Abstract
Purpose
To assess current uterine fibroid embolisation (UFE) practice in European countries and determine the clinical environment for UFE in different hospitals.
Material and Methods
In May 2009, an invitation for an online survey was sent by e-mail to all members of the Cardiovascular and Interventional Radiologic Society of Europe, representing a total number of 1,250 different candidate European treatment centres. The survey covered 21 questions concerning local UFE practice.
Results
A total of 282 respondents completed the questionnaire. Fifteen questionnaires were excluded because they were doubles from centres that had already returned a questionnaire. The response rate was 267 of 1,250 centres (21.4%). Ninety-four respondents (33%) did not perform UFE and were excluded, and six centres were excluded because demographic data were missing. The remaining 167 respondents from different UFE centres were included in the study. Twenty-six percent of the respondents were from the United Kingdom (n = 43); 16% were from Germany (n = 27); 11% were from France (n = 18); and the remaining 47% (n = 79) were from other European countries. Most centres (48%, n = 80) had 5 to 10 years experience with UFE and performed 10 to 50 procedures annually (53% [n = 88]) of respondents). Additional demographic data, as well as specific data on referral of patients, UFE techniques used, and periprocedural and postprocedural, care will be provided.
Conclusion
Although UFE as an alternative treatment for hysterectomy or myomectomy is widespread in Europe, its impact on the management of the patient with symptomatic fibroids seems, according to the overall numbers of UFE procedures, somewhat disappointing. Multiple factors might be responsible for this observation.
Keywords: Uterine fibroids, Uterine fibroid embolisation, Survey
Introduction
Uterine fibroids are the most common benign tumours in women of childbearing age. Symptomatic fibroids can cause a diversity of symptoms, which can be divided into four categories: bleeding symptoms (irregular and/or heavy menstrual bleeding), pain (in the pelvic region and the back), bulk-related symptoms (pressure on bladder and bowel as well as increase in abdominal circumference), and subfertility [1]. These symptoms often lead to medical or surgical treatments.
During the last two decades, minimally invasive therapeutic options for uterine fibroids have increased considerably. Uterine fibroid embolisation (UFE) was introduced in 1994 and is currently a well acknowledged and proven alternative to surgical treatment [2, 3].
UFE is a percutaneous trancatheter embolisation technique using embolisation material to occlude the (end-)arteries supplying the fibroid. Devascularisation causes infarction and consequently decreased fibroid size, which may result in effective alleviation of symptoms.
However, after the introduction of UFE as an alternative to more invasive approaches, a real widespread breakthrough, especially in general interventional radiology (IR), did not occur.
Information on UFE still seems to be unavailable to a large number of women in Europe, and many gynaecologists do not provide the option of UFE, or they inaccurately inform patients, using misleading facts. Specific data on the number of centres and interventionalists performing UFE in Europe and the number of UFE procedures per centre do not exist.
This publication reports the outcome of a survey among European interventional radiologists concerning UFE treatment. The purpose of this study was to determine actual data on the current clinical practice of UFE in European countries.
Materials and Methods
In May 2009, we designed a survey to assess current UFE practice in European countries. All professionally active European members of the Cardiovascular and Interventional Radiologic Society of Europe (CIRSE) were invited by e-mail to participate in this study. The total number of different candidate treatment facilities in Europe was 1,250. The questionnaire consisted of 21 questions concerning local clinical practice of UFE and related topics (see Appendix for complete questionnaire). The online button-driven questionnaire was designed for easy handling with a simple set-up to be sure that as many interventional radiologists as possible would participate in this study and could be able to complete all fields.
