alcohol dehydrogenase I [26], [37], tobacco mosaic virus coat protein [37], [44], Phb from rabbit skeletal muscle [5], [23], [29], [37], [43], [45], aspartate aminotransferase from pig heart mitochondria [46] and creatine kinase from rabbit skeletal muscle [7]. On the basis of these experimental data a new model of protein aggregation was proposed [12], [23], [36], [37]. According to this model nuclei are assembled rather quickly in the start aggregates and further growth of aggregates is due to sticking of the start aggregates and aggregates of higher order. It was demonstrated that the size of the start aggregates is independent of the concentration of the protein involved in aggregation. This fact allowed us to draw an analogy between the formation of the start aggregates and micelle formation. In the latter case the micelles of a definite size are formed when the critical micelle concentration is achieved. Such an analogy offers an explanation of why the formation of start aggregates proceeds according to the all-or-none principle.
The idea on the formation of primary aggregates in the course of protein aggregation was also used by Durand and coworkers.When studying thermal aggregation of β-lactoglobulin at pH 7.0 by size exclusion chromatography, Durand et al. [47]–[51] showed that there was a clear separation between a narrow peak that corresponded to residual native protein and a broad peak that corresponded to the aggregates. This observation implied that the aggregates of the minimum size contained many monomers and that no or very few stable oligomers were formed. The authors supposed that the first stage of thermal aggregation of β-lactoglobulin was the step of the formation of “primary aggregates”.
Shiraki and coworkers [52] studied heat-induced aggregation of lysozyme at around the isoelectric point. Dynamic light scattering and transmission electron microscopy revealed that aggregation occurred in a two-step process: formation of start aggregates, followed by further growth mediated by their sticking with diffusion-limited cluster-cluster aggregation.
Recent investigations of dithiothreitol-induced aggregation of alpha-lactalbumin and insulin using dynamic light scattering convincingly demonstrate that the aggregation process proceeds through the stage of formation of the start aggregates [53], [54].
It is notable that according to present views the assembly of amyloid fibrils proceeds through the stage of the formation of protofibrils. Association of protofibrils results in the formation of fibrils and then clusters of fibrils and bundles [55], [56]. It is evident that protofibrils are equivalent to the start aggregates in the case of aggregation producing amorphous aggregates (this analogy was discussed in Golub et al. [23]).
Screening of the Ligands Affecting Denaturation or Aggregation of Phb and GDH on the Basis of the Data of the Aggregation Kinetics
Consider the use of this new approach to characterization of the aggregation rate for the quantitative estimation of the ability of various agents to suppress protein aggregation. Our investigations have shown that Phb is a convenient model for testing the compounds affecting protein denaturation and aggregation [4], [5], [25], [29], [45], [57]–[60]. Fig. 11 shows the effect of some agents on Phb aggregation. The suppression of aggregation in the presence of 1 mM AMP is accompanied by the increase in the T 0 value from 47.2°C (curve 1; the control) to 51.4°C (curve 2; 1 mM AMP) and the decrease in parameter K agg from (1.83±0.02)⋅105 (curve 1) to (1.20±0.02)⋅105 (counts/s)⋅(°C)−2 (curve 2). Based on the data on kinetics of thermal inactivation of Phb and DSC data [58], [60], we can conclude that the anti-aggregation ability of allosteric activator AMP is due to the stabilization of the native Phb molecule. The acceleration of Phb aggregation in the presence of HP-β-CD (curve 3 in Fig. 11) is accompanied by the decrease in the T 0 value to 46.1°C and the increase in parameter K agg to (3.27±0.03)⋅105 (counts/s)⋅(°C)−2. This action of HP-β-CD is caused by the destabilization of the Phb molecule as a result of binding of HP-β-CD to the native form of the enzyme and early intermediates of Phb unfolding as suggested by the data on the kinetics of thermal inactivation and DSC data [45]. It is known that osmolytes stabilize the protein structure [61]–[63]. Therefore, when testing the effect of osmolytes on protein aggregation, one can expect that osmolytes will reveal the protective action. Fig. 11 demonstrates that a markedly expressed retardation of thermal aggregation of Phb is observed in the presence of 1 M TMAO. The T 0 value increases to 55.1°C and the K agg value decreases to (1.54±0.02)⋅105 (counts/s)⋅(°C)−2 (curve 4). At last, it is of interest to discuss the effect of the agents of protein nature responsible for suppression of protein aggregation in the cell, namely small heat shock proteins. Meremyanin et al. [25], [29] showed that α-crystallin, one of the representatives of small heat shock protein family, effectively hindered thermal aggregation of Phb. Curve 5 in Fig. 11 presents an initial part of the dependence of the light scattering intensity on temperature for Phb aggregation measured in the presence of α-crystallin at the concentration of 1 mg/ml. The protective action of α-crystallin is reflected in the increase in the T 0 value (to 52.6°C) and the marked decrease in the K agg value (to (0.27±0.01)⋅105 (counts/s)⋅(°C)−2). The R 2 values for approximation of the curves 1–5 shown in Fig. 11 by Eq. (4) were found to be 0.981, 0.954, 0.987, 0.986 and 0.972, respectively. The values of parameters of T 0 and K agg obtained during testing of the effect of various agents on Phb aggregation are given in Table 1.
