Abstract
Objective
The purpose of this study was to determine whether obese individuals with reduced adipose tissue inflammation exhibit a more favorable cardiovascular risk profile.
Background
Obesity is associated with a low-grade state of chronic inflammation that may be causally related to cardiometabolic disease.
Methods
Using immunohistochemistry, we categorized obese individuals dichotomously as having inflamed fat (n=78) or non-inflamed fat (n=31) based on the presence (+) or absence (-) of macrophage crown-like structures (CLS) in subcutaneous abdominal fat biopsy samples. We compared their metabolic, vascular, and adipose tissue characteristics to lean subjects (n=17).
Results
Inflamed CLS+ obese individuals displayed higher plasma insulin, HOMA, triglycerides, glucose, blood pressure, hs-CRP, LDL-C, and lower HDL-C and brachial artery flow-mediated dilation (FMD) compared to leans (p<0.05). Adipose mRNA expression of inflammatory genes including CD68, leptin, MMP-9, CD163, and CD8A were significantly greater and VEGF lower in the CLS+ group (p<0.05). In contrast, obese subjects with non-inflamed fat exhibited a mixed clinical phenotype with lower insulin resistance, reduced proatherogenic gene expression, and preserved vascular function as in lean subjects. In multiple linear regression adjusting for age and gender, CLS status (beta = -0.28, p=0.008) and waist circumference (beta = -0.25, p =0.03) were independent predictors of FMD.
Conclusion
These findings lend support to the novel concept that factors in addition to absolute weight burden, such as qualitative features of adipose tissue, may be important determinants of cardiovascular disease. Therapeutic modulation of the adipose phenotype may represent a target for treatment in obesity.
Keywords: obesity, endothelium, inflammation, vasodilation, vasculature
Introduction
There is growing recognition that many overweight individuals maintain a relatively favorable cardiometabolic profile even in extreme obesity. Determinants of a metabolically healthier obese phenotype are poorly understood and likely multifactorial relating to differences in body fat distribution, physical activity, and adipose metabolism (1). In addition, recent data suggest that inflammation of adipose tissue orchestrated by monocyte/macrophage infiltration and overproduction of proatherogenic cytokines may mediate metabolic and vascular disease in human obesity (2, 3). Chronic activation of the immune system has been strongly implicated in the pathogenesis of obesity-associated disorders including type 2 diabetes mellitus, cancer, and cardiovascular disease, and is a growing target of interest for therapeutic intervention (4).
We and others have previously shown that proinflammatory changes in fat are linked to metabolic stress and endothelial dysfunction (2, 5, 6). The goals of the present study were to determine whether qualitative differences exist between the adipose tissue of obese versus normal weight individuals, and to investigate whether overweight patients with reduced adipose inflammation are polarized toward a lean phenotype.
Methods
Study subjects
We enrolled overweight adult men and women (age≥18 years) with a body mass index (BMI) of ≥25 kg/m2 receiving care at the Boston Medical Center. We also recruited lean adults (BMI <25 kg/m2) through advertisements to the general public. Subjects with unstable medical conditions or pregnancy were excluded. The study was approved by Boston Medical Center IRB and all subjects gave written informed consent. Blood pressure, heart rate, height, weight, BMI, and waist circumference (WC) were recorded for each subject and all biochemical analyses quantified from fasting blood samples.
Subcutaneous adipose tissue collection
From each subject, we collected abdominal subcutaneous adipose tissue via percutaneous needle biopsy technique or directly harvested during gastric bypass surgery, as previously described (2, 7). All subjects were in a fasting state for ≥12 hours prior to biopsy. Each subject provided a single biopsy specimen from the subcutaneous region for analysis.
