Abstract
Aim:
To determine factors associated with poor outcome in children suffering traumatic head injury (HI).
Materials and Methods:
A retrospective study over an 8-year period including 454 children with traumatic HI admitted in the Intensive Care Unit of a university hospital (Sfax-Tunisia). Basic demographic, clinical, biological and radiological data were recorded on admission and during the ICU stay. Prognosis was defined according Glasgow outcome scale (GOS) performed after hospital discharge by ICU and pediatric physicians.
Results:
There were 313 male (68.9%) and 141 female patients. Mean age (±SD) was 7.2±3.8 years, the main cause of trauma was traffic accidents (69.4%). Mean Glasgow coma scale (GCS) score was 8±3, mean injury severity score (ISS) was 26.4±8.6, mean pediatric trauma score (PTS) was 4±2 and mean pediatric risk of mortality (PRISM) was 11.1±8. The GOS performed within a mean delay of 7 months after hospital discharge was as follow: 82 deaths (18.3%), 5 vegetative states (1.1%), 15 severe disabilities (3.3%), 71 moderate disabilities (15.6%) and 281 good recoveries (61.9%). Multivariate analysis showed that factors associated with poor outcome (death, vegetative state or severe disability) were: PRISM ≥24 (P=0.03; OR: 5.75); GCS ≤8 (P=0.04; OR:2.42); Cerebral edema (P=0.03; OR:2.23); lesion type VI according to Traumatic Coma Data Bank Classification (P=0.002; OR:55.95); Hypoxemia (P=0.02; OR:2.97) and sodium level >145 mmol/l (P=0.04; OR: 4.41).
Conclusions:
A significant proportion of children admitted with HI were found to have moderate disability at follow-up. We think that improving prehospital care, establishing trauma centers and making efforts to prevent motor vehicle crashes should improve the prognosis of HI in children.
Keywords: Acute head injury, children, Glasgow coma scale score, intensive care unit, multivariate analysis, prognosis, trauma
INTRODUCTION
Traumatic brain injury (TBI) is an important cause of morbidityand mortality in children and adolescents. It results in considerablehealth care cost and, for many survivors, permanent disability.[1] The underlying pathophysiology highlights the importance not only of the primary injury, but alsoof the secondary processes occurring after injury, that may leadto cerebral hypoxia and ischemia.[2] Secondary brain injury is the leading cause of in-hospital death after TBI.[2,3] Moreover, the childhood outcome varies from center to center depending on the availability of modern neurosurgical and neuroradiological facilities and qualified expertise.[4] For children, a head injury (HI) can lead to persistent cognitive and neurobehavioral deficits, intellectual, academic and personality adjustment problems and family stress.[5] Neuropsychiatric sequelae outstrip the neurophysical(such as ataxia or incontinence) as the major cause of disability.Problems with memory, attention, executive function, behavioralcontrol and regulation of mood, associated with frontal and temporal lobes injury, are particularly troublesome.[5]
The outcome measurement is fundamental to the effective evaluation of clinical management of any illness. In a disease process such as HI, which has an infinite variation in severity and which is influenced by countless variables, objective measures of outcome are critical in the assessment of treatment regimens. The Glasgow outcome scale (GOS) is the most widely used method for assessing outcome after HI. It is also used to assess the outcome of many other neurosurgical disorders.[6]
In Tunisia, each year, nearly 13,000 victims of motor-vehicle crashes are recorded, and about 1500 patients die according to the National Guard statistical data.[7] Pediatric morbidity and mortality due to head trauma is increasing because of high rate of road traffic accidents. Survivors are susceptible to irreversible neurological damage that represents an important socioeconomic problem.[8,9] The outcome of childhood HI in children was not previously studied.
The aim of the present study was to evaluate outcome of traumatic head-injured children referred to our medico-surgical Intensive Care Unit (ICU), and to define a predictive factors which can be used in routine practice in general ICUs as an indicator of poor prognosis according GOS score.[6]
MATERIALS AND METHODS
This study was approved by an Internal Review Boards
Patients
In this retrospective study, we included all consecutive patients with TBI aged less than 15 years and admitted to ICU of university hospital, during an 8-year period.The data were recorded from the patient clinical notes with multiple contributors. Our department is a 22-bed medical surgical ICU in a teaching hospital of 510 beds that serves as first line medical center for an urban population of one million inhabitants and as a referral center for a larger population coming from south Tunisia. The total number of admissions in our unit is about 1200 per year. In our department, three beds are reserved to pediatrics intensive care.
Patients were admitted directly from the scene of the accident within 6 hours of injury. They were all examined and scored according to Glasgow coma scale(GCS) score on arrival and underwent computed cerebral tomography (CT) scan as soon as feasible.
Methods
The patients’ medical files were retrospectively reviewed, and the following data were collected: age, gender, vital signs (heart rate, respiratory rate, systolic and diastolic blood pressure), body temperature in °C, GCS score,[10] injury severity score (ISS),[11] pediatric trauma score (PTS),[12] pediatric risk of mortality (PRISM) score,[13] cause of injury, pupil response, motor deficit, convulsion, use of mechanical ventilation, presence of shock or arterial hypotension,[14] cardiac arrest, fluid intake volume, brain CT-scan result and use of catecholamine.
Biochemical parameters measured on admission and during the ICU stay were arterial blood gases and acid-base state, hemoglobin concentration, platelets count, serum glucose and sodium levels, blood urea and urine specific gravity.
Plain radiographic studies of the neck were done in all patients. Cranial CT-Scan was done in all but 8 patients due to unavailability of CT-scan, for these patients a brain magnetic resonance imaging (MRI) was performed on admission. Cranial CT-Scan results were stratified by a university radiologist according to the “Traumatic Coma Data Bank Computed Tomography Classification” for Severe Head Injury”[15] as following:
Grade 1: No visible intracranial pathology.