The first questions referred to demographic data concerning the treatment facility in which respondents were working. The next question was if UFE was performed by the responding interventional radiologist. In case the answer was “no,” the questionnaire was excluded from the database. The remaining respondents were asked about the interventional group in their facility (number of interventional radiologists and how many of them performed UFE). Respondents had to indicate the time period when their hospital staff started performing UFE as well as the number of UFE procedures performed per year. Respondents were asked to describe the referral pattern of patients (self-referral, e.g., directly to IR, referral by gynaecologist, or referral by general practitioner) categorized as percentages adding up to 100%. The questionnaire also assessed who was responsible for preprocedural and postprocedural patient care (radiologist, gynaecologist, or both). The type of pain management, such as patient-controlled analgesia (PCA), epidural analgesia, or other—as well as the duration of hospitalisation—were also inventoried. Specific procedure-related questions, such as preferred vascular access (unifemoral, bifemoral, or other), use of microcatheters, type of embolic agents (gelatin sponge, spherical or nonspherical embolic material) used, and the advocated embolisation end point (complete stasis, sluggish flow, or pruned tree appearance), were asked. The use of magnetic resonance imaging (MRI) for preprocedural and postprocedural evaluation was also discussed. The last items were aimed at the future expectations of the respondents concerning Magnetic Resonance-guided Focused Ultrasound (MRgFUS) as a new treatment alternative for uterine fibroids, and we asked if the treatment facility had a Web site to allow screening of the Web sites for dedicated information on UFE treatment. Participants were asked to complete the online questionnaire before the end of July 2009. To avoid bias, we decided to include only one survey per treatment centre. In case more than one questionnaire was returned from the same facility, we decided to include the first submitted survey in the study and exclude the duplicates.
Results
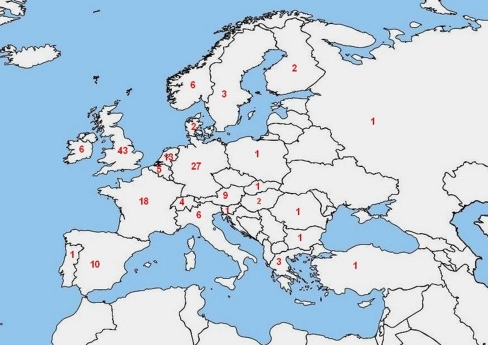
A total of 282 respondents returned the completed questionnaire (Table 1). Fifteen questionnaires were excluded because they were duplicates from treatment centres that had already returned a questionnaire. The response rate was therefore 267 of 1,250 candidate treatment centres (21.4%). Ninety-four respondents (33%) did not perform UFE and were consequently excluded from the study. Six questionnaires were excluded because essential demographic data were missing. Further contact efforts to obtain these missing data did not result in sufficient completion of the information, thus making rejection of these questionnaires inevitable. The remaining 167 respondents, all from different UFE centres, were included in the study. The geographic distribution of the respondents covered 24 countries in Europe, including Turkey. Figure 1 shows the number of included treatment facilities per country.
Table 1.
Number of treatment centres per European country and response rate
| Countries | No. of treatment centres | No. of respondents | No. of exclusions | No. of included centres (%) | |
|---|---|---|---|---|---|
| No UFE | Duplicates | ||||
| Austria | 54 | 14 | 5 | 0 | 9 (16.7) |
| Belgium | 27 | 8 | 2 | 1 | 5 (18.5) |
| Bulgaria | 7 | 2 | 1 | 0 | 1 (14.3) |
| Croatia | 6 | 0 | 0 | 0 | 0 (0) |
| Cyprus | 1 | 0 | 0 | 0 | 0 (0) |
| Czech Republic | 35 | 2 | 2 | 0 | 0 (0) |
| Denmark | 9 | 5 | 3 | 0 | 2 (22.2) |
