A 15-year-old male, diagnosed with aplastic anemia three years earlier, presented with epigastric pain of three days duration. There was history of repeated blood transfusions in the past. Clinical examination was unremarkable. His full blood counts showed normocytic hypochromic anemia. His renal and liver function tests and sonography of abdomen were normal. Magnetic resonance imaging (MRI) of the upper abdomen showed diffuse hypointense signal of cortices of both kidneys on T1-weighted and T2-weighted images [Figures 1 and 2]. The liver also showed hypointense signal on both sequences. Normal signal intensity of kidney and liver are shown in Figure 3. The pancreas and spleen showed normal signal intensity. Based on the imaging characteristics, a diagnosis of renal and hepatic iron deposition was made.
Figure 1.
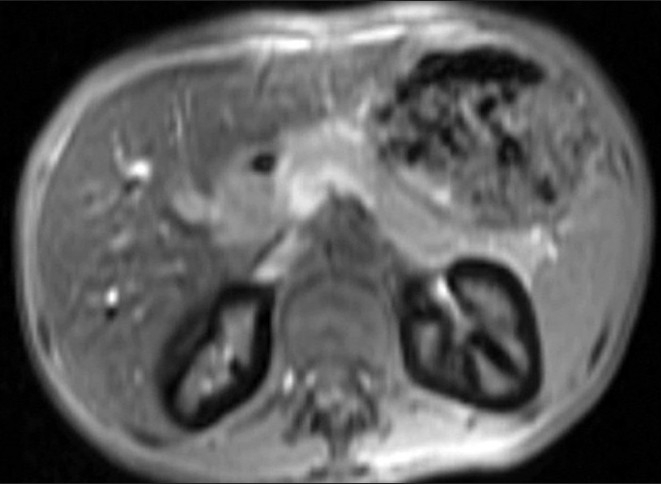
T1-weighted fast spoiled gradient echo (FSPGR) axial image shows diffuse hypointense signal of bilateral renal cortices. The liver is also hypointense. The spleen and pancreas show normal signal intensity
Figure 2.
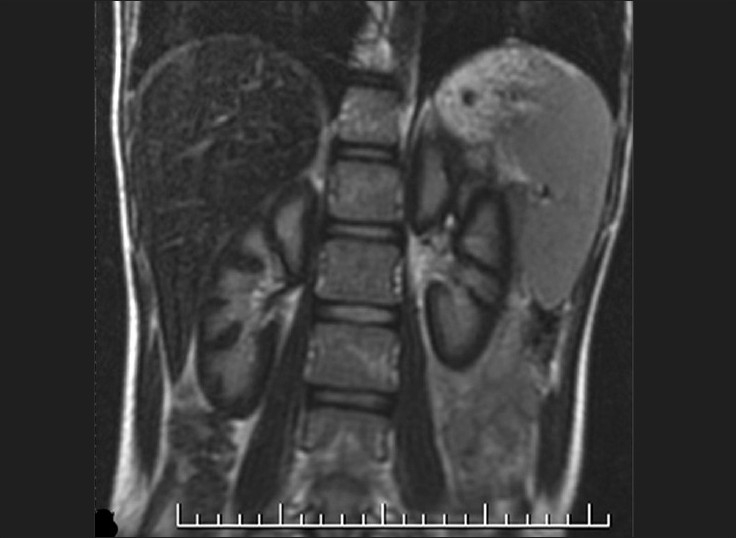
Fast spin echo (FSE) T2-weighted coronal image shows hypointense signal of bilateral renal cortices. The renal medulla is normal
Figure 3.
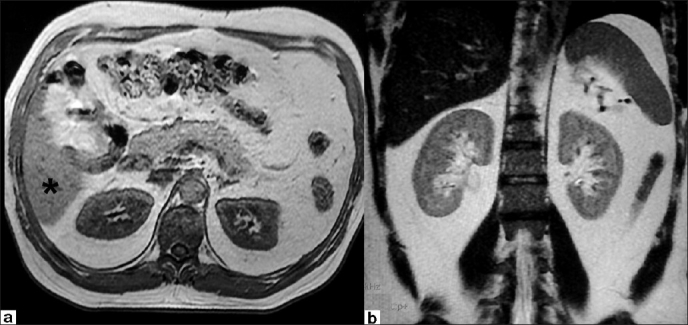
T1-weighted FSPGR axial (a) and FSE T2-weighted coronal (b) images showing normal isointense signal of renal cortices. The normal isointense signal of the liver is seen on T1-weighted images (asterix)
The common sites of iron deposition in hemolytic anemia are liver and heart where it occurs due to iron overload as a result of repeated blood transfusions.[1] Kidneys are less common sites, seen often in patients with sickle cell anemia and paroxysmal nocturnal hemoglobinuria.[1,2] Deposition of iron (in the form of hemosiderin) in kidneys is considered to be specific for intravascular hemolysis. This presumably could have occurred in our patient as a result of antibodies developing due to repeated transfusions. MRI is very sensitive in the detection of renal iron as hemosiderin is paramagnetic and shortens the T2 relaxation times.[1,2] Diffuse hypointense signal of the renal cortex on T1-weighted and T2-weighted images is characteristic. Serum ferritin is often used to assess iron overload in transfusion-dependent patients. However, it is less useful in the evaluation of chronic hemolysis and iron deposition. Detection of renal and hepatic iron deposition by MRI is thus a useful indicator of ongoing systemic iron overload. This finding may sometimes be an early indicator of renal dysfunction in patients receiving regular blood transfusions.[3]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Schein A, Enruquez C, Coates TD, Wood JC. Magnetic resonance detection of kidney iron deposition in sickle cell disease: A marker of chronic hemolysis. J Magn Reson Imaging. 2008;28:698–704. doi: 10.1002/jmri.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzukawa K, Ninomiya H, Mitsuhashi S, Anno I, Nagasawa T, Abe T. Demonstration of the deposition of hemosiderin in the kidneys of patients with paroxysmal nocturnal hemoglobinuria by magnetic resonance imaging. Int Med. 1993;32:686–90. doi: 10.2169/internalmedicine.32.686. [DOI] [PubMed] [Google Scholar]
- 3.Koliakos G, Papachristou F, Koussi A, Perifanis V, Tsatra I, Souliou E, et al. Urine biochemical markers of early renal dysfunction are associated with iron overload in beta-thalassemia. Clin Lab Haematol. 2003;25:105–9. doi: 10.1046/j.1365-2257.2003.00507.x. [DOI] [PubMed] [Google Scholar]