Grade 2: Cisterns present with midline shift 0-5mm; no high or mixed density lesion >25ml.
Grade 3: Cisterns compressed or absent with midline shift 0-5mm.
Grade 4: Midline shift >5 mm or surgically evacuated lesion and nonevacuated high or mixed density lesion >25ml
Neurological state was assessed using the GCS score at the site of accident and again on hospital arrival before the use of sedative but after resuscitation : the preintubation GCS (used in our analysis). All patients were intubated, ventilated and received sedation with thiopental sodium 50 mg /kg/day or by the association Fentanyl-Midazolam as necessary. Patients with diabetes mellitus and/or the use of glucose containing fluid given intravenously were registered. Corticosteroids were not used for treatment of cerebral edema. In our ICU, the bed head was kept elevated in all patients. Mannitol was used when Cerebral CT-Scan showed cerebral edema and/or herniation; however, hypertonic saline is not used in our practice. Mild hyperventilation (PaCO2 = 30-35 mmHg) is applied in all patients with severe traumatic HI and requiring mechanical ventilation. Before January 2000, barbiturate therapy (thiopental sodium 50 mg/kg/day) was used in all patients admitted for severe traumatic HI requiring mechanical ventilation. Nevertheless this therapy was abandoned since this date. In our practice anticonvulsants were used only if the patient developed seizure. Hypothermia therapy was not used in our study. All patients with suspected intra cranial hypertension or with herniation syndrome received mannitol. In our ICU, therapies were directed by repeated CT-Scan.
When extracranial pathology was suspected, appropriate investigations were performed.
All clinical, biological and radiological parameters and relevant therapeutic measures were registered on admission and during the ICU stay.
For each patient, we daily recorded mean of Na, Kandblood sugar levels(BSL)in addition tothe peak/trough results. Moreover, we have recorded the development of secondary systemic insults (SSI) on admission and during ICU stay. SSI were divided into subgroups of respiratory (hypoxemia, hypercapnia, hypocapnia),[16,17] circulatory (hypotension or arterial hypertension),[18,19] metabolic/ electrolytic SSI (anemia, hyper or hypoglycemia, hyponatremia, diabetes insipidus)[16,20,21] and hyperthermia. During the ICU stay all complications were recorded: nosocomial infections,[22] pneumonia,[23] tract urinary infection[23] meningitis,[22] and septicemia.[24]
GOS[6] was performed after hospital discharge by an ICU and pediatric physicians. This score was previously used to assess prognosis in children suffering head trauma.[25] In all cases, data were recorded via children's medical record (Neurosurgery and ICU medical record) after hospital discharge. When some data are missed, childhood families were contacted by phone. Missing data were treated by, by parents questioning and/or by patient's re-evaluation.
According to GOS score, patients can be divided into five groups with increasing severity ranging from good recovery to death. Our patients were divided into two groups:
GOS (I): Patients with favorable outcomes involving patients having a good recovery and/or moderate disability.
GOS (II): Patients with unfavorable outcome involving patients having severe disability or persistent vegetative state or deaths
Statistical analysis
Categorical data were expressed in proportion and subgroups (GOS (I) and GOS (II) were analyzed by the χ2 test.
Continuous variables were expressed as means (±SD) and subgroups evaluated by Student's t-test. Risk factors were evaluated in univariate analysis and by multivariate analysis by a multiple logistic stepwise regression procedure. Odds ratios (ORs) were estimated from the b coefficients obtained, with respective 95% confidence intervals (CI 95%).
PRISM, PTS, ISS and GCS Score were used to predict bad outcome and were analyzed using Receiver Operating Characteristic (ROC) curves. The area under the ROC curve which was estimated by the method of Hanley and McNeill[26] provides a measure of overall mortality of the test. For comparable data a p value less than 0.05 was considered as statistically significant.
RESULTS
During the study period, 454 children were admitted in our ICU for traumatic HI. They were all included in this study.
This represented 33% of all pediatric ICU admissions and 84% of pediatric post-traumatic cases admitted in our ICU. The transport and the stabilization of vital functions were performed by a prehospital system and/or fire fighters in 37% of cases. However, in 63% of cases, the transport was performed by patient's family.
Of all patients coming, 32.3% were from Sfax city or its delegations. However, a significant number of children (67.7%) were referred to us from south Tunisia cities. There were 313 male (68.9%) and 141 female patients with a mean age (±SD) of 7.2±3.8 years
The demographic and clinical parameters on admission are shown in Table 1.
Table 1.
Demographic and clinical parameters on admission of all study population
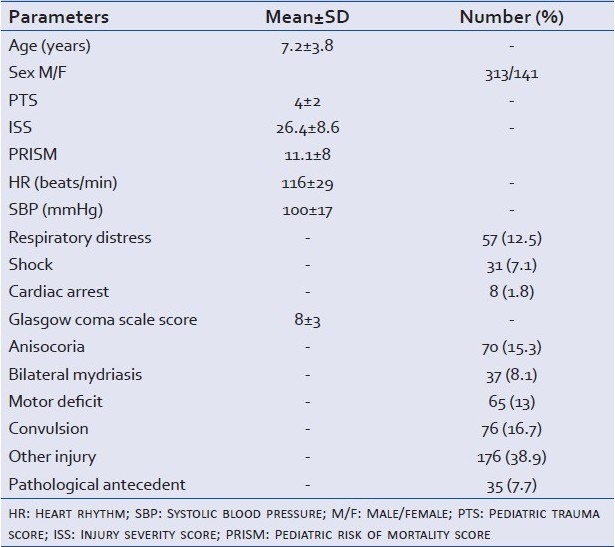
Trauma was caused by traffic accident (69.4%), domestic accidents (29.7%), or was due to assault (0.2%).
Extracranial pathology was present in 38.7% of patients including ribs or long bones fracture (25.1%), chest trauma (9.5%), abdominal trauma (14.3%), pelvic trauma (4.4%) and spinal trauma (1.3%).