Adipose tissue histology and RT-PCR
Macrophages in adipose tissue were identified using cell-specific stains targeted to CD68 (DakoCytomation, Carpinteria, CA). Based on the presence (+) or absence (-) of macrophage crown-like structures (CLS), obese subjects were dichotomously categorized as CLS+ if any adipose tissue macrophage clusters were present in any examined field (inflamed fat), or CLS- if clusters were absent (non-inflamed fat) as previously validated (2, 8, 9). All lean subjects were CLS-. For mRNA expression analyses, samples were homogenized using a MagNa Lyser tissue homogenizer (Roche Applied Science, Indianapolis, IN). Quantitative RT-PCR reactions were performed using a high throughput instrument (BioMark, Fluidigm, San Francisco, CA) with Gene Expression Assays (Applied Biosystems, Foster City, CA) and DynamicArray chips (Fluidigm, San Francisco, CA) (10).
Vascular studies
For each subject, brachial artery ultrasound studies were performed in a fasting state. Brachial vasomotor responses were examined using a noninvasive, standardized method of ultrasound using a Toshiba Powervision 6000 system, as previously described (2, 11). Flow-mediated dilation (FMD) following a 5-minute cuff occlusion in an upper arm position and nitroglycerin-mediated dilation (NMD) of the brachial artery served as measures of endothelium-dependent and -independent dilation, respectively. Sublingual nitroglycerin (0.4 mg) was omitted if contraindicated or the subject declined.
Statistical analysis
Analyses were completed using SAS for Windows, version 9.1 (SAS Institute Inc., Cary, NC, USA). Data are presented as mean ± SD, or median with interquartile range, or proportions (%). Categorical group differences were examined using the Chi-square test or Fisher's exact test as appropriate. Kolgomorov-Smirnov tests, histograms and normal probability plots were used to determine whether continuous variables were normally distributed or skewed. Natural log transformation was applied only to continuous variables not meeting normality which specifically were glucose, HOMA, insulin, triglycerides, as well as adipose expression of IL-8, catalase, peroxiredoxin-1, guanylate cyclase I α/β, IL-6 and IL-1β. The latter four genes did not reach normality despite transformation and were analyzed by nonparametric methods. Group differences for continuous variables were examined using analysis of variance (ANOVA) with Tukey post-hoc analysis, and the Kruskal-Wallis test was used for skewed variables. Univariate associations between vascular parameters or HOMA and clinical data were examined in the obese using Pearson's correlation. Alternatively, Spearman's rank correlation was used for skewed data. Multiple linear regression was used to determine whether CLS status was independently associated with FMD. Univariate clinical correlates of FMD with significance level of P<0.1 were included in the model. For all analyses, P value <0.05 was considered statistically significant.
Results
Clinical and histological data
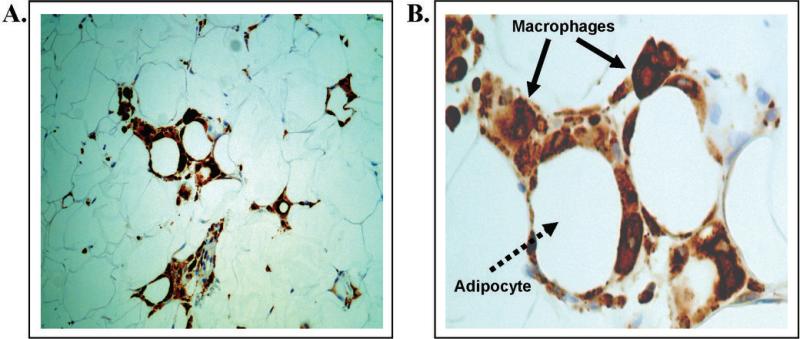
A total of 109 obese subjects (mean age 42 ±11, 86% female) and 17 lean individuals (mean age 33 ± 12, 77% female) completed this study. The clinical characteristics of all participants are displayed in table 1. The majority of obese individuals (72%) demonstrated evidence of adipose inflammation characterized by tissue presence of macrophage crown-like structures in subcutaneous fat (CLS+, n=78) as shown in figure 1, that were absent in 28% of overweight subjects (CLS-, n=31). In contrast, all leans were non-inflamed (CLS-). As expected, the lean group had significantly lower BMI, WC, plasma insulin, HOMA, LDL-C, HbA1c, triglycerides, glucose, hs-CRP, hypertension, diabetes prevalence, medication use, and higher HDL-C levels compared to the CLS+ obese (p<0.05). However, despite the same degree of adiposity, gender distribution, and age range as the CLS+ group, insulin levels and HOMA were significantly lower in CLS- obese, exhibiting values that were intermediate to the lean and inflamed overweight groups (p<0.05).
Table 1.
Clinical characteristics
| Lean (n=17) | Obese (CLS-) (n=31) | Obese inflamed (CLS+) (n=78) | |
|---|---|---|---|
| Age (yrs) | 33 ± 12 | 40 ± 10 | 43 ± 12* |
| Female (%) | 77 | 97 | 82 |
| BMI (kg/m2) | 22 ± 1 | 45 ± 7* | 45 ± 9* |
| Waist circumference (cm) | 76 ± 1 | 127 ± 6* | 130 ± 7* |
| Weight (kg) | 63 ± 7 | 118 ± 20* | 127 ± 29* |
| Insulin (mU/ml) | 2, 1 | 10.5, 7* | 14, 12*† |
| HOMA | 0.5, 0.2 | 2.3, 1.7* | 4.1, 3.4*† |
| Triglycerides (mg/dl) | 62, 34 | 83, 43* | 107, 89* |
| Glucose (mg/dl) | 86,10 | 92, 21* | 100, 26* |
| HDL-C (mg/dl) | 67 ± 18 | 49 ± 9* | 49 ± 14* |
| LDL-C (mg/dl) | 97 ± 24 | 115 ± 26* | 121 ± 31* |
| Total cholesterol (mg/dl) | 178 ± 29 | 183 ± 29 | 194 ± 36 |
| HbA1c (%) | 5.3 ± 0.3 | 6.1 ± 1.0* | 6.3 ± 1.1* |
| hs-CRP (mg/dl) | 0.9 ± 0.9 | 5.1 ± 3.9* | 8.5 ± 9.5* |
| Diabetes (%) | 0 | 26* | 36* |
| Hypertension (%) | 0 | 36* | 46* |
| Hypoglycemic use (%) | 0 | 13 | 32* |
| Lipid lowering use (%) | 0 | 13 | 26* |
| Active smoker (%) | 0 | 16 | 8 |
Data are presented as means ± SD or median, interquartile range.
p<0.05 vs. lean;
p<0.05 vs. CLS- obese
Figures 1. Representative histology of inflamed human subcutaneous adipose tissue as demonstrated using light microscopy.
CD68+ macrophages (brown color) are organized into multiple “crown-like-structures” that encircle necrotic adipocytes as a hallmark of chronic local inflammation in human fat tissue. A. left panel, 20x power; B. right panel, 40x power, solid arrows indicate macrophages, dotted arrow illustrates an adipocyte.
Vascular parameters
Vascular function data for all three groups are displayed in table 2. Brachial artery FMD (n=126) was significantly impaired in the CLS+ subjects compared to both lean and CLS- groups demonstrating that subjects with reduced adipose inflammation display endothelium-dependent vasodilation similar to those with normal weight. In contrast, endothelium-independent NMD (n=67) was significantly reduced in obese groups compared to the leans (p<0.05). As shown in table 3, univariate correlates of brachial artery FMD in the obese were WC, HOMA, HbA1c, and adipose IL-6 and CCL2 expression. HOMA correlated positively with WC, BMI, HbA1c, triglycerides, plasma hs-CRP, adipose IL-1β expression and negatively with HDL-C and FMD. In multiple linear regression with adjustment for age, gender, and HOMA, CLS status (CLS+ vs. CLS-; beta = - 0.28, p = 0.008) and WC (beta = -0.25, p = 0.03) were the only significant predictors of FMD (model R2 = 0.19).
Table 2.
Vascular data
| Lean | Obese (CLS-) | Obese inflamed (CLS+) | |
|---|---|---|---|
| Systolic BP (mmHg) | 117 ± 11 | 129 ± 14* | 130 ± 16* |
| Diastolic BP (mmHg) | 67 ± 6 | 75 ± 9* | 73 ± 10* |
| Brachial diameter (mm) | 3.4 ± 0.7 | 3.8 ± 0.5 | 4.2 ± 0.7*† |
| FMD (%) | 12.5 ± 6 | 12.4 ± 4 | 9.2 ± 4*† |
| NMD (%) | 22.4 ± 10 | 15.2 ± 5* | 13.2 ± 6* |
FMD = Flow-mediated dilation; NMD = Nitroglycerin-mediated dilation
p<0.05 vs. lean;
p<0.05 vs. CLS- obese
Table 3.
Clinical correlations
| Correlates of FMD | Coefficient | P |
|---|---|---|
| Waist circumference | -0.26 | 0.0l |
| HOMA | -0.20 | 0.05 |
| HbAlc | -0.26 | 0.00l |
| CCL2 | -0.25 | 0.0l |
| IL-6 | -0.25 | 0.02 |
| Correlates of HOMA | ||
|---|---|---|
| Waist circumference | 0.43 | <0.001 |
| BMI | 0.36 | 0.001 |
| HbAlc | 0.42 | <0.001 |
| Triglycerides | 0.57 | <0.001 |
| Hs-CRP | 0.26 | 0.02 |
| IL-1β | 0.23 | 0.03 |
| HDL cholesterol | -0.33 | <0.001 |
FMD = Flow-mediated dilation; HOMA = Homeostasis model assessment
Adipose tissue gene expression
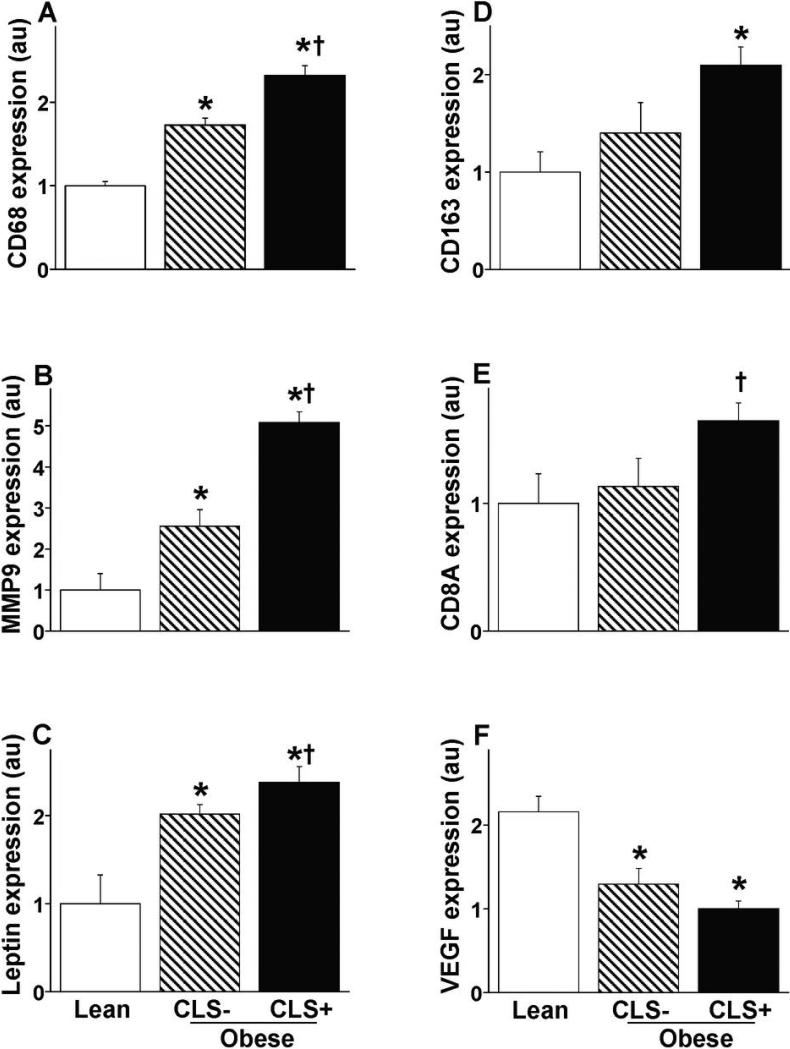
We identified specific genes that were differentially expressed between subcutaneous adipose tissue of all three groups. As shown in figures 2A-E, CD68, leptin, matrix metalloproteinase-9 (MMP-9), CD163 (M2 macrophage marker), and CD8A mRNA expression were greatest in the CLS+ obese group and lowest in leans, with intermediate values in CLS- subjects (P<0.05 for all inter-group differences). Lean individuals also exhibited significantly lower mRNA expression of IL-8 (neutrophil chemotaxis), chemokine C-C motif ligand 2 (CCL-2), IL-6, TNF-α, IL-1β, and MRC1 (M2 marker, CD 206 mannose receptor), and higher expression of vascular endothelial growth factor-A (VEGF-A) (figure 2F) compared to both obese groups (p<0.05). Increased oxidative stress has been implicated in mechanisms of obesity-related metabolic disorders (12). In this regard, we observed decreased expression of antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and peroxiredoxin-1 (PRDX1) and markers of local NO bioavailability (eNOS and guanylate cyclase I α/β) in the adipose tissue of obese as compared to lean subjects (p<0.05). Conversely, we observed increased expression of pro-oxidant enzymes including NADPH oxidase cytochrome b (p<0.01) and NADPH oxidase 4 (NOX4) (p=0.06) in the adipose tissue of obese. The findings support a milieu of increased oxidative stress in association with obesity however our measured tissue variables did not specifically correlate with systemic vascular responses.
Figures 2. Adipose tissue mRNA gene expression.
Lean subjects and obese individuals are stratified by CLS status for A) CD68; B) MMP-9; C) Leptin; D) CD 163; E) CD8A; and F) VEGF-A.
* p<0.05 vs. lean; † p<0.05 vs. CLS- obese.
Discussion
In the present study, we demonstrate that obese individuals display more proatherogenic vascular, metabolic, and adipose tissue profiles as compared to lean subjects. The key finding was that for the same degree of severe obesity, individuals with reduced adipose inflammation exhibited an “intermediate” clinical phenotype with arterial function similar to normal weight subjects. We observed parallel trends in adipocytokine expression that mirrored systemic profiles suggesting a biological connection. In this regard, the findings prompt speculation that aspects of cardiovascular disease mechanisms may have origins within the adipose microenvironment.
Animal models consistently show that excess adiposity induces a chronic state of immune system activation characterized by adipose tissue influx of macrophages, neutrophils, and T lymphocytes that stimulate adipocytokine production implicated in the temporal development of insulin resistance (13). We now recognize that the consequence of adipose inflammation likely extends beyond metabolic disturbance to cause vascular injury and atherosclerosis. For example, adipose tissue inflammation induces endothelial activation and alters blood flow in the microcirculation of obese mice (14). Inflamed perivascular fat preferentially localizes to atherosclerotic regions and adipocytokines impair vasomotor function (15). These findings show that functional properties of blood vessels are adversely modulated by the state of the adipose microenvironment. It is thus plausible to speculate that disease at the adipose level may extend and reflect in the systemic vasculature, as this concept is supported by our finding that obese patients with reduced adipose inflammation displayed favorable arterial responses as in lean subjects. This may hold clinical significance as prospective studies consistently show that endothelial dysfunction predicts cardiovascular events (16).
The degree of adipose immune activation in humans is more variable than in genetically modified experimental animals, and this heterogeneity in tissue phenotype provides a window of opportunity to investigate how adipose changes relate to clinical disease. In this regard, we demonstrated that a specific pattern of macrophage build-up in fat is associated with metabolic and endothelial dysfunction (2, 7). The key issue of what sets off the inflammatory cascade in fat is not well understood and likely multifactorial relating to adipocyte hypertrophy and dysfunction, oxidative stress, toxic lipolysis, and deficient neovascular remodeling (17). Adipose activation and adipocytokine overproduction may have its systemic consequences. For example, we demonstrated over-expression of MMP9 which plays a key role in matrix turnover and remodeling as part of the activated tissue profile. Plasma metalloproteinase concentrations are increased in the obese and clinical studies show that fat is a significant source (5). As MMP9 destabilizes atherosclerotