Abstract
Background:
Fine-needle aspiration biopsy (FNA) of the abdominal fat pad is a minimally invasive procedure to demonstrate tissue deposits of amyloid. However, protocols to evaluate amyloid in fat pad aspirates are not standardized, especially for detecting scant amyloid in early disease.
Materials and Methods:
We studied abdominal fat pad aspirates from 33 randomly selected patients in whom subsequent tissue biopsy, autopsy, and/or medical history for confirmation of amyloidosis (AL) were also available. All these cases were suspected to have early AL, but had negative results on abdominal fat pad aspirates evaluated by polarizing microscopy of Congo Red stained sections (CRPM). The results with CRPM between four reviewers were compared in 12 cases for studying inter observer reproducibility. 24 cases were also evaluated by ultrastructural study with electron microscopy (EM).
Results:
Nine of thirty-three (27%) cases reported negative by polarizing microscopy had amyloidosis. Reanalysis of 12 mixed positive-negative cases, showed considerable inter-observer variability with frequent lack of agreement between four observers by CRPM alone (Cohen's Kappa index of 0.1, 95% CI -0.1 to 0.36). EM showed amyloid in the walls of small blood vessels in fibroadipose tissue in four out of nine cases (44%) with amyloidosis.
Conclusion:
In addition to poor inter-observer reproducibility, CRPM alone in cases with scant amyloid led to frequent false negative results (9 out of 9, 100%). For improved detection of AL, routine ultrastructural evaluation with EM of fat pad aspirates by evaluating at least 15 small blood vessels in the aspirated fibroadipose tissue is recommended. Given the high false negative rate for CRPM alone in early disease, routine reflex evaluation with EM is highly recommended to avert the invasive option of biopsying various organs in cases with high clinical suspicion for AL.
Keywords: Amyloidosis, congo red, abdominal fat pad aspiration, fine-needle aspiration biopsy, FNA, electron microscopy, ultrastructural evaluation, polarizing microscopy
INTRODUCTION
Amyloidosis (AL) comprises of a heterogeneous group of disorders that manifest symptoms caused by the extracellular deposition of misfolded insoluble proteins in various tissues and organs.[1,2] Classification of AL is based on precursor plasma proteins as well as the location of the deposits: Systemic versus localized, and inherited versus acquired.[2,3] Light chain AL (also known as primary/immunoglobulin light chain AL) is deposition of monoclonal immunoglobulin light chains and accounts for the majority of patients with AL in the United States. The clinical symptomatology include proteinuria, nephrotic syndrome, restrictive heart failure, anemia, macroglossia, carpal tunnel syndrome, neuropathy, hepatomegaly, and other symptoms depending on the organs involved.[4] Light chain AL can be associated with monoclonal gammopathy of undetermined significance (MGUS), Waldenstrom's macroglobulinemia, and lymphoplasmacytic disorders. It is reported in up to 15% of myeloma cases.[2,5,6]
Up to 27 distinct proteins of variable sizes, amino acid sequences, and structures are associated with the development of AL.[5,6] Although several unrelated proteins are observed, they all produce a common beta- fibrillar configuration into antiparallel beta pleated sheets, which produce a distinct X-ray diffraction pattern with two characteristic signals. These insoluble polymeric fibrils are deposited as extracellular toxic protein aggregates in tissue. They are identifiable as straight, unbranching, randomly criss -crossing, 10-12 nμ diameter fibrils under electron microscopy (EM).[7] They are also identifiable by a characteristic apple-green birefringence under a polarized microscope in Congo red stained sections (CRPM).[6]
The gold standard for tissue diagnosis of AL had been the biopsy of affected organs such as the kidney, liver, or heart. Although these biopsies have good diagnostic value, they are invasive at relatively higher cost with relatively frequent complications than fine needle aspiration biopsy (FNA). In the 1960s, rectal, gingival, and bone marrow biopsies became a common method for diagnosing amyloid as comparatively less invasive alternatives.[8,9] The biopsy of rectal mucosa, which is an easily accessible site of the GI tract, has a sensitivity of 80%.[8,10] Since 1973, FNA of abdominal fat pad has been utilized as a minimally invasive, convenient, safe, economical, and simple procedure for the tissue diagnosis of AL.[11] This procedurally easy method with Congo red staining has a sensitivity of 52-88% and specificity greater than 95%, which, on average, was equal to or better than rectal biopsies.[10,12–15] However, some studies have reported lower sensitivity of fat pad aspirates with Congo red staining.[16] This may be due to multiple variables including type of patient population, severity of disease with scant versus abundant amyloid, experience level of the interpreters, microscope type, polarizer quality, room darkness, and time spent to detect amyloid. An additional limitation is the inter-observer variability in the interpretation of the fat pad aspirate with Congo red staining alone.[9,11,13] The use of immunohistochemistry and Congo red fluorescence has been reported to increase the sensitivity.[17,18] Given this variability, it is important to select appropriate methods of detection and sample processing protocols. This is especially critical for detecting scant amyloid in fat pad aspirates from cases with early disease. In patients with monoclonal gammopathy, the management algorithm has significant clinical and economic implications if association of synchronous amyloidosis is not established accurately.