In this study, brain CT-Scan was performed on admission in 446 patients; however, for eight patients the brain was explored on admission by MRI.
Four hundred and twenty-four patients (93.4%) needed intubation on the scene of the accident and the remaining patients were intubated at their admission, mechanical ventilation (mean duration 4.3±5.6 days) and sedation on admission according to the protocols detailed above.
On admission, 91(20%) patients needed craniotomy, the most observed surgical interventions were: evacuation of a subdural hematoma (N=8), evacuation of an extradural hematoma (N=30), lobectomy (N=3), cerebrospinal fluid drainage (N=3), elevation of depressed skull fracture (N=36) and decompressive craniectomy (N=1). Moreover, 40 needed immediately extracranial surgery.
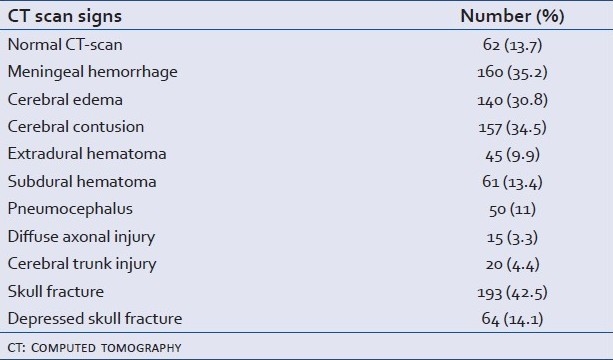
The results of brain CT-scan are presented in Table 2. According to Marshall tomographic grading “Traumatic Coma Data Bank classification” we had 31.9% type I, 41% type II, 9.9% type III, 1.3% type IV, 12.1% type V and 3.7% type VI.
Table 2.
Cerebral CT scan findings in our series
During the ICU stay, 314 patients (69.2%) had complications: 83 patients (18.3%) developed nosocomial infections in 108 episode: pneumonia 58(53.7%), tract urinary infection 16(14.8%), meningitis 9(8.3%), septicemia 8(7.4%) and inner ear infection or sinusitis 9(8.4%).
Moreover, 172 patients (37.8%) developed arterial hypotension requiring fluid resuscitation, catecholamine were used in 28 patients (6.1%).
A total of 219 patients (48.2%) had rhabdomyolysis (CPK >500 IU/l).[27] Hyponatremia (<130 mmol/l) was present in 77(17%), hypernatremia (>145 mmol/l) in 54(11.9%), diabetes insipidus in 5(1.1%), fat embolism in 2(0.4%), stage III or IV ulcer[28] in 15(3.3%), neurogenic pulmonary edema in 8(1.8%) and gastrointestinal hemorrhage in 6(1.3%).
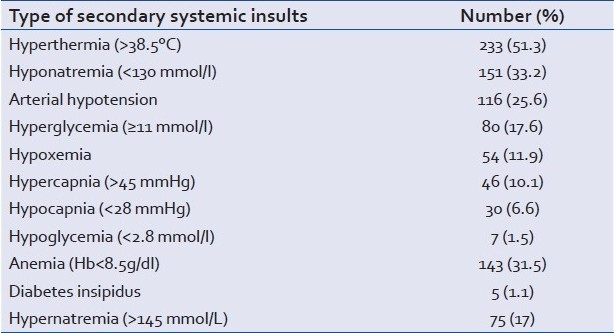
During the ICU stay 377 patients (83%) developed SSIs. Table 3 shows the frequency of each SSI.
Table 3.
Frequency of each secondary systemic insult
Mean ICU stay was 6.8±11.8 days and mean hospital stay was 11.9±15.8 days.
A total of 82 patients (18.1%) died. Regarding the time of death, the mortality percentage was 45.1% in the first 24-48 hours, 35.3% between 3 and 7 days and only 19.6% thereafter . Brain herniation (diagnosed clinically) was the main cause of mortality (80.4%). The GOS performed within a mean delay at 7±16 months after hospital discharge (range 0.5 and 96 months) were as follow: 82 deaths (18.1%), five vegetative state (1.1%) and 281 good recovery (61.9%) [Table 4]. Moreover, neuropsychiatric sequelae were observed in 143 patients (31.4%). The most observed sequel were motor deficit in 69 patients (15%), post-concussion syndrome including headaches, personality changes (anxiety, irritability), dizziness or impaired memory and concentration was observed in 36 patients (9.7%), dysarthria in 29(7.8%), lack of motor coordination and/or difficulty balancing in 23(6.2%), blurred vision or tired eyes in 21(5.7%), sensorial deficit in 13(3.5%), post-traumatic epilepsy in 11(3%), scholarly difficulties in 10(2.2%) and incontinence and/or bowel disorders in 7(2%).
Table 4.
Outcome in patient with severe head injury according to the Glasgow outcome scale

Univariate analysis showed that factors associated with a bad outcome according the GOS 2 were: low PTS on admission, high ISS, high PRISM, presence of shock, menigeal hemorrhage, cerebral edema, high blood sugar level on admission and bilateral mydriasis [Table 5]. Moreover, the majority SSIswere associated with a poor outcome [Table 6].
Table 5.
Factors associated with bad outcome in univariate analysis
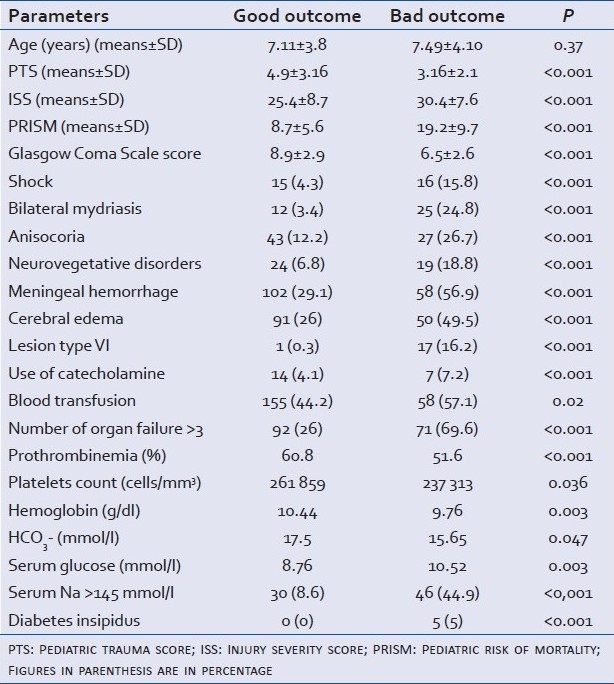
Table 6.