Figure 11. Effect of different agents on Phb aggregation.
The initial parts of the dependences of the light scattering intensity on temperature for aggregation of Phb (0.5 mg/ml) in the presence of the following agents: (1) control, (2) 1 mM AMP, (3) 0.19 M HP-β-CD, (4) 1 M TMAO and (5) α-crystallin at the concentration of 1 mg/ml. Points are the experimental data. The solid curves were calculated from Eq. (4).
Table 1. Parameters of aggregation of Phb (0.5 mg/ml) in the presence of different ligands (0.08 M Hepes-buffer, pH 6.8, containing 0.1 M NaCl).
| Ligand | T 0 (°C) | K agg [(counts/s)⋅(°C)−2] |
| - | 47.2±0.1 | (1.83±0.02)⋅105 |
| AMP (1 mM) | 51.4±0.1 | (1.20±0.02)⋅105 |
| HP-β-CD (19 mM) | 46.1±0.1 | (3.27±0.03)⋅105 |
| TMAO (1 M) | 55.1±0.1 | (1.54±0.02)⋅105 |
| α-crystallin (1 mg/ml) | 52.6±0.1 | (0.27±0.01)⋅105 |
Fig. 12 shows the effect of specific ligands (NADH, ADP, L-glutamate and L-leucine) on aggregation of GDH studied in the regime of heating with a constant rate. The values of parameters T 0 and K agg are given in Table 2. The increase in parameter K agg in the presence of 0.2 mM NADH means that NADH displays the destabilizing action on the enzyme. Judging from the K agg values, the enhancement of GDH stability is observed in the presence of allosteric activators, ADP and L-leucine, and in the presence of substrate, L-glutamate. ADP (0.5 mM) eliminates the destabilizing effect of NADH (0.2 mM). NAD has no effect on GDH aggregation (see Table 2).
Figure 12. Effect of different agents on GDH aggregation.
The initial parts of the dependences of the light scattering intensity on temperature for aggregation of GDH (0.12 mg/ml) in the presence of the following agents: (1) control; (2) 0.2 mM NADH; (3) 50 mM L-glutamate; (4) 0.2 mM NADH+0.5 mM ADP; (5) 50 mM L-leucine and (6) 0.5 mM ADP. Points are the experimental data. The solid curves were calculated from Eq. (4).
Table 2. Parameters of aggregation of GDH (0.12 mg/ml) in the presence of different ligands (0.1 M Na-phosphate buffer, pH 7.6).
