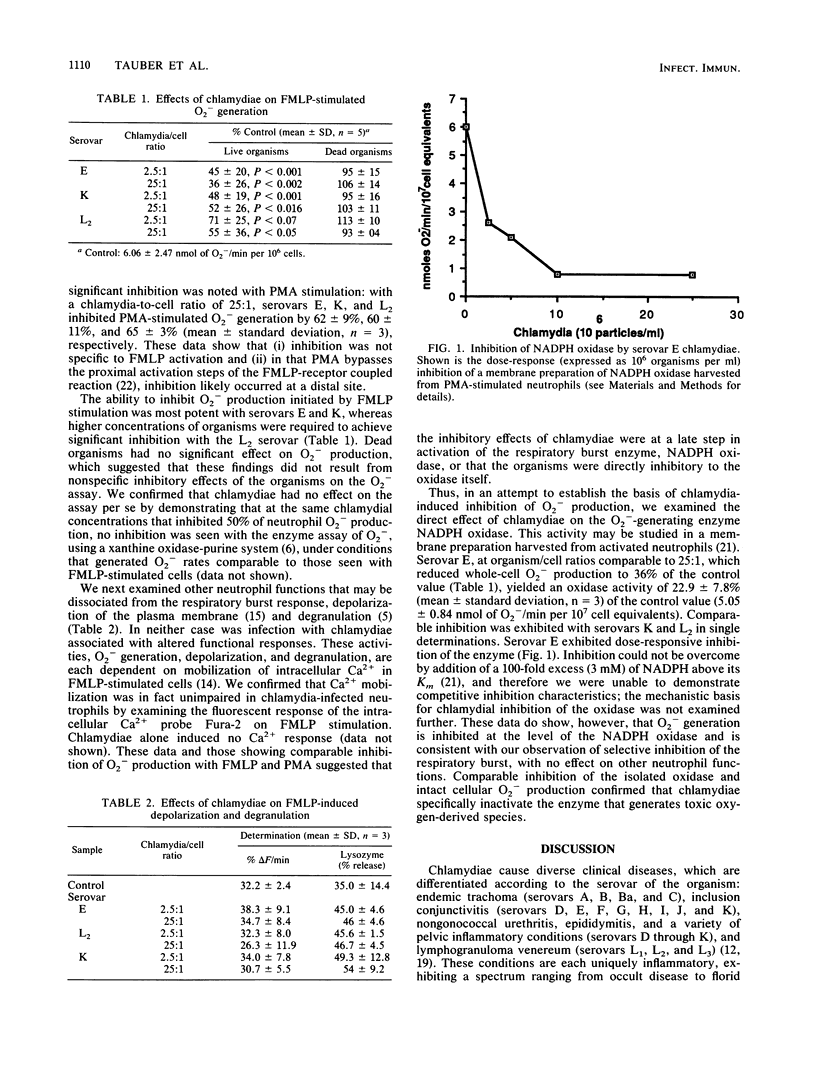
Abstract
The effects of Chlamydia trachomatis (serovars E, K, and L2) on human neutrophil activation were examined with respect to the organisms both as primary agonists and as agents that modulate cell responses to a second stimulus. Unopsonized chlamydiae alone, at ratios of 1.5 to 100 organisms per cell, failed to elicit changes in intracellular calcium or membrane depolarization or to stimulate the respiratory burst or degranulation during 60 min of incubation. Each of these functions except the respiratory burst was also normally activated when chlamydia-infected neutrophils were subsequently stimulated with formylmethionyl leucine phenylalanine or phorbol myristate acetate; the respiratory burst was inhibited 30 to 65%. Inhibition was dependent on live organisms and was maximal within 5 min of incubation. The organisms had no effect on the superoxide (O2-) assay, and the site of chlamydial inhibition was determined at the level of the NADPH oxidase itself, not at an intermediate step in the activation cascade. The mechanism of enzyme inactivation could not be determined. These results show that unopsonized chlamydiae do not elicit responses from infected neutrophils and suggest that microbicidal mechanisms other than those dependent on elaboration of toxic oxygen-derived species are required to inactivate chlamydiae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Lewis J. C., Lyles D. S., Heller K. A., Mills E. L., Bass D. A. Inhibition of neutrophil lysosome-phagosome fusion associated with influenza virus infection in vitro. Role in depressed bactericidal activity. J Clin Invest. 1982 Jun;69(6):1393–1397. doi: 10.1172/JCI110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard J., Levitt D. Chlamydia trachomatis (L2 serovar) binds to distinct subpopulations of human peripheral blood leukocytes. Clin Immunol Immunopathol. 1986 Feb;38(2):150–160. doi: 10.1016/0090-1229(86)90134-0. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Tauber A. I. Failure to detect superoxide in human neutrophils stimulated with latex particles. Pediatr Res. 1983 Apr;17(4):281–284. doi: 10.1203/00006450-198304000-00011. [DOI] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B. Inhibition of phagolysosome fusion is localized to Chlamydia psittaci-laden vacuoles. Infect Immun. 1981 May;32(2):889–896. doi: 10.1128/iai.32.2.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K. L., Collamer M., Auerbach M., Myers J. B., Pavlotsky N., Tauber A. I. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988 Aug 15;141(4):1295–1301. [PubMed] [Google Scholar]