| Finland | 15 | 3 | 1 | 0 | 2 (13.3) |
| France | 85 | 20 | 0 | 2 | 18 (21.2) |
| Germany | 289 | 37 | 9 | 1 | 27 (9.3) |
| Greece | 67 | 18 | 15 | 0 | 3 (4.5) |
| Hungary | 19 | 2 | 0 | 0 | 2 (10.5) |
| Iceland | 1 | 0 | 0 | 0 | 0 (0) |
| Ireland | 14 | 9 | 2 | 1 | 6 (42.9) |
| Italy | 50 | 12 | 6 | 0 | 6 (12.0) |
| Latvia | 1 | 0 | 0 | 0 | 0 (0) |
| Luxembourg | 6 | 1 | 1 | 0 | 0 (0) |
| Malta | 1 | 0 | 0 | 0 | 0 (0) |
| Norway | 23 | 9 | 3 | 0 | 6 (26.1) |
| Poland | 27 | 3 | 0 | 2 | 1 (3.7) |
| Portugal | 11 | 2 | 1 | 0 | 1 (9.1) |
| Romania | 6 | 5 | 0 | 4 | 1 (16.7) |
| Russia | 14 | 1 | 0 | 0 | 1 (7.1) |
| Serbia | 7 | 1 | 1 | 0 | 0 (0) |
| Slovakia | 4 | 1 | 0 | 0 | 1 (25.0) |
| Slovenia | 6 | 1 | 0 | 0 | 1 (16.7) |
| Spain | 52 | 12 | 1 | 1 | 10 (19.2) |
| Sweden | 13 | 4 | 1 | 0 | 3 (23.1) |
| Switzerland | 42 | 11 | 6 | 1 | 4 (9.5) |
| The Netherlands | 109 | 30 | 16 | 1 | 13 (11.9) |
| Turkey | 61 | 6 | 5 | 0 | 1 (1.6) |
| Ukraine | 1 | 0 | 0 | 0 | 0 (0) |
| United Kingdom | 189 | 57 | 13 | 1 | 43 (22.8) |
| Missing demographic data | 6 | ||||
| Total | 1,250 | 282 | 94 | 15 | 167 (13.4) |
Fig. 1.
Number of included treatment facilities per European country
Twenty-six percent (n = 43) of the respondents were from the United Kingdom; 16% (n = 27) were from Germany; 11% (n = 18) were from France; and the remainder (53%, n = 79) were from other European countries. Fifty-two percent (n = 86) of the respondents worked in an academic centre, and the remaining 48% (n = 81) worked in a general hospital setting or private practice. The majority of the respondents (65%, n = 108) worked in a group with ≥1 interventional radiologist performing UFE. Only 1% (n = 2) of the respondents had >15 years of experience with UFE; 25% (n = 42) had 10–15 years of experience; the majority (48%, n = 80) had 5–10 years of experience; and 26% (n = 43) had introduced UFE during the last 5 years.
Table 2 lists the number of UFE procedures, per year and per country, classified into five categories: ≤0, 10 to 50, 50 to 100, 100 to 200, and ≥200 UFE treatments annually. Most centres (53%, n = 88) performed between 10 and 50 treatments on an annual basis. Extreme numbers of UFE treatments were provided by two treatment facilities (one in France and one in Romania): They both performed approximately 500 procedures/year.
Table 2.
Annual number of UFE procedures (classified into five categories) performed in treatment facilities per country
| Countries (n) | ≤10 | 10 to <50 | 50 to <100 | 100 to <200 | ≥200 |
|---|---|---|---|---|---|
| Austria (n = 9) | 2 | 7 | 0 | 0 | 0 |
| Belgium (n = 5) | 1 | 4 | 0 | 0 | 0 |
| Bulgaria (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Denmark (n = 2) | 0 | 2 | 0 | 0 | 0 |
| Finland (n = 2) | 1 | 1 | 0 | 0 | 0 |
| France (n = 18) | 5 | 10 | 1 | 1 | 1 |
| Germany (n = 27) | 11 | 14 | 1 | 1 | 0 |
| Greece (n = 3) | 2 | 1 | 0 | 0 | 0 |
| Hungary (n = 2) | 0 | 0 | 2 | 0 | 0 |
| Ireland (n = 6) | 1 | 3 | 1 | 1 | 0 |
| Italy (n = 6) | 6 | 0 | 0 | 0 | 0 |
| Norway (n = 6) | 3 | 3 | 0 | 0 | 0 |
| Poland (n = 1) | 0 | 0 | 1 | 0 | 0 |
| Portugal (n = 1) | 0 | 1 | 0 | 0 | 0 |
| Romania (n = 1) | 0 | 0 | 0 | 0 | 1 |
| Russia (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Slovakia (n = 1) | 0 | 1 | 0 | 0 | 0 |
| Slovenia (n = 1) | 1 | 0 | 0 | 0 | 0 |
| Spain (n = 10) | 4 | 6 | 0 | 0 | 0 |
| Sweden (n = 3) | 2 | 1 | 0 | 0 | 0 |
| Switzerland (n = 4) | 3 | 1 | 0 | 0 | 0 |
| The Netherlands (n = 13) | 7 | 5 | 0 | 1 | 0 |
| Turkey (n = 1) | 1 | 0 | 0 | 0 | 0 |
| United Kingdom (n = 43) | 8 | 28 | 5 | 1 | 1 |
| Total number (%) of treatment facilities (n = 167) | 60 (36) | 88 (53) | 11 (6) | 5 (3) | 3 (2) |