| Ligand | T 0 (°C) | K agg [(counts/s)⋅(°C)−2] |
| - | 34.3±0.1 | (8.7±0.1)⋅102 |
| NADH (0.2 mM) | 35.0±0.1 | (11.6±0.2)⋅102 |
| NAD (0.2 mM) | 34.5±0.1 | (8.4±0.1)⋅102 |
| ADP (0.5 mM) | 39.3±0.1 | (4.7±0.1)⋅102 |
| NADH (0.2 mM)+ADP(0.5 mM) | 36.4±0.1 | (4.7±0.1)⋅102 |
| L-Leucine (50 mM) | 36.0±0.1 | (2.8±0.1)⋅102 |
| L-Glutamate (50 mM) | 32.8±0.1 | (4.9±0.1)⋅102 |
The effect of ADP on aggregation of GDH was studied in greater detail. Fig. 13A shows the initial parts of the dependences of the light scattering intensity on temperature obtained for GDH aggregation at various concentrations of ADP. The values of parameters T 0 and K agg as a function of the ADP concentration are represented in Fig. 13B and C. A sharp increase in parameter T 0 is observed in the presence of 0.05 mM ADP, namely from 35.2°C at [ADP] = 0 to 38.7°C at [ADP] = 0.05 mM (Fig. 13B). The changes in parameter T 0 in the interval of ADP concentration from 0.05 to 0.5 mM are insignificant. The average value of T 0 in this interval of ADP concentrations is equal to 39.1±0.2°C. A decrease in parameter T 0 is observed at the ADP concentrations higher than 0.5 mM. As it follows from Fig. 13C, the increase in ADP concentration is accompanied by the diminishing of parameter K agg. The dependence of K agg on ADP concentration in the interval 0.05–0.5 mM was analyzed using the following equation:
| (10) |
where K agg,0 is the value of K agg at [ADP] = 0, and K diss is the microscopic dissociation constant for the ADP-GDH complex. The K diss value was found to be 0.52±0.07 mM. It is reasonable to suppose that this value of K diss corresponds to the temperature of 39.1°C.
Figure 13. Effect of ADP on GDH aggregation.
(A) The initial parts of the dependences of the light scattering intensity on temperature for aggregation of GDH (0.16 mg/ml) in the presence of the following concentrations of ADP: (1) 0, (2) 0.05, (3) 0.1, (4) 0.5 and (6) 2 mM. (B and C) The dependences of parameters T 0 and K agg calculated from Eq. (4) on the ADP concentration, respectively. The solid curve in panel C was calculated from Eq. (10) at K diss = 0.52 mM.
Conclusion
This article has described a new approach to testing the agents affecting the stability of proteins. The approach is based on the quantitative analysis of the initial increment in the light scattering intensity caused by aggregation of the denatured protein molecules in the experiments where aggregation is studied in the regime of the elevation of temperature with a constant rate. The proposed approach allows estimating the temperature at which the initial increase in the light scattering intensity is registered (T 0) and establishing parameter K agg, which characterizes the rate of aggregation. Temperature T 0 is a moment of the origination of the start aggregates. Such aggregation systems may be used for testing the compounds affecting protein stability as a result of direct binding to the native protein molecule (for example, substrates and modifiers of the enzymes); the compounds stabilizing the protein structure owing to the effects of excluded volume (for example, osmolytes); and the compounds possessing chaperone-like activity (for example, small heat shock proteins). If we want to select agents affecting exclusively the stage of denaturation, we should demonstrate that these agents have no effect on the stage of aggregation. In turn, this would require additional aggregation systems where a preliminary denatured protein undergoes thermal aggregation. A protein denatured by ultraviolet radiation may be used for this purpose [64]. Test-systems based on aggregation of UV-irradiated proteins allow registering the direct action of the agents under study on the stages of aggregation.
When discussing the practical utility of the method of study of protein-ligand interactions proposed in the present work, we should like to note, first of all, that this method may be used for characterization of the proteins themselves. The results of investigation of protein stability in buffer solutions of different composition may be useful for selection of optimal buffer for purification procedure and storage of the proteins under study. The protein mutant forms obtained by genetic engineering may also be characterized by the method under discussion. Furthermore, screening against cofactors, substrate analogs, effectors and metals may be used to establish the conditions that favor protein stability. These stabilizing agents may be used during protein crystallization to improve the crystallization rate. Finally, an important line of application of the screening procedure based on the measurements of the light scattering intensity increase, which accompanies protein aggregation, is the search for compounds of potential medicinal significance.