The objective of this study was to evaluate the utility of ultrastructural studies by EM to complement negative results with CRPM.[19] In addition, inter-observer variability in interpretation of CRPM was also evaluated.
MATERIALS AND METHODS
Abdominal fat pad aspirates from 33 randomly selected patients with availability of subsequent tissue biopsy, autopsy, and/or medical history for confirmation of AL were studied after approval from the institutional review board (IRB). All these cases had negative results on abdominal fat pad aspirates evaluated by CRPM.
The indication for evaluation was clinically suspected early AL in patients with plasma cell monoclonal gammopathy based on serum or urine immunofixation or serum free light chain assay (Freelite, The Binding Site, UK). Further correlation with organ biopsies, bone marrow biopsies, autopsies, biochemical tests, and organ response to plasma cell directed chemotherapy were available to evaluate the true rate of AL in the suspected cohort.
FNAs were performed under local anesthesia with an 18 gauge needle by 7 cytopathologists (with 3-24 years experience) by the protocol reported recently as a video article.[20] In 24 cases, representative fibroadipose tissue was submitted concurrently in 10% formalin for cell block preparation and in glutaraldehyde fixative for EM studies. EM was not performed in 9 cases by some cytopathologists. The cell block preparations were made from specimens received as coagulum of clotted fibroadipose tissue or as microfragments of fibroadipose tissue. The coagulum allows for easy processing for cell block preparation with minimal loss of diagnostic fibro fatty tissue fragments in the aspirated specimen with tendency to float even after centrifugation.[20] In cases with lack of coagulum, the specimens were filtered through filter paper and fibro fatty tissue fragments caught on the filter paper were scraped off for cell block preparation. All 33 cases had adequate aspirates for evaluation with many fibrovascular fragments. Amyloid was evaluated by examining 10 μm thick, Congo red stained sections (not more than 1 month old) of formalin fixed paraffin embedded cell-blocks under polarized microscopy in all 33 cases [Figure 1].
Figure 1.
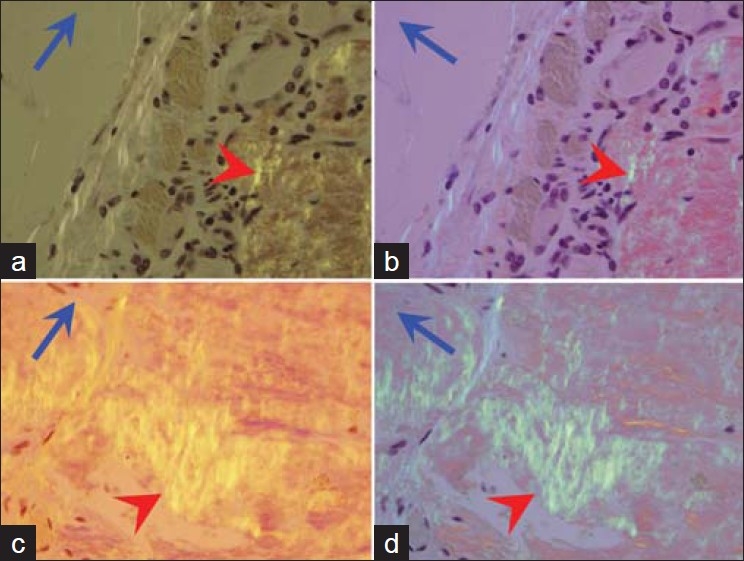
Amyloid (red arrowheads) with orange yellow birefringence under polarized light. The color changes to apple green when the axis of polarizer (blue arrows) is changed by 90 degree (a, b). Compare with the positive control in c and d. [Congo red stained 10 μ sections of formalin fixed paraffin embedded cell block]
Ultra-structural studies were also performed in 24 cases by scrutinizing the walls of at least 15 small blood vessels for the presence of amyloid fibrils with diameter of 8-12 nμ in multiple epoxy embedded ultrathin sections [Figure 2]. Number of blood vessels to be evaluated under EM was arbitrarily chosen as 15 blood vessels as all positive specimens in our study had at least 15 blood vessels altogether in different EM blocks from a particular case. The results with EM and CRPM were compared.