n Number of treatment facilities
In Table 3, data on preprocedural, periprocedural, and postprocedural care management are listed. In the majority of cases (76%), patients were referred by a gynaecologist for UFE, and only in a small minority (4%) were referred by a general practitioner. In the remaining cases (20%), patients referred themselves directly to the interventional radiologist. Preprocedural care was generally performed by a combination of a gynaecologist and radiologist (42%, n = 70). Preprocedural MRI was considered a standard procedure by 56% (n = 90) of the responding radiologists. Pain management was preferably performed (76%, n = 122) using PCA. Some facilities (15%, n = 24) used epidural analgesia, and 9% (n = 14) employed other pain-management protocols, such as intravenous medication (n = 8), oral medication (n = 1), a combination of PCA and epidural analgesia (n = 2), general anaesthesia (n = 1), superior hypogastric plexus nerve block (n = 1), and neuroleptic medication (n = 1). Half of the respondents (50%, n = 81) admitted their patients for one overnight stay after UFE treatment. Only 1% (n = 1) performed UFE on an outpatient basis, and 3% (n = 5) admitted their patients for a total of four overnight stays. Postprocedural care was most frequently (55%, n = 91) provided by a combination of a gynaecologist and radiologist. The majority of respondents (72%, n = 120) followed-up their patients up for 3 or 6 months, and 8% (n = 14) did so for >12 months.
Table 3.
Preprocedural, periprocedural, and postprocedural care-related information
| % | N | |
|---|---|---|
| Referral for UFE (n = 165) | ||
| Self-referral | 20 | – |
| Gynaecologist | 76 | – |
| General practitioner | 4 | – |
| Preprocedural care (n = 166) | ||
| Radiologist | 17 | 28 |
| Gynaecologist | 41 | 68 |
| Combination | 42 | 70 |
| Pain management (n = 160) | ||
| Patient-controlled analgesia (PCA) | 76 | 122 |
| Epidural analgesia | 15 | 24 |
| Other | 9 | 14 |
| Length of hospital stay (n = 163) | ||
| Outpatient treatment | 1 | 1 |
| 1 night | 50 | 81 |
| 2 nights | 33 | 54 |
| 3 nights | 13 | 22 |
| 4 nights | 3 | 5 |
| Postprocedural care (n = 166) | ||
| Radiologist | 21 | 35 |
| Gynaecologist | 24 | 40 |
| Combination | 55 | 91 |
| Postprocedural follow-up schedule (n = 166) | ||
| No follow-up | 4 | 6 |
| Follow-up for 3 months | 41 | 69 |
| Follow-up for 6 months | 31 | 51 |
| Follow-up for 12 months | 16 | 26 |
| Follow-up for >12 months | 8 | 14 |
| Planned MRI scan (n = 161) | ||
| Preprocedure | 56 | 90 |
| 3 months postprocedure | 18 | 29 |
| 6 months postprocedure | 21 | 34 |
| 9 months postprocedure | 0 | 0 |
| 12 months postprocedure | 4 | 7 |
| >12 months postprocedure | 1 | 1 |
n Number of respondents who answered the specific question
In Table 4, UFE procedure-related details are listed. A large majority (81%, n = 134) of the respondents preferred unifemoral arterial access, whereas 17% (n = 28) chose bifemoral arterial puncture, and only 2% (n = 3) preferred brachial arterial access. Spherical embolic material was the favoured embolic agent in 77% (n = 127) of the responding radiologists. The frequency of using microcatheters during UFE procedures varied among the respondents. Only 3% (n = 5) indicated that they never use them; 36% (n = 60) use them only when considered necessary; and 34% (n = 56) always employ microcatheters during UFE. Fourteen percent (n = 24) used the so-called “pruned tree” appearance on fluoroscopic imaging as the UFE end point. The user frequency of the end points “complete stasis” and “sluggish flow” was quite similar (41%, n = 68 vs. 45%, n = 74).