Acknowledgments
The authors thank Igor Yudin for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the Russian Foundation for Basic Research (grants 11-04-00932-?, 11-04-01271-a and 11-04-01350-?), the Program “Molecular and Cell Biology” of the Presidium of the Russian Academy of Sciences and Dmitry Zimin Dynasty Foundation, the Federal Target Program “Scientific and Scientific-Pedagogical Personnel of Innovative Russia” in 2009–2013 (the state contract No. P1356). The funders had no role in study design,data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Senisterra GA, Markin E, Yamazaki K, Hui R, Vedadi M, et al. Screening for ligands using a generic and high-throughput light-scattering-based assay. J Biomol Screen. 2006;11:940–948. doi: 10.1177/1087057106294699. [DOI] [PubMed] [Google Scholar]
- 2.Senisterra GA, Ghanei H, Khutoreskaya G, Dobrovetsky E, Edwards AM, et al. Assessing the stability of membrane proteins to detect ligand binding using differential static light scattering. J Biomol Screen. 2010;15:314–320. doi: 10.1177/1087057109357117. [DOI] [PubMed] [Google Scholar]
- 3.Kurganov BI. Kinetics of heat aggregation of proteins. Biochemistry (Mosc) 1998;63:364–366. [PubMed] [Google Scholar]
- 4.Eronina TB, Chebotareva NA, Bazhina SG, Makeeva VF, Kleymenov SY, et al. Effect of proline on thermal inactivation, denaturation and aggregation of glycogen phosphorylase b from rabbit skeletal muscle. Biophys Chem. 2009;141:66–74. doi: 10.1016/j.bpc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Eronina TB, Chebotareva NA, Bazhina SG, Kleymenov SY, Naletova IN, et al. Effect of GroEL on thermal aggregation of glycogen phosphorylase b from rabbit skeletal muscle. Macromol Biosci. 2010;10:768–774. doi: 10.1002/mabi.200900396. [DOI] [PubMed] [Google Scholar]
- 6.Maloletkina OI, Markossian KA, Asryants RA, Semenyuk PI, Kurganov BI. Effect of 2-hydroxypropyl-β-cyclodextrin on thermal inactivation, denaturation and aggregation of glyceraldehyde-3-phosphate dehydrogenase from rabbit skeletal muscle. Inter J Biol Macromol. 2010;46:487–492. doi: 10.1016/j.ijbiomac.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Maloletkina OI, Markossian KA, Belousova LV, Kleimenov SY, Orlov VN, et al. Thermal stability and aggregation of creatine kinase from rabbit skeletal muscle. Effect of 2-hydroxypropyl-β-cyclodextrin. Biophys Chem. 2010;148:121–130. doi: 10.1016/j.bpc.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Roberts CJ. Non-native protein aggregation. Pathways, kinetics, and shelf-life prediction. In: Murphy RM, Tsai AM, editors. Misbehaving Proteins: Protein Misfolding, Aggregation, and Stability. New York: Springer; 2006. pp. 17–46. [Google Scholar]
- 9.Weiss WFIV, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98:1246–1277. doi: 10.1002/jps.21521. [DOI] [PubMed] [Google Scholar]
- 10.Kurganov BI, Kornilaev BA, Chebotareva NA, Malikov VP, Orlov VN, et al. Dissociative mechanism of thermal denaturation of rabbit skeletal muscle glycogen phosphorylase b. Biochemistry. 2000;39:13144–13152. doi: 10.1021/bi000975w. [DOI] [PubMed] [Google Scholar]
- 11.Sabbaghian M, Ebrahim-Habibi A, Nemat-Gorgani M. Thermal aggregation of a model allosteric protein in different conformational states. Int J Biol Macromol. 2009;44:156–162. doi: 10.1016/j.ijbiomac.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Khanova HA, Markossian KA, Kurganov BI, Samoilov AM, Kleimenov SYu, et al. Mechanism of chaperone-like activity. Suppression of thermal aggregation of βL-crystallin by alpha-crystallin. Biochemistry. 2005;44:15480–15487. doi: 10.1021/bi051175u. [DOI] [PubMed] [Google Scholar]
- 13.De Young LR, Fink AL, Dill KA. Aggregation of globular proteins. Acc Chem Res. 1993;26:614–620. [Google Scholar]