Figure 2.
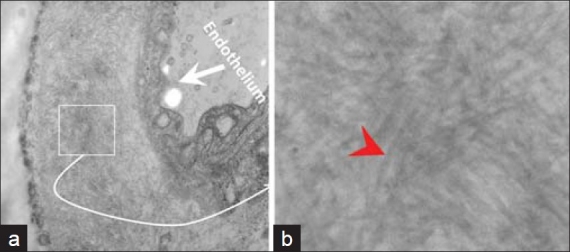
The anterior fat pad aspirate showed amyloid (small box) in the wall of small blood vessels (a). The non-branching random amyloid fi brils with 8.6 nμ diameter were consistent with amyloid (red arrowhead) (b). [Epoxy embedded Glutaraldehyde fi xed section, stained with uranyl acetate and lead citrate. Ultrastructure]
In order to further evaluate inter-observer reproducibility, Congo red stained cell block sections (not more than 1 month old) from 4 positive cases mixed randomly with 8 negative cases. Inter-observer reproducibility and concordance for CRPM among four pathologists (without color blindness issues) were assessed and analyzed by Cohen's kappa statistic. The post-training experience level of all four pathologists (2 cytopathologists and 2 surgical pathologists) ranged from 3 to 24 years. The inter observer reproducibility study was conducted after initial consensus practice session between all four pathologists.
RESULTS
CRPM alone was initially interpreted as negative in all the 33 cases. However, 9 out of these 33 cases (27%) were confirmed as having AL based on subsequent tissue biopsy, autopsy, and/or medical history. In 4 of these 9 confirmed cases (44%), AL could be detected by EM of fat pad aspirates [Figure 3].
Figure 3.
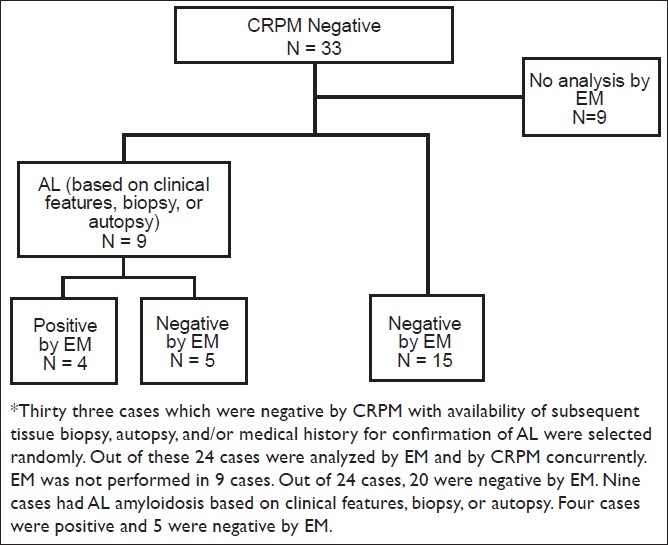
Case enrollment and results for the study to evaluate the role of electron microscopy and congo red stained cell block sections in detection of amyloid in abdominal fat pad aspirates in early amyloidosis*
The results of inter observer comparison among the four pathologists with CRPM showed variable sensitivity (25-75%) and specificity (50-100%). Kappa index of 0.1273 (95% CI -0.1037 to 0.3583) was consistent with poor inter-observer agreement between four observers for evaluation with CRPM alone. One of the cases with autopsy confirmed amyloid cardiomyopathy was positive with EM on anterior fat pad aspirate. This case was interpreted unequivocally as positive with Congo red by 3 of 4 pathologists (1 pathologist was equivocal) during evaluation for inter observer study. Similarly, some of the unequivocally negative cases shuffled with positive cases were interpreted randomly as false positive by four interpreters. The false positive results were most likely due to the superficial resemblance of blue birefringence of collagen fibers with apple green birefringence of amyloid in Congo red stained sections (by 4 pathologists without color blindness), especially when interpreting cases with scant amyloid.