Association between each secondary systemic insult and outcome
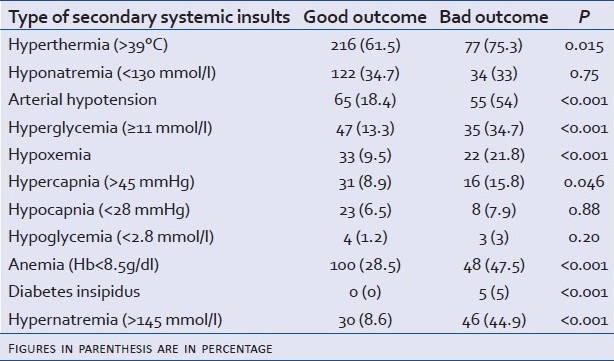
Finally in our study, sodium level >145 mmol/l on ICU admission and during ICU stay was associated with poor outcome (P<0.0001). Moreover a severe hypornatremia <125 mmol/l was also associated with poor outcome (P<0.01) [Figure 1].
Figure 1.
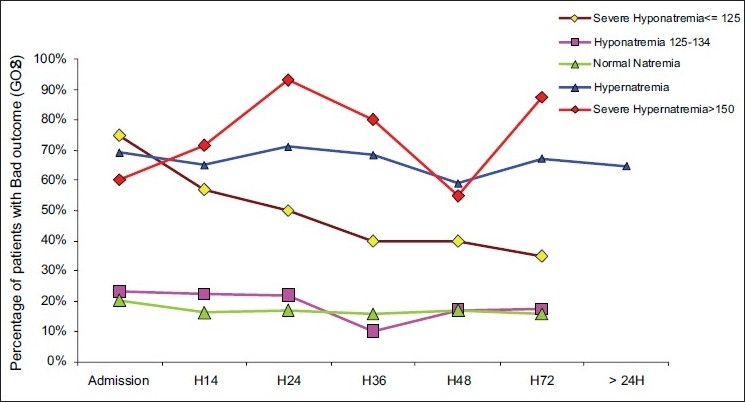
Association between dysnatremia [on admission; 12 hours after ICU admission (H12); ….] and bad outcome
In addition a blood sodium level >145 mmol/l was significantly associated with hyperglycemia (P=0.04), high value of PRISM (21.5±11.7 vs. 10.2±7; P<0.001); high value of ISS (31.5±9.3 vs 26.1±8.5; P<0.001) and a low value of GCS on admission (6.9±3.2 vs. 8.5±2.9; P=0.002).
The multivariate analysis showed that factors associated with a poor prognosis (GOS 2) were PRISM ≥24 (P=0.03; OR:5.75); GCS score ≤8 (P=0.04; OR:2.42); Cerebral edema (P=0.03; OR: 2.23); lesion type VI according Traumatic Coma Data Bank Classification (P=0.002; OR: 55.95); Hypoxemia (P=0.02; OR:2.97) and sodium level >145 mmol/l (P=0.04; OR:4.41).
A significant association was found between PRISM score and GOS(II), this model had a high discriminative power . In fact, PRISM score >24 was associated with poor outcomewith a sensitivity of 50%, a specificity of 81% and an area under the ROC curve at 0.83 [Figure 2]. Moreover, PRISM score >24 was associated with a positive predictive value of GOS (II) of 96.5%and a negative predictive value of 82.9%.
Figure 2.
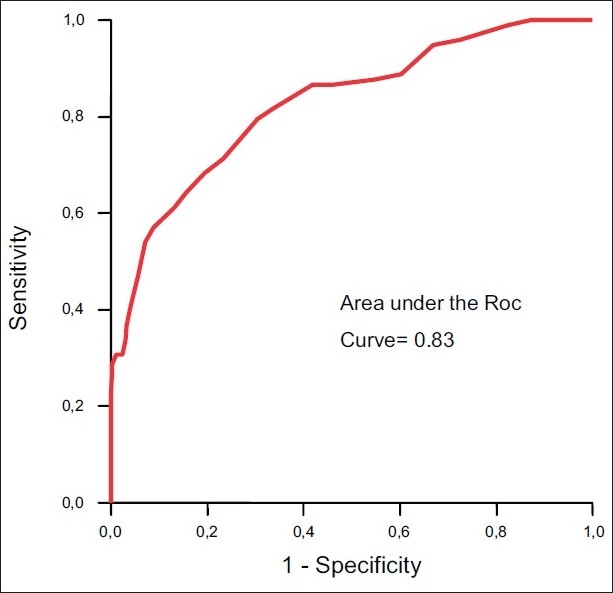
Receiver operating characteristic curve (ROC curve) for ability of PRISM to predict the outcome. The area under the curve was 0.83 indicating a good capability of the model to discriminate between good and poor outcome
In addition, a low of GCS score on admission was associated with a poor outcome. In fact GCS score ≤8 was associated with bad outcome with a sensitivity of 50%, a specificity of 81% and an area under the ROC curve at 0.73. PTS was also discriminating with areas below the ROC curve at 0.73. However, ISS was not enough discriminating with areas below the ROC curve at 0.65.