- 14.Sugunakar YP, Przybycien TM. Simulations of reversible protein aggregate and crystal structure. Biophys J. 1996;70:2888–2902. doi: 10.1016/S0006-3495(96)79859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speed MA, King J, Wang DIC. Polymerization mechanism of polypeptide chain aggregation. Biotechnol Bioeng. 1997;54:333–343. doi: 10.1002/(SICI)1097-0290(19970520)54:4<333::AID-BIT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Ferrone F. Analysis of protein aggregation kinetics. Meth Enzymol. 2003;309:256–274. doi: 10.1016/s0076-6879(99)09019-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Kurganov BI. Kinetics of heat- and acidification-induced aggregation of firefly luciferase. Biophys Chem. 2003;106:97–109. doi: 10.1016/s0301-4622(03)00134-0. [DOI] [PubMed] [Google Scholar]
- 18.Philo JS, Arakawa T. Mechanisms of protein aggregation. Curr Pharm Biotechnol. 2009;10:348–351. doi: 10.2174/138920109788488932. [DOI] [PubMed] [Google Scholar]
- 19.Morris AM, Watzky MA, Finke RG. Protein aggregation kinetics, mechanism, and curve-fitting: a review of the literature. Biochim Biophys Acta. 2009;1794:375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Ferrone FA, Wetzel R. Huntington's disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci U S A. 2002;99:11884–11889. doi: 10.1073/pnas.182276099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignatova Z, Gierasch LM. Aggregation of a slow-folding mutant of a beta-clam protein proceeds through a monomeric nucleus. Biochemistry. 2005;44:7266–74. doi: 10.1021/bi047404e. [DOI] [PubMed] [Google Scholar]
- 22.Cellmer T, Douma R, Huebner A, Prausnitz J, Blanch H. Kinetic studies of protein L aggregation and disaggregation. Biophys Chem. 2007;125:350–359. doi: 10.1016/j.bpc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Golub N, Meremyanin A, Markossian K, Eronina T, Chebotareva N, et al. Evidence for the formation of start aggregates as an initial stage of protein aggregation. FEBS Lett. 2007;581:4223–4227. doi: 10.1016/j.febslet.2007.07.066. [DOI] [PubMed] [Google Scholar]
- 24.Khanova HA, Markossian KA, Kleimenov SYu, Levitsky DI, Chebotareva NA, et al. Effect of α-crystallin on thermal denaturation and aggregation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biophys Chem. 2007;125:521–531. doi: 10.1016/j.bpc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Meremyanin AV, Eronina TB, Chebotareva NA, Kleimenov SY, Yudin IK, et al. Effect of α-crystallin on thermal aggregation of glycogen phosphorylase b from rabbit skeletal muscle. Biochemistry (Mosc) 2007;72:518–528. doi: 10.1134/s0006297907050082. [DOI] [PubMed] [Google Scholar]
- 26.Markossian KA, Golub NV, Khanova HA, Levitsky DI, Poliansky NB, et al. Mechanism of thermal aggregation of yeast alcohol dehydrogenase I. Role of intramolecular chaperone. Biochim Biophys Acta. 2008;1784:1286–1293. doi: 10.1016/j.bbapap.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Noda L, Kuby SA, Lardy H. ATP-creatine transphosphorylase. Meth Enzymol. 1955;2:11505–11610. [Google Scholar]
- 28.Putilina T, Skouri-Panet F, Prat K, Lubsen NH, Tardieu A. Subunit exchange demonstrates a differential chaperone activity of calf α-crystallin towards βLOW- and individual γ-crystallins. J Biol Chem. 2003;278:13747–13756. doi: 10.1074/jbc.M208157200. [DOI] [PubMed] [Google Scholar]
- 29.Meremyanin AV, Eronina TB, Chebotareva NA, Kurganov BI. Kinetics of thermal aggregation of glycogen phosphorylase b from rabbit skeletal muscle: mechanism of protective action of α-crystallin. Biopolymers. 2008;89:124–134. doi: 10.1002/bip.20872. [DOI] [PubMed] [Google Scholar]
- 30.Kastenschmidt LL, Kastenschmidt J, Helmreich E. Subunit interactions and their relationship to the allosteric properties of rabbit muscle phosphorylase b. Biochemistry. 1968;7:3590–3607. doi: 10.1021/bi00850a037. [DOI] [PubMed] [Google Scholar]