DISCUSSION
AL is a rapidly fatal, progressive, systemic illness with wide spectrum of organ involvement. The median survival of patients with light chain AL is estimated at 12 months and is further decreased in the presence of advanced cardiac involvement.[21] Although treatment for light chain AL is difficult, chemotherapy and/or autologous peripheral blood stem cell transplant (aimed at decreasing or suppressing amyloidogenic monoclonal proteins) are the standard of care and are effective in achieving a clonal response in 50-60% cases.[22]
An accurate tissue diagnosis of light chain AL is essential prior to chemotherapy, since the treatment is inappropriate, ineffective, and detrimental in other types of AL. Diagnosis of light chain AL involves confirmation of a clonal gammopathy and detection of AL in tissue. The diagnostic key step is the detection and confirmation of amyloid protein in tissue. This is usually achieved through biopsies of the abdominal fat pad, bone marrow, rectum, or if necessary other clinically involved organs such as the heart, kidney, or liver.[22] The abdominal fat pad aspirate serves as a simple, minimally invasive test in cases with clinical suspicion and helps avoid riskier and more invasive biopsies of affected organs.
The most frequent indication for the fat pad aspirate in this study was to confirm clinical suspicion of AL in patients with a monoclonal gammopathy established by serum/urine immunofixation electrophoresis (IFE) or by detecting free light chain in serum. Compared to the direct biopsy of the involved organs, abdominal fat pad aspiration is safer, minimally invasive, less expensive, and preferred approach by patients and providers alike.[23] In addition, any other biopsy procedures including gum biopsy, rectal biopsy, bone marrow biopsy, or other organ biopsies may not only incur morbidity but add to the cost with lower sensitivity to detect scant amyloid in cases with early disease. This approach may add the cost and discomfort due to possibility of multiple attempts and repeats. Fat pad aspiration is inexpensive without significant morbidity.
Although CRPM is commonly used for detecting amyloid in the fat pad aspirates, there are several pitfalls with this method as highlighted by the current study. All of our 33 cases, suspected for early disease with relatively scant amyloid, were negative by CRPM alone. In 9 patients with confirmed AL amyloidosis, additional EM with ultra-structural studies on fat pad aspirates detected amyloid in 4 cases (44 %). This suggests that reflex EM should be performed in cases of early disease with scant amyloid. Depending on the approach to evaluate the specimen for amyloid, it may be subjected to cytology smear preparation, cell block preparation, and /or processing for electron microscopy. In a series of 151 patients, reported by Gertz et al., the subcutaneous fat aspirate was falsely negative in 28% of cases.[22] A recent study also reported lower sensitivity of fat pad aspiration for amyloid with Congo red stain.[16] Another study comparing EM, immuno- electron microscopy, and Congo red staining to evaluate abdominal fat pad specimens of suspected cardiac amyloidosis cases, reported detection of amyloid in 100% of cases by EM and in 93% cases by Congo red staining.[24] Our study on cases with early disease with scant amyloid could detect it in 44% (4 out of 9) cases by EM, all of which were negative by CRPM, thus averting subsequent invasive organ biopsy. Many factors including interpreter experience, sample adequacy, and staining techniques can lead to false results with CRPM of fat pad aspirates. Reflex testing for EM is recommended to decrease the chances of false negative results.
Another concern revealed by our study was the inter observer variability in evaluation by CRPM of fat pad aspirates from cases with scant amyloid. The sensitivity (25-75%) and specificity (50-100%) of this technique varied widely between four observers. With these inconsistencies associated with application of CRPM alone, EM of abdominal fat pad aspirates for detection of scant amyloid in early disease is recommended. This may not be critical in cases with late stage disease with abundant amyloid deposits. Remarkably, 44% (4 out of 9) cases of AL could be diagnosed accurately with EM as compared to 100% false negative results (9 out of 9) with CRPM alone. In addition, the false positivity due to superficial resemblance of focal blue birefringence associated with collagen tissue with apple green birefringence of amyloid may be confirmed further by EM to overcome the disparity related to the inter-observer variation with CRPM for detection of scant amyloid. Inconsistency related to the focal blue birefringence of collagen fibers in Congo red stained sections may be more challenging under routine surgical pathology-cytopathology setting with additional unanticipated factors such as color blindness and limited understanding of uncommonly used polarizing microscopy.