- Kanofsky J. R., Wright J., Miles-Richardson G. E., Tauber A. I. Biochemical requirements for singlet oxygen production by purified human myeloperoxidase. J Clin Invest. 1984 Oct;74(4):1489–1495. doi: 10.1172/JCI111562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Monnickendam M. A., Pearce J. H. Immune responses and chlamydial infections. Br Med Bull. 1983 Apr;39(2):187–193. doi: 10.1093/oxfordjournals.bmb.a071814. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Byrne G. I., Rothermel C. D., Cartelli D. M. Lymphokine enhances oxygen-independent activity against intracellular pathogens. J Exp Med. 1983 Jul 1;158(1):234–239. doi: 10.1084/jem.158.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Register K. B., Davis C. H., Wyrick P. B., Shafer W. M., Spitznagel J. K. Nonoxidative antimicrobial effects of human polymorphonuclear leukocyte granule proteins on Chlamydia spp. in vitro. Infect Immun. 1987 Oct;55(10):2420–2427. doi: 10.1128/iai.55.10.2420-2427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register K. B., Morgan P. A., Wyrick P. B. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infect Immun. 1986 Jun;52(3):664–670. doi: 10.1128/iai.52.3.664-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P., Wakefield D., Graham D. M., Easter J. F., Penny R. The chemiluminescence response of normal human leukocytes to chlamydia trachomatis. Diagn Immunol. 1985;3(3):119–125. [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Tauber A. I. Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood. 1987 Mar;69(3):711–720. [PubMed] [Google Scholar]
- Tosi M. F., Hammerschlag M. R. Chlamydia trachomatis selectively stimulates myeloperoxidase release but not superoxide production by human neutrophils. J Infect Dis. 1988 Aug;158(2):457–460. doi: 10.1093/infdis/158.2.457. [DOI] [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Chen W. J., Kuo C. C. Degradation of Chlamydia trachomatis in human polymorphonuclear leukocytes: an ultrastructural study of peroxidase-positive phagolysosomes. Infect Immun. 1986 Aug;53(2):427–431. doi: 10.1128/iai.53.2.427-431.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong E. C., Klebanoff S. J., Kuo C. C. Toxic effect of human polymorphonuclear leukocytes on Chlamydia trachomatis. Infect Immun. 1982 Aug;37(2):422–426. doi: 10.1128/iai.37.2.422-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner S. L. Isolation and characterization of macrophage phagosomes containing infectious and heat-inactivated Chlamydia psittaci: two phagosomes with different intracellular behaviors. Infect Immun. 1983 Jun;40(3):956–966. doi: 10.1128/iai.40.3.956-966.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvillich M., Sarov I. Interaction between human polymorphonuclear leucocytes and Chlamydia trachomatis elementary bodies: electron microscopy and chemiluminescent response. J Gen Microbiol. 1985 Oct;131(10):2627–2635. doi: 10.1099/00221287-131-10-2627. [DOI] [PubMed] [Google Scholar]