- 31.Scopes RK, Stoter A. Purification of all glycolytic enzymes from one muscle extract. Meth Enzymol. 1982;90:479–490. doi: 10.1016/s0076-6879(82)90175-6. [DOI] [PubMed] [Google Scholar]
- 32.Kirschenbaum DM. Molar absorptivity and A 1% 1 cm values for proteins at elected wavelengths of the ultraviolet and visible region. Int J Protein Res. 1972;4:63–73. doi: 10.1111/j.1399-3011.1972.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 33.Privalov PL, Potekhin SA. Scanning microcalorimetry in studying temperature-induced changes in proteins. Meth Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- 34.Scientist for Experimental Data Fitting. Microsoft Windows Version 2.0. Salt Lake City: Micro-Math Inc; 1995. [Google Scholar]
- 35.Markossian KA, Khanova HA, Kleimenov SY, Levitsky DI, Chebotareva NA, et al. Mechanism of thermal aggregation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 2006;45:13375–13384. doi: 10.1021/bi0610707. [DOI] [PubMed] [Google Scholar]
- 36.Markossian KA, Kurganov BI, Levitsky DI, Khanova HA, Chebotareva NA, et al. Mechanisms of chaperone-like activity. In: Obalinsky TR, editor. Protein Folding: New Research. New York: Nova Science Publishers Inc; 2006. pp. 89–171. [Google Scholar]
- 37.Markossian KA, Yudin IK, Kurganov BI. Mechanism of suppression of protein aggregation by alpha-crystallin. Int J Mol Sci. 2009;10:1314–1345. doi: 10.3390/ijms10031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chrunyk BA, Evans J, Lillquist J, Young P, Wetzel R. Inclusion body formation and protein stability in sequence variants of interleukin-1β. J Biol Chem. 1993;268:18053–18061. [PubMed] [Google Scholar]
- 39.Chrunyk BA, Wetzel R. Breakdown in the relationship between thermal and thermodynamic stability in an interleukin-1β point mutant modified in a surface loop. Protein Eng. 1993;6:733–738. doi: 10.1093/protein/6.7.733. [DOI] [PubMed] [Google Scholar]
- 40.Raibekas AA. Estimation of protein aggregation propensity with a melting point apparatus. Anal Biochem. 2008;380:331–332. doi: 10.1016/j.ab.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Duy C, Fitter J. Thermostability of irreversible unfolding alpha-amylases analyzed by unfolding kinetics. J Biol Chem. 2005;280:37360–37365. doi: 10.1074/jbc.M507530200. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Roberts CJ. Lumry-Eyring nucleated-polymerization model of protein aggregation kinetics. 2. Competing growth via condensation and chain polymerization. J Phys Chem B. 2009;113:7020–7032. doi: 10.1021/jp8083088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markossian KA, Golub NV, Chebotareva NA, Asryants RA, Naletova IN, et al. Comparative analysis of the effects of alpha-crystallin and GroEL on the kinetics of thermal aggregation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Protein J. 2010;29:11–25. doi: 10.1007/s10930-009-9217-9. [DOI] [PubMed] [Google Scholar]
- 44.Panyukov Y, Yudin I, Drachev V, Dobrov E, Kurganov B. The study of amorphous aggregation of tobacco mosaic virus coat protein by dynamic light scattering. Biophys Chem. 2007;127:9–18. doi: 10.1016/j.bpc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Eronina TB, Chebotareva NA, Kleymenov SY, Roman SG, Makeeva VF, et al. Effect of 2-hydroxypropyl-β-cyclodextrin on thermal stability and aggregation of glycogen phosphorylase b from rabbit skeletal muscle. Biopolymers. 2010;93:986–993. doi: 10.1002/bip.21508. [DOI] [PubMed] [Google Scholar]
- 46.Golub NV, Markossian KA, Sholukh MV, Muranov KO, Kurganov BI. Study of kinetics of thermal aggregation of mitochondrial aspartate aminotransferase by dynamic light scattering: protective effect of alpha-crystallin. Eur Biophys J. 2009;38:547–556. doi: 10.1007/s00249-009-0403-7. [DOI] [PubMed] [Google Scholar]
- 47.Aymard P, Gimel JC, Nicolai T, Durand D. Experimental evidence for a two-step process in the aggregation of beta-lactoglobulin at pH 7. J Chim Phys. 1996;93:987–997. [Google Scholar]