Moreover blood glucose level on admission was significantly higher in patients with bad outcome (8.76±4.3 vs. 10.52±6.9 mmol/l; P=0.003) when compared with patients with a good outcome (GOS(I)).
As shown in Figure 3, the outcome was significantly correlated with the number of organ failure (P<0.001). In fact, the bad rate was increased from 30.4% in patients with a number of organ failure less than 3-69.6% in those having more than three organ failure [Figure 3].
Figure 3.
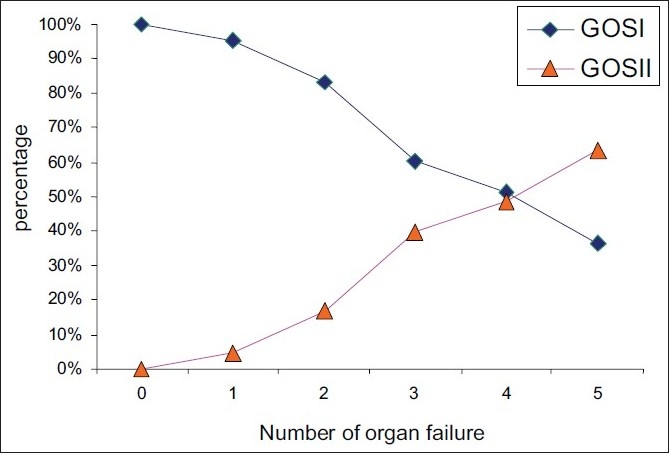
Association between the number of organ failure and outcome
Finally, according to “Traumatic Coma Data Bank” classification, mortality rate was at 11.7% in type I group, 16.6% in type II, 42% in type III, 66% in type IV, 27% in type V and 94% in type VI (P<0.0001) [Figure 4].
Figure 4.
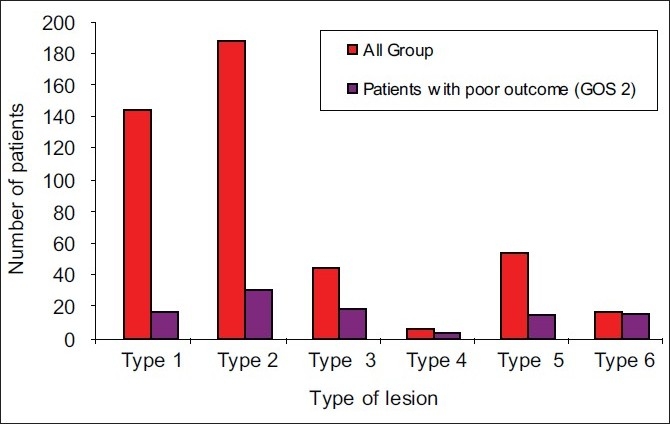
Outcome according to Traumatic Coma Databank classification of lesions
DISCUSSION
HI is the most frequent cause of mortality and morbidity in childhood. Indeed, several studies reported that there is a fair chance of recovery after severe brain injury in children.[29–32]
The measurement of outcome is fundamental to the effective evaluation of the clinical management of any illness. The GOS is widely used for assessing outcome after HI and offers the opportunity to diagnose functional disability or cognitive deficit.[5,6,33–35] In our study, the GOS scale showed a high percentage of good outcomes(77.5%) 7 months after injury in accordancewith other studies.[5,33–36] However, neuropsychiatric sequelae were observed in 143 patients (33%).
In our patients, extracranial injuries were present in 38.7% and may explain in part the high frequency of rhabdomyolysis. Despite the unavailability of specific monitoring tools (jugular venous saturation, intracranial pressure monitoring and transcranial Doppler sonography) in our ICU, the mortality rate was 18.1%, which is somewhat similar to those reported in other studies[4,37,38] and in particular in developed countries.[37–39]
Several investigators have stated that age is a good indicator of mortality in TBI.[9,40] The influence of age on children outcome is controversial.[39–44] Some authors consider that younger children have better chance of survival, tolerate longer periods of coma or decerebration and have fewer life-threatening complications when compared with older persons . [9,39,40] However, other authors reported opposite findings.[40] As in other studies,[37,38] our analysis does not identify age as a predictorof outcome, (P=0.37). However, we cannot conclude that age has no effect on outcome. In fact, important features of outcome such as neurobehavioral troubles were not fully studied in our patients. In fact, the GOS scale was performed within a mean delay at 7 months after hospital discharge, so we must mention that even ‘good recovery’ after pediatric brain trauma can be followed by poor long-term functional outcome.
The main factors correlated with outcome were the severityof injury as assessed by the GCS score.[10] Several studies have shown that there is a good correlation between GCS score and neurological outcome.[9,37,39] In our study, we found that GCS score ≤8 was associated with a poor outcome in multivariate analysis (P=0.04; OR: 2.42).
Pupil reflexes recovery is a useful predictor of recovery after brain trauma.[45,46] In a prospective study Riter et al.[46] found that in patients with GCS score under 8, death or a vegetative state occurred in 39% of patients with two reactive pupils, in 66% when there was only one reactive pupil, and in 85% when both pupils were nonreactive. Fearnside et al.[47] showed a significant difference in mortality whether both pupils reacted or not (P<0.05). In fact, pupil reactivity is related to cerebral blood flow.[46] In our study anisocoria and bilateral mydriasis was clearly associated with bad outcome in univariate analysis (P<0.001).
The ISS is a commonly used scoring system in traumatology.[48] Some studies have not found ISS to be a good outcome predictor, even in cases with serious injuries.[49] Others have found it to be a good predictor of poor prognosis.[45,50] We demonstrated a significant relationship between the ISS and the prognosis in univariate analysis.