Table 4.
UFE procedure-related information
| % | N | |
|---|---|---|
| Preferred arterial access (n = 165) | ||
| Unifemoral | 81 | 134 |
| Bifemoral | 17 | 28 |
| Brachial | 2 | 3 |
| Embolic agent (n = 166) | ||
| Gelatin sponge | 1 | 2 |
| Spherical embolic material | 77 | 127 |
| Nonspherical embolic material | 22 | 37 |
| Use of microcatheters (n = 166) | ||
| Never | 3 | 5 |
| Seldom | 36 | 60 |
| Regularly | 27 | 45 |
| Always | 34 | 56 |
| End point used (n = 166) | ||
| Complete stasis | 41 | 68 |
| Sluggish flow | 45 | 74 |
| Pruned tree | 14 | 24 |
n Number of respondents who answered the specific question
Ninety percent (n = 151) of the treatment facilities had a Web site. We screened these Web pages for patient information on UFE. Nineteen percent (n = 31) contained dedicated passive or interactive treatment information for patients as well as physicians. Table 5 lists the annual number of treatments per centre in five categories for centres with and without a dedicated UFE Web site. This table illustrates that centres providing UFE information on a Web site have higher treatment numbers than facilities not operating an active Web site.
Table 5.
Number of UFE treatments per category for centres with and without a dedicated UFE Web site
| No. of UFE procedures/year | % UFE Web site presence (n) | |
|---|---|---|
| No (136) | Yes (31) | |
| ≤10 | 41 (56) | 13 (4) |
| 10 to <50 | 51 (69) | 61 (19) |
| 50 to <100 | 6 (8) | 10 (3) |
| 100 to <200 | 1 (2) | 10 (3) |
| ≥200 | 1 (1) | 7 (2) |
Future expectations for MR-HIFU as a thermoablative therapy for fibroids were indicated as “very promising” by five % (n = 9) of the respondents; 30% (n = 50) thought it was “promising,” and 28% (n = 47) did not see an important role for this treatment in the future. The remaining 36% (n = 60) had no opinion about this topic.
Discussion
Since the publications of Ravina et al. in the early 1990s on premyomectomy transcatheter embolisation to minimize blood loss during surgery, the surprising effects on decreased fibroid size and symptoms became evident and led to the first reports on UFE as a single treatment for symptomatic fibroids [2, 4]. After a worldwide introduction as a possible alternative to hysterectomy and many additional studies on this treatment, UFE now seems to fit into the treatment options available to women suffering from uterine fibroids.
The Cochrane review from 2006 cited that UFE resulted in the same patient satisfaction rate as surgery (myomectomy or hysterectomy) [5]. The length of hospital stay was decreased after UFE, and the return to daily activities was faster. Because UFE seemed to result in a higher minor complication rate and more unscheduled visits and readmission rates, the statement was made that additional focus on long-term follow-up was necessary to determine the real impact of UFE [6].
As a result, retrospective cohort studies examined and compared the results of UFE and hysterectomy, which lead to satisfying conclusions concerning safety, expectations, and cost-effectiveness over a longer follow-up period [7, 8]. Furthermore, randomized studies comparing UFE and surgery (hysterectomy or myomectomy) have been completed in the meantime, resulting in publications in major scientific journals [9–12]. However, in gynaecological papers, the experimental character of UFE was only recently abandoned. Short, medium, and long-term follow-up data are currently available, leading to a more positive attitude from our gynaecological colleagues [3, 13, 14].
Guidelines from different medical specialities currently consent that UFE is a valuable alternative to surgical management of symptomatic fibroids in carefully selected and informed patients [6, 15]. Bratby et al. quoted that level 1 evidence has established the role of UFE as a proper alternative treatment [16]. Last but not least, Bradley et al. stated that UFE is effective, safe, and durable and should be considered a true alternative to hysterectomy [3]. Apparently, the Cochrane review from 2006 is considerably outdated and must be revised as soon as possible.