- 48.Le Bon C, Nicolai T, Durand D. Kinetics of aggregation and gelation of globular proteins after heat-induced denaturation. Macromolecules. 1999;32:6120–6127. [Google Scholar]
- 49.Durand D, Gimel JC, Nicolai T. Aggregation, gelation and phase separation of heat denatured globular proteins. Physica A. 2002;304:253–265. [Google Scholar]
- 50.Baussay K, Bon LC, Nicolai T, Durand D, Busnel JP. Influence of the ionic strength on the heat-induced aggregation of the globular protein beta-lactoglobulin at pH 7. Int J Biol Macromol. 2004;34:21–28. doi: 10.1016/j.ijbiomac.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Pouzot M, Nicolai T, Durand D, Benyahia L. Structure factor and elasticity of a heat-set globular protein gel. Macromolecules. 2004;37:614–620. [Google Scholar]
- 52.Tomita S, Yoshikawa H, Shiraki K. Arginine controls heat-induced cluster-cluster aggregation of lysozyme at around the isoelectric point. Biopolymers. 2011 doi: 10.1002/bip.21637. doi: 10.1002/bip.21637. [DOI] [PubMed] [Google Scholar]
- 53.Bumagina ZM, Gurvits BY, Artemova NV, Muranov KO, Yudin IK, et al. Mechanism of suppression of dithiothreitol-induced aggregation of bovine alpha-lactalbumin by alpha-crystallin. Biophys Chem. 2010;146:108–117. doi: 10.1016/j.bpc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Bumagina Z, Gurvits B, Artemova N, Muranov K, Kurganov B. Paradoxical acceleration of dithiothreitol-induced aggregation of insulin in the presence of a chaperone. Int J Mol Sci. 2010;11:4556–4579. doi: 10.3390/ijms11114556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manno M, Craparo EF, Podestà A, Bulone D, Carrotta R, et al. Kinetics of different processes in human insulin amyloid formation. J Mol Biol. 2007;366:258–274. doi: 10.1016/j.jmb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh P, Kumar A, Datta B, Rangachari V. Dynamics of protofibril elongation and association involved in Aβ42 peptide aggregation in Alzheimer's disease. BMC Bioinformatics. 2010;11(Suppl 6):1–19. doi: 10.1186/1471-2105-11-S6-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kornilaev BA, Kurganov BI, Eronina TB, Livanova NB. Effect of specific ligands on thermal inactivation of muscle glycogen phosphorylase b. Biochemistry (Mosc) 1996;61:628–634. [PubMed] [Google Scholar]
- 58.Kornilaev BA, Kurganov BI, Eronina TB, Livanova NB. Thermal inactivation of skeletal muscle phosphorylase b: protective effect of the specific ligands. Biochem Mol Biol Int. 1996;38:921–927. [PubMed] [Google Scholar]
- 59.Kornilaev BA, Kurganov BI, Eronina TB, Chebotareva NA, Livanova NB, et al. Mechanism of heat denaturation of muscle glycogen phosphorylase b. Mol Biol (Mosc) 1997;31:82–89. [PubMed] [Google Scholar]
- 60.Kornilaev BA, Kurganov BI, Livanova NB, Eronina TB, Orlov VN, et al. Heat resistance of muscle glycogen phosphorylase b in the presence of specific ligands. Doklady Biochemistry. 1997;1352:1–3. [PubMed] [Google Scholar]
- 61.Bolen DW. Protein stabilization by naturally occurring osmolytes. In: Murphy KP, editor. Methods in Molecular Biology, vol. 168: Protein Structure, Stability, and Folding. Totowa, USA: Humana Press; 2001. pp. 17–35. [DOI] [PubMed] [Google Scholar]
- 62.Chebotareva NA, Kurganov BI, Livanova NB. Biochemical effects of molecular crowding. Biochemistry (Mosc) 2004;69:1239–1251. doi: 10.1007/s10541-005-0070-y. [DOI] [PubMed] [Google Scholar]
- 63.Street TO, Bolen DW, Rose GD. A molecular mechanism for osmolyte-induced protein stability. Proc Natl Acad Sci U S A. 2006;103:13997–14002. doi: 10.1073/pnas.0606236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muranov KO, Maloletkina OI, Poliansky NB, Markossian, Kleymenov SY, et al. Mechanism of aggregation of UV-irradiated βL-crystallin. Exp Eye Res. 2011;92:76–86. doi: 10.1016/j.exer.2010.11.005. [DOI] [PubMed] [Google Scholar]