The PRISM score has been widely used as a severity score in critically ill children, in various clinical situations.[13] However, only few studies using the PRISM score in pediatric trauma patients are available.[48] These studies showed that PRISM score was an accurate tool for predicting outcome .[43,48,51] In our study, we showed, with multivariate analysis that PRISM score was a reliable tool for predicting poor outcome, as demonstrated by the high value (0.83) of the area under ROC curve. Our results are in agreement with previous studies.[13,48,52]
The CT-scan is also helpful to reflect the HIs severity and predict clinical course. The influence of the type of cerebral lesions on cranial pressure and mortality has been evaluated variously in the literature.[53–55] However, according to the “Traumatic Coma Data Bank” classification, some lesions appear to carry a poor prognosis.[56,57] In our study the presence cerebral edema was associated with mortality in multivariate analysis (P= 0.03; OR: 2.23).
The negative influence of the SSIs on the brain is well-documented. In our study, the development of SSI was associated with a poor prognosis in univariate analysis. In addition, the mortality rate is greatly associated with the number of SSIs.
A significant correlation between glucose levels, severity of head trauma, pupillary reaction and maximum intracranial pressure during the first 24 hours after trauma was well-documented.[58] Our study shows that high level of blood glucose on admission was associated with poor outcome. This result was previously reported in other studies.[58,59]
Hypoxia has also been indicated asearly predictor of poor outcome after moderate and severe HI for the additional risk of secondary brain injury: hypoxia was recorded in 27–55% of subjects on the scene, inthe ambulance or on arrival at the Emergency Department.[60,61] In our study, hypoxia was recorded only in 54 patients (11.9%),and was associated with poor outcome in double analysis.
Hyponatremia is associated with cerebral edema that increases secondary injury as identified in our study. Moreover, therapeutic hypernatremia in “neurotrauma” is considered as neuroprotective (eg., use of hypertonic saline.). However, in our study a Na+ >145 mmol/l on ICU admission and during ICU stay was associated with a poor outcome. This can be explained by the high frequency of hyperglycemia and the high risk of this group, characterized by a low GCS and a high value of PRISM and ISS on admission. Moreover, hypernatremia leads to brain shrinkage. Brain shrinkage induced by hypernatremia can cause vascularrupture, with cerebral bleeding, subarachnoid hemorrhage andpermanent neurological damage.[62] So, hypernatremia can depress lesion caused by brain trauma.
On the other hand, hypotonic hyponatremia can be responsible of cerebral edema.[63] Because the surroundingcranium limits expansion of the brain, intracranial hypertensiondevelops, with a risk of brain injury. This leads to aggravation of post-traumatic lesion.
Despite the retrospective nature of this study, we were able to define some simple variables that are predictive of poor prognosis and that are merely based on easily measurable clinical, CT Scan, biochemical and laboratory parameters that may be used either at the scene of the accident (clinical) or in the emergency department of any hospital with available facilities. According to the National Guard statistical data, seatbelt is used only in 22% and only 60% of motorbike drivers wear helmet in our population.[7]
Limitations of study
We must mention that our study has several limitations. All retrospective studies as our study suffer from incomplete or inconsistent information. Furthermore, in our ICU the diagnosis of raised intracranial pressure was performed on clinical findings (pupil response, motor deficit, convulsion…) and brain CT-scan results. Moreover, the GOS scale was retrospectively performed within a mean delay at 7 months after hospital discharge and we think that the frequency of neurobehavioral impairments is under estimated. In fact, these complications were retrospectively noted after hospital discharge by an ICU and pediatric physicians and not by a pediatric neurologist. Moreover, some missing data were obtained on the basis of parents questioning and not by clinical examination of patient so validity and reliability of this information is highly questionable . Moreover, we must mention that even ‘good recovery’ after pediatric brain trauma can be followed by poor long-term functional outcome. In fact, the tool that we use to assess prognosis (GOS scale) can be considered as a blunt instrument, especially for survivors who are not vegetative or severely impaired. In fact, neurobehavioral impairments are common in children with HI[64] and can have substantial impact on children's academic and social function and quality of life. In fact, numerous studies have investigated the nature of neuropsychological sequelae following pediatric TBI in school-aged children and adolescents. These studies suggested that, even years after injury, deficits can appear in terms of attentional capacity, memory and learning, psychomotor skills and executive functions.[36,43,64]
Future directions of study
As neurobehavioral impairments were under estimated in our study, further studies are needed to analyse neurobehavioral outcomes in children suffering traumatic HI.