Acquisition of adequate fat pad material with enough blood vessels for detection of amyloid is critical. Depending on the gauge, needles are categorized into fine (21-25G), intermediate (18-20G), and large (e.g. - 14G).[25] The needles utilized for performing anterior fat pad aspiration in the reported literature are of variable gauges ranging from intermediate (18 to 20G) to fine (21 to 22G).[16] It is not uncommon to use wider gauge needles, such as 18G, to sample additional material for preparation of cell blocks during FNAB of mass lesions after retrieving optimum material for cytopathologic evaluation with fine gauge needles, such as 25G. A critical component of an adequate fat pad aspirate is the retrieval of enough material for cell block preparation and electron microscopy. Since cohesive fibroadipose tissue fragments do not aspirate well with fine needles during fat aspiration procedures, wider gauge needles (such as 18G) should be preferred to yield adequate material.[20] Presence of just fat droplets is not adequate. As observed in this study, aspirate should have fibrovascular fragments with at least 15 blood vessels available for evaluation under EM. Performance of anterior fat pad FNA for AL with 18G needle along with triaging and processing of specimen including submission of the specimen for cell block preparation as coagulum with fibroadipose tissue was recently reported as an open access video article.[20]
In summary, during evaluation of fat pad aspirates, early AL is likely to be missed more frequently by CRPM alone than by a combination of CRPM and EM. The inter observer reproducibility for CRPM was poor with frequent false positive and false negative interpretations, especially in cohort of patients expected to be in early stages with scant amyloid. To achieve higher sensitivity and specificity in cases suspected for early AL, a routine EM with evaluation of at least 15 small blood vessel walls in the aspirated fibroadipose tissue retrieved by FNA biopsy with 18 G needles is recommended.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board.
EDITORIAL / PEER-REVIEW STATEMENT
CytoJournal editorial team thanks the academic editor. Dina R. Mody, M.D., Professor & Director of Cytopathology, Department of Pathology, The Methodist Hospital, Houston, Texas, USA, for organizing and completing the double-blind peer-review process for this manuscript as per Cytojournal's peer-review policy (http://www.cytojournal.com/prp.asp).
Acknowledgments
This study was presented in part at the 96th Annual Meeting of United States and Canadian Academy of Pathology, March 24-30, 2007, San Diego, CA.
Footnotes
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/11/82278
Contributor Information
Sumana Devata, Email: sdevata@mcw.edu.
Parameswaran Hari, Email: phari@mcw.edu.
Natalia Markelova, Email: navmar@hotmail.com.
Rongshan Li, Email: rli@plusdx.com.
Richard Komorowski, Email: rkomorow@mcw.edu.
Vinod B. Shidham, Email: vshidham@med.wayne.edu.
REFERENCES
- 1.Gillmore JD, Hawkins PN, Pepys MB. Amyloidosis: A review of recent diagnostic and therapeutic developments. Br J Haematol. 1997;99:245–56. doi: 10.1046/j.1365-2141.1997.303194.x. [DOI] [PubMed] [Google Scholar]
- 2.Seldin DC, Skinner M. Amyloidosis. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 17th ed. New York: McGraw-Hill Companies; 2008. [Last cited on 2010,Aug 28]. Available from: http://www.accessmedicine.com . [Google Scholar]
- 3.Falk RH, Comenzo RL, Skinner M. The Systemic Amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 4.Scully RE, Mark EJ, McNelly WF, Ebeling SH, Ellender SM. Case records of the Massachusetts General Hospital Weekly clinicopathological exercises Case 3-2000 A 66-year-old woman with diabetes, coronary disease, orthostatic hypotension and the nephrotic syndrome. N Engl J Med. 2000;342:264–73. doi: 10.1056/NEJM200001273420408. [DOI] [PubMed] [Google Scholar]