This current European survey showed interesting findings on clinical UFE practice in a variety of treatment centres in different European countries. It was nevertheless interesting to discover the marked variation in current UFE practice across European centres in terms of distribution, approach, treatment care, and numbers. The top five of countries with the highest number of UFE centres were (starting with the highest) the United Kingdom, Germany, France, The Netherlands, and Spain. UFE facilities were not exclusively restricted to academic centres (52%), as probably could be expected, but were also present in general hospital and/or private settings. Our survey also illustrates that although in 2009 UFE was widespread throughout European countries, the majority of centres (53%) performed only between 10 and 50 UFE procedures/year. Only 5% performed >100 cases annually. Most respondents (65%) were active in a group of interventionalists performing UFE, providing potential 24-h/7-day coverage of patient care. The overall impression that UFE is not new in Europe was expressed by the fact that the majority of centres (74%) had >5 years of experience with UFE.
Participation with gynaecologists was performed by the majority of interventionalists in both preprocedural (42%) and postprocedural (55%) patient care. Preprocedural and postprocedural care solely by IR was noted in only 17 and 21% of centres, respectively. Gynaecologists were the main referrers for UFE treatment. The preferred pain-management in the majority of centres (76%) was PCA, as expected, and most centres (50%) admitted the patients for one overnight stay after the procedure.
In terms of facility-related efficacy estimation, it was disappointing to note that only 24% of centres followed-up their patients for ≥12 months, 31% for only 6 months, and the majority (41%) for only 3 months. Although the use of preprocedural and postprocedural MRI is advocated widely to properly map and follow-up a UFE candidate, only 56% of centres employed a pre-UFE MRI-planning protocol. Postprocedural MRI follow-up was even more disappointing, probably related to the poor follow-up intervals as stated previously. Contrast-enhanced MRI is by all means the only reliable imaging modality to obviate sufficient devascularisation, e.g., a technically successful embolisation.
The fact that most centres (81%) used a unifemoral arterial access for UFE minimizes the concern about potential adverse events occurring at the puncture sites, and using the Waltman loop manoeuvre might also be of great help in cases of steep aortic bifurcation issues. Although 66% of interventional radiologists do not use microcatheters, or use them only if necessary, coaxial use of microcatheters might be important to avoid vascular spasms, resulting in inadequate devascularisation of fibroids, thus leading to inferior clinical results. The chosen embolisation end point may depend on the type of embolisation material used. In the minority of centres, the pruned-tree appearance on fluoroscopic images is still used as the end point indicator. Sluggish flow in the uterine artery, e.g., the Shlansky-Goldberg method (stasis during five heartbeats) [17], was employed in equal frequency as total stasis of contrast medium in the uterine artery. Concerning embolisation material, no solid conclusive data are available to date on superior clinical outcome after UFE with a certain type embolic agent, although studies are pointing in the direction of calibrated microspheres [18–21]. It was therefore interesting to notice that a large majority of centres (77%) favoured the use of these microspheres. Gelatin sponge was still employed in 1% of the centres. Although the role of the industry in promoting spherical embolic agents cannot be underestimated, the advantages of calibrated microspheres during UFE procedures, especially when using microcatheters, are evident. Less clogging of microcatheters and better prediction of the level of devascularisation might result in a smooth, swift, and successful UFE procedure.
Some treatment centres have dedicated fibroid clinics for preprocedural consultation and postembolisation clinical and radiologic follow-up. These centres of excellence often employ well-designed and properly executed public relations focused on potential patients and referring physicians. Kroencke stated the importance of using the media to enhance patient awareness of treatment options [22]. We should not underestimate the inventiveness of modern patients in their search for alternative treatment options independent from their treating physician. Not only the Internet, but also magazines, radio, and television, can be used by treatment centres to reach potential patients. The creation of an interactive Web site is a unique opportunity to do so. This study indicated that treatment centres operating a Web site containing dedicated UFE information performed more procedures than centres that do not use Web site possibilities, thus emphasizing the efficacy of such strategies. However, the presence of such a Web site might not be the only explanation for this finding.