CONCLUSIONS
TBI is the most common cause of death and acquired disability among children and young adults in developed countries. A significant proportion of children admitted with mild HI were found to have moderate disability at follow-up. However, the outcome measure (GOS) is a very blunt instrument, especially for survivors who are not vegetative or severely impaired. There is now a large body of literature showing that neurobehavioral impairments are common in children with injuries in moderate to severe range, and have substantial impact on children's academic, social function and quality of life.[64] The GOS is not sensitive in such disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Flaada JT, Leibson CL, Mandrekar JN, Diehl N, Perkins PK, Brown AW, et al. Relative risk of mortality after traumatic brain injury: A population-based study of the role of age and injury severity. J Neurotrauma. 2007;24:435–45. doi: 10.1089/neu.2006.0119. [DOI] [PubMed] [Google Scholar]
- 2.Signorini DF, Andrews PJ, Jones PA, Wardlaw JM, Miller JD. Adding insult to injury: The prognostic value of early secondary insults for survival after traumatic brain injury. J Neurol Neurosurg Psychiatry. 1999;66:26–31. doi: 10.1136/jnnp.66.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–9. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- 4.Odebode TO, Abubakar AM. Childhood head injury: Causes, outcome, and outcome predictors. A Nigerian perspective. Pediatr Surg Int. 2004;20:348–52. doi: 10.1007/s00383-004-1196-5. [DOI] [PubMed] [Google Scholar]
- 5.Hawley CA, Ward AB, Magnay AR, Long J. Outcomes following childhood head injury: A population study. J Neurol Neurosurg Psychiatry. 2004;75:737–42. doi: 10.1136/jnnp.2003.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennett B. Outcome of severe damage to the central nervous system.Scale, scope and philosophy of the clinical problem. Ciba Found Symp. 1975;34:3–21. doi: 10.1002/9780470720165.ch2. [DOI] [PubMed] [Google Scholar]
- 7.Bahloul M, Chelly H, Ben Hmida M, Ben Hamida C, Ksibi H, Kallel H, et al. Prognosis of traumatic head injury in South Tunisia: A multivariate analysis of 437 cases. J Trauma. 2004;57:255–61. doi: 10.1097/01.ta.0000083004.35231.1e. [DOI] [PubMed] [Google Scholar]
- 8.Diamond PT. Brain injury in the Commonwealth of Virginia: An analysis of Central Registry data, 1988-1993. Brain Inj. 1996;10:413–9. doi: 10.1080/026990596124278. [DOI] [PubMed] [Google Scholar]
- 9.Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient's age.A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–16. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- 10.Simpson D, Reilly P. Pediatric coma scale. Lancet. 1982;2:450. doi: 10.1016/s0140-6736(82)90486-x. [DOI] [PubMed] [Google Scholar]
- 11.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 12.Tepas JJ, 3rd, Mollitt DL, Talbert JL, Bryant M. The pediatric trauma score as a predictor of injury severity in the injured child. J Pediatr Surg. 1987;22:14–8. doi: 10.1016/s0022-3468(87)80006-4. [DOI] [PubMed] [Google Scholar]
- 13.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Granry JC, Dubé L, Terminassian A, Frebet E, Le Rolle T. Multimodal monitoring of head injuries in children. Ann Fr Anesth Reanim. 2002;21:148–56. doi: 10.1016/s0750-7658(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 15.Vos PE, van Voskuilen AC, Beems T, Krabbe PF, Vogels OJ. Evaluation of the traumatic coma data bank computed tomography classification for severe head injury. J Neurotrauma. 2001;18:649–55. doi: 10.1089/089771501750357591. [DOI] [PubMed] [Google Scholar]
- 16.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. JAMA. 1978;240:439–42. [PubMed] [Google Scholar]
- 17.Pfenninger EG, Lindner KH. Arterial blood gases in patients with acute head injury at the accident site and upon hospital admission. Acta Anaesthesiol Scand. 1991;35:148–52. doi: 10.1111/j.1399-6576.1991.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 18.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Pietropaoli JA, Rogers FB, Shackford SR, Wald SL, Schmoker JD, Zhuang J. The deleterious effects of intraoperative hypotension on outcome in patients with severe head injuries. J Trauma. 1992;33:403–7. doi: 10.1097/00005373-199209000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Greger NG, Kirkland RT, Clayton GW, Kirkland JL. Central diabetes insipidus.22 years’ experience. Am J Dis Child. 1986;140:551–4. doi: 10.1001/archpedi.1986.02140200061028. [DOI] [PubMed] [Google Scholar]
- 21.Born JD, Hans P, Smitz S, Legros JJ, Kay S. Syndrome of inappropriate secretion of antidiuretic hormone after severe head injury. Surg Neurol. 1985;23:383–7. doi: 10.1016/0090-3019(85)90212-5. [DOI] [PubMed] [Google Scholar]
- 22.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 23.Marquette CH, Georges H, Wallet F, Ramon P, Saulnier F, Neviere R, et al. Diagnostic efficiency of endotracheal aspirates with quantitative bacterial cultures in intubated patients with suspected pneumonia.Comparison with the protected specimen brush. Am Rev Respir Dis. 1993;148:138–44. doi: 10.1164/ajrccm/148.1.138. [DOI] [PubMed] [Google Scholar]
- 24.Bone RC. Sepsis, the sepsis syndrome, multi-organ failure: A plea for comparable definitions. Ann Intern Med. 1991;114:332–33. doi: 10.7326/0003-4819-114-4-332. [DOI] [PubMed] [Google Scholar]
- 25.Melo JR, Di Rocco F, Blanot S, Laurent-Vannier A, Reis RC, Baugnon T, Sainte-Rose C, et al. Acute hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. Acta Neurochir (Wien) 2010;152:1559–65. doi: 10.1007/s00701-010-0680-z. [DOI] [PubMed] [Google Scholar]
- 26.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 27.Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982;61:141–52. doi: 10.1097/00005792-198205000-00002. [DOI] [PubMed] [Google Scholar]
- 28.AHCPR Guidelines: Treatment of Pressure Ulcers. Department of Health and Human Services. Washington, DC, U.S: AHCPR Publication #95-0652; 1994. U.S. Department of Health and Human Services; pp. 12–3. [Google Scholar]
- 29.Fabbri A, Servadei F, Marchesini G, Stein SC, Vandelli A. Early predictors of unfavourable outcome in subjects with moderate head injury in the emergency department. J Neurol Neurosurg Psychiatry. 2008;79:567–73. doi: 10.1136/jnnp.2007.120162. [DOI] [PubMed] [Google Scholar]
- 30.Kumaraswamy N, Naziah A, Abdullah J, Ariff MMed AR, Abdullah MR, Ghazaime G. Outcome of children with traumatic brain injury in rural Malaysia. J Clin Neurosci. 2002;9:251–5. doi: 10.1054/jocn.2001.1047. [DOI] [PubMed] [Google Scholar]
- 31.Clifton GL, Kreutzer JS, Choi SC, Devany CW, Eisenberg HM, Foulkes MA, et al. Relationship between Glasgow Outcome Scale and neuropsychological measures after brain injury. Neurosurgery. 1993;33:34–9. doi: 10.1227/00006123-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Fleminger S, Ponsford J. Long term outcome after traumatic brain injury. BMJ. 2005;331:1419–20. doi: 10.1136/bmj.331.7530.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–8. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 34.van der Naalt J, van Zomeren AH, Sluiter WJ, Minderhoud JM. One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J Neurol Neurosurg Psychiatry. 1999;66:207–13. doi: 10.1136/jnnp.66.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks DN, Hosie J, Bond MR, Jennett B, Aughton M. Cognitive sequelae of severe head injury in relation to the Glasgow Outcome scale. J Neurol Neurosurg Psychiatry. 1986;49:549–53. doi: 10.1136/jnnp.49.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagannathan J, Okonkwo DO, Yeoh HK, Dumont AS, Saulle D, Haizlip J, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2:240–9. doi: 10.3171/PED.2008.2.10.240. [DOI] [PubMed] [Google Scholar]
- 37.White JR, Farukhi Z, Bull C, Christensen J, Gordon T, Paidas C, et al. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29:534–40. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Chiaretti A, Piastra M, Pulitanò S, Pietrini D, De Rosa G, Barbaro R, et al. Prognostic factors and outcome of children with severe head injury: An 8-year experience. Childs Nerv Syst. 2002;18:129–36. doi: 10.1007/s00381-002-0558-3. [DOI] [PubMed] [Google Scholar]
- 39.Tilford JM, Simpson PM, Yeh TS, Lensing S, Aitken ME, Green JW, et al. Variation in therapy and outcome for pediatric head trauma patients. Crit Care Med. 2001;29:1056–61. doi: 10.1097/00003246-200105000-00037. [DOI] [PubMed] [Google Scholar]
- 40.Barlow KM, Thomson E, Johnson D, Minns RA. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116:e174–85. doi: 10.1542/peds.2004-2739. [DOI] [PubMed] [Google Scholar]
- 41.Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc. 1997;3:581–91. [PubMed] [Google Scholar]
- 42.Johnson DL, Krishnamurthy S. Severe pediatric head injury: Myth, magic, and actual fact. Pediatr Neurosurg. 1998;28:167–72. doi: 10.1159/000028643. [DOI] [PubMed] [Google Scholar]
- 43.Campbell CG, Kuehn SM, Richards PM, Ventureyra E, Hutchison JS. Medical and cognitive outcome in children with traumatic brain injury. Can J Neurol Sci. 2004;31:213–9. doi: 10.1017/s0317167100053853. [DOI] [PubMed] [Google Scholar]
- 44.Anderson V, Spencer-Smith M, Leventer R, Coleman L, Anderson P, Williams J, et al. Childhood brain insult: Can age at insult help us predict outcome? Brain. 2009;132:45–56. doi: 10.1093/brain/awn293. [DOI] [PubMed] [Google Scholar]
- 45.Hill DA, Delaney LM, Roncal S. A chi-square automatic interaction detection (CHAID) analysis of factors determining trauma outcomes. J Trauma. 1997;42:62–6. doi: 10.1097/00005373-199701000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Ritter AM, Muizelaar JP, Barnes T, Choi S, Fatouros P, Ward J, et al. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 1999;44:941–8. doi: 10.1097/00006123-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury.A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7:267–79. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 48.Cantais E, Paut O, Giorgi R, Viard L, Camboulives J. Evaluating the prognosis of multiple, severely traumatized children in the intensive care unit. Intensive Care Med. 2001;27:1511–7. doi: 10.1007/s001340101039. [DOI] [PubMed] [Google Scholar]
- 49.Fandino J, Stocker R, Prokop S, Trentz O, Imhof HG. Cerebral oxygenation and systemic trauma related factors determining neurological outcome after brain injury. J Clin Neurosci. 2000;7:226–33. doi: 10.1054/jocn.1999.0202. [DOI] [PubMed] [Google Scholar]
- 50.Meldon SW, Reilly M, Drew BL, Mancuso C, Fallon W., Jr Trauma in the very elderly: A community-based study of outcomes at trauma and nontrauma centers. J Trauma. 2002;52:79–84. doi: 10.1097/00005373-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Thakker JC, Splaingard M, Zhu J, Babel K, Bresnahan J, Havens PL. Survival and functional outcome of children requiring endotracheal intubation during therapy for severe traumatic brain injury. Crit Care Med. 1997;25:1396–401. doi: 10.1097/00003246-199708000-00030. [DOI] [PubMed] [Google Scholar]
- 52.Castello FV, Cassano A, Gregory P, Hammond J. The Pediatric Risk of Mortality (PRISM) Score and Injury Severity Score (ISS) for predicting resource utilization and outcome of intensive care in pediatric trauma. Crit Care Med. 1999;27:985–8. doi: 10.1097/00003246-199905000-00041. [DOI] [PubMed] [Google Scholar]
- 53.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9:S287–92. [PubMed] [Google Scholar]
- 54.Marshall LF, Toole BM, Bowers SA. The National Traumatic Coma Data Bank.Part 2: Patients who talk and deteriorate: implications for treatment. J Neurosurg. 1983;59:285–8. doi: 10.3171/jns.1983.59.2.0285. [DOI] [PubMed] [Google Scholar]
- 55.Eisenberg HM, Gary HE, Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, et al. Initial CT findings in 753 patients with severe head injury.A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–98. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- 56.Hukkelhoven CW, Steyerberg EW, Habbema JD, Farace E, Marmarou A, Murray GD, et al. Predicting outcome after traumatic brain injury: Development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22:1025–39. doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- 57.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 58.Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–43. doi: 10.1097/00006123-200002000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;75:545–51. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- 60.Bochicchio GV, Sung J, Joshi M, Bochicchio K, Johnson SB, Meyer W, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–4. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 61.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Weastmead Head Injury Project outcome in severe head injury: a comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7:267–79. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 62.Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–9. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 63.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 64.Kramer ME, Chiu CY, Walz NC, Holland SK, Yuan W, Karunanayaka P, et al. Long-term neural processing of attention following early childhood traumatic brain injury: fMRI and neurobehavioral outcomes. J Int Neuropsychol Soc. 2008;14:424–35. doi: 10.1017/S1355617708080545. [DOI] [PMC free article] [PubMed] [Google Scholar]