- 5.Pettersson T, Konttinen YT. Amyloidosis – recent developments. Semin in Arthritis Rheum. 2010;39:356–68. doi: 10.1016/j.semarthrit.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 7.Makin SO, Lerpell LC. Structures for amyloid fibrils. FEBS J. 2005;272:5950–61. doi: 10.1111/j.1742-4658.2005.05025.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Gameren II, Hazenberg C, Van Rijswijk MH. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006;54:2015–21. doi: 10.1002/art.21902. [DOI] [PubMed] [Google Scholar]
- 9.Guy CD, Jones CK. Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: Specificity, positive predictive value, and diagnostic pitfalls. Diagn Cytopathol. 2001;24:181–5. doi: 10.1002/1097-0339(200103)24:3<181::aid-dc1037>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Libbey CA, Skinner M, Cohen AS. Use of Abdominal Fat Tissue Aspirate in the Diagnosis of Systemic Amyloidosis. Arch Intern Med. 1983;14:1549–52. [PubMed] [Google Scholar]
- 11.Westermark P, Stenkvist B. A new method for the diagnosis of systemic amyloidosis. Arch Intern Med. 1973;132:522–3. [PubMed] [Google Scholar]
- 12.Gertz MA, Li C, Shirahama T, Kyle RA. Utility of subcutaneous fat aspiration for the diagnosis of systemic amyloidosis (Immunoglobulin Light Chain) Arch Intern Med. 1988;148:929–33. [PubMed] [Google Scholar]
- 13.Westermark P. Diagnosing Amyloidosis. Scand J Rheumatol. 1995;24:327–9. doi: 10.3109/03009749509095175. [DOI] [PubMed] [Google Scholar]
- 14.Duston MA, Skinner M, Meenan RF, Cohen AS. Sensitivity, specificity, and predictive value of abdominal fat aspiration for the diagnosis of amyloidosis. Arthritis Rheum. 1989;32:82–5. doi: 10.1002/anr.1780320114. [DOI] [PubMed] [Google Scholar]
- 15.Blum A, Sohar E. The diagnosis of amyloidosis: Ancillary procedures. Lancet. 1962;279:721–3. doi: 10.1016/s0140-6736(62)91658-6. [DOI] [PubMed] [Google Scholar]
- 16.Halloush R, Lavrovskaya E, Mody D, Lager D, Truong LD. Diagnosis and typing of systemic amyloidosis: The role of abdominal fat pad fine needle aspiration. [Last cited on 2010, Aug 28];CytoJournal. 2009 6:24. doi: 10.4103/1742-6413.58950. Available from: http://www.cytojournal.com/text.asp?2009/6/1/24/58950 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linke RP. Highly sensitive diagnosis of amyloid and various amyloid syndromes using Congo red fluorescence. Virchow Arch. 2000;436:439–48. doi: 10.1007/s004280050471. [DOI] [PubMed] [Google Scholar]
- 18.Giorgadze TA, Shiina N, Baloch ZW, Tomaszewski JE, Gupta PK. Improved detection of amyloid in fat pad aspiration: An evaluation of Congo red stain by fluorescent microscopy. Diagn Cytopathol. 2004;31:300–6. doi: 10.1002/dc.20131. [DOI] [PubMed] [Google Scholar]
- 19.Shidham VB, Kumar N, Cihlar K, Varsegi G, Markelova N, Li R, et al. Fine needle aspiration of abdominal fat pad for diagnosis of early amyloidosis: How can the clinical role of the test be improved? [Last cited on 2010, Aug 28];Modern Pathology. 2007 20(Supplement 2):1A–380A. Available from: http://www.nature.com/modpathol/journal/v20/n2s/pdf/3800802a.pdf. Abstract no. 362 . [Google Scholar]
- 20.Shidham VB, Hunt B, Jaradeh SS, Barboi A, Devata S, Hari P. Performing and Processing FNA of Anterior Fat Pad for Amyloid. [Last cited on 2010, Aug 28];J Vis Exp. 2010 44 doi: 10.3791/1747. Available from: http://www.jove.com/details.stp? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and Laboratory Features in 229 Cases. Mayo Clin Proc. 1983;58:665–83. [PubMed] [Google Scholar]
- 22.Gertz MA, Merlini G, Treon SP. Amyloidosis and Waldenstrom's Macroglobulinemia. Hematology Am Soc Hematol Educ Program. 2004;1:257–82. doi: 10.1182/asheducation-2004.1.257. [DOI] [PubMed] [Google Scholar]
- 23.Duston MA, Sinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration: Analysis of Four Years′ experience. Am J Med. 1987;82:412–4. doi: 10.1016/0002-9343(87)90439-6. [DOI] [PubMed] [Google Scholar]
- 24.Arbustini E, Vrga L, Concardi M, Pallandini G, Obici L, Merlini G. Electron and Immuno-electron Microscopy of Abdominal Fat Identifies and Characterizes Amyloid Fibrils in Suspected Cardiac Amyloidosis. Amyloid. 2002;9:108–14. [PubMed] [Google Scholar]
- 25.DeMay RM. American Society of Clinical Pathology. Chicago: ASCP Press; 1996. Fine Needle Aspiration Biopsy, The Art and Science of Cytopathology; p. 465. [Google Scholar]