Another important point in building a UFE practice is that interventional radiologists must become accustomed to treating patients in a clinical environment. UFE treatments must be performed by a multidisciplinary team, including gynaecologists and anaesthesiologists. It is extremely important, as quoted by Keeling et al., that interventional radiologists see the patient in the first place instead of a uterine fibroid that must be embolised [23]. Although quality of care is becoming increasingly important, it is no longer acceptable to meet the patient in the angiographic suite for the first time, perform the embolisation procedure, and never see her again.
Concerning new therapeutic developments, it was amazing to notice that Magnetic Resonance-guided Focused Ultrasound was categorized as a “very promising” respectively “promising” new treatment option by only 5% respectively 30% of the centres. Thirty-six percent did not see any role for MRgFUS in the future. However, recent papers show that MRgFUS can be a treatment option with satisfying results for a selection of patients [24–26]. The low levels of confidence we found might be partially biased by uneasy feelings toward a possible competitive treatment for UFE. Another reason could be that MRgFUS devices are expensive to acquire and therefore many of the respondents’ facilities will never have the opportunity to obtain one. Moreover, current focused ultrasound technology can treat only relatively small volumes of fibroid tissue at a time, and respondents might see this is an essential factor limiting the number of patients that can be treated with this technique.
A limitation of this study was the low response rate (21.4%). All CIRSE members received an invitation to participate in this online questionnaire; however, only a relatively small percentage responded. Therefore, we do not have the illusion of possessing a solid and complete data set on this subject because not all centres performing UFE completed the questionnaire. A low response rate is always a problem when conducting a survey, and it tends to be even lower when using an electronic survey instead of a survey sent by postal mail [27, 28]. Therefore, the results published here are not an absolute view on UFE practice in Europe. It is possible that the nonresponding interventional centres do not perform UFE except, for instance, only nonvascular interventions. Another possibility is that some of the centres performing UFE are not members of CIRSE and therefore did not receive a survey invitation. Moreover, a key issue in survey research is nonresponse bias, which occurs when respondents differ in meaningful ways from nonrespondents. It is possible that the responders are more actively involved in this subject than nonresponders and are therefore more willing to participate in this survey.
We conclude with the statement that UFE as an alternative treatment for hysterectomy or myomectomy is widespread in Europe. However, the impact on the management of the patient with symptomatic fibroid seemed, according to the overall numbers of UFE procedures, disappointing. A more active attitude toward clinical IR, together with effective public relations, might establish a more solid fundament for UFE treatment in the future.
Acknowledgments
We thank the CIRSE Central Office for their support.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Appendix

References
- 1.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 2.Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346(8976):671–672. doi: 10.1016/S0140-6736(95)92282-2. [DOI] [PubMed] [Google Scholar]
- 3.Bradley LD. Uterine fibroid embolization: a viable alternative to hysterectomy. Am J Obstet Gynecol. 2009;201:127–135. doi: 10.1016/j.ajog.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Ravina JH, Bouret JM, Fried D, et al. Value of preoperative embolization of uterine fibroma: report of a multicenter series of 31 cases. Contracept Fertil Sex. 1995;23:45–49. [PubMed] [Google Scholar]
- 5.Gupta JK, Sinha A, Lumsden MA et al (2006) Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev (1). Art. No.: CD 005073. doi:10.1002/14651858.CD005073.pub2. [DOI] [PubMed]
- 6.Marshburn PB, Matthews ML, Hurst BS. Uterine artery embolization as a treatment option for uterine myomas. Obstet Gynecol Clin North Am. 2006;33:125–144. doi: 10.1016/j.ogc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Dutton S, Hirst A, McPherson K, et al. A UK multicentre retrospective cohort study comparing hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids (HOPEFUL study): main results on medium-term safety and efficacy. BJOG. 2007;114:1340–1351. doi: 10.1111/j.1471-0528.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirst A, Dutton S, Wu O et al (2008) A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study. Health Technol Assess 12:1–248, iii [DOI] [PubMed]
- 9.Edwards RD, Moss JG, Lumsden MA, et al. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360–370. doi: 10.1056/NEJMoa062003. [DOI] [PubMed] [Google Scholar]
- 10.Hehenkamp WJ, Volkers NA, Birnie E, et al. Symptomatic uterine fibroids: treatment with uterine artery embolization or hysterectomy—results from the randomized clinical Embolisation versus Hysterectomy (EMMY) Trial. Radiology. 2008;246:23–32. doi: 10.1148/radiol.2463070260. [DOI] [PubMed] [Google Scholar]
- 11.Pinto I, Chimeno P, Romo A, et al. Uterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment—a prospective, randomized, and controlled clinical trial. Radiology. 2003;226:425–431. doi: 10.1148/radiol.2262011716. [DOI] [PubMed] [Google Scholar]
- 12.Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73–85. doi: 10.1007/s00270-007-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohle PN, Voogt MJ, De VJ, et al. Long-term outcome of uterine artery embolization for symptomatic uterine leiomyomas. J Vasc Interv Radiol. 2008;19:319–326. doi: 10.1016/j.jvir.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Popovic M, Berzaczy D, Puchner S, et al. Long-term quality of life assessment among patients undergoing uterine fibroid embolization. AJR Am J Roentgenol. 2009;193:267–271. doi: 10.2214/AJR.08.1841. [DOI] [PubMed] [Google Scholar]
- 15.Hovsepian DM, Siskin GP, Bonn J, et al. Quality improvement guidelines for uterine artery embolization for symptomatic leiomyomata. J Vasc Interv Radiol. 2009;20(Suppl):S193–S199. doi: 10.1016/j.jvir.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Bratby MJ, Belli AM. Radiological treatment of symptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:717–734. doi: 10.1016/j.bpobgyn.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Society of Interventional Radiology (2003) 28th Annual scientific meeting program. SIR, Fairfax, VA, pp 62–73
- 18.Spies JB, Allison S, Flick P, et al. Polyvinyl alcohol particles and tris-acryl gelatin microspheres for uterine artery embolization for leiomyomas: results of a randomized comparative study. J Vasc Interv Radiol. 2004;15:793–800. doi: 10.1097/01.RVI.0000136982.42548.5D. [DOI] [PubMed] [Google Scholar]
- 19.Siskin GP, Beck A, Schuster M, et al. Leiomyoma infarction after uterine artery embolization: a prospective randomized study comparing tris-acryl gelatin microspheres versus polyvinyl alcohol microspheres. J Vasc Interv Radiol. 2008;19:58–65. doi: 10.1016/j.jvir.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Rasuli P, Hammond I, Al-Mutairi B, et al. Spherical versus conventional polyvinyl alcohol particles for uterine artery embolization. J Vasc Interv Radiol. 2008;19:42–46. doi: 10.1016/j.jvir.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Golzarian J, Lang E, Hovsepian D, et al. Higher rate of partial devascularization and clinical failure after uterine artery embolization for fibroids with spherical polyvinyl alcohol. Cardiovasc Intervent Radiol. 2006;29:1–3. doi: 10.1007/s00270-005-0243-5. [DOI] [PubMed] [Google Scholar]
- 22.Kroencke TJ (2009) How to promote IR to patients and referring physicians. CIRSE annual meeting abstract book, Lisbon, Portugal, pp 237–278
- 23.Keeling AN, Reekers JA, Lee MJ. The clinical practice of interventional radiology: a European perspective. Cardiovasc Intervent Radiol. 2009;32:406–411. doi: 10.1007/s00270-009-9503-0. [DOI] [PubMed] [Google Scholar]
- 24.Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110(2 Pt 1):279–287. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 25.LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol. 2010;194:274–280. doi: 10.2214/AJR.09.2842. [DOI] [PubMed] [Google Scholar]
- 26.Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—results of different treatment protocols. Radiology. 2007;243:885–893. doi: 10.1148/radiol.2433060267. [DOI] [PubMed] [Google Scholar]
- 27.Akl EA, Maroun N, Klocke RA, et al. Electronic mail was not better than postal mail for surveying residents and faculty. J Clin Epidemiol. 2005;58:425–429. doi: 10.1016/j.jclinepi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Seguin R, Godwin M, MacDonald S, et al. E-mail or snail mail? Randomized controlled trial on which works better for surveys. Can Fam Physician. 2004;50:414–419. [PMC free article] [PubMed] [Google Scholar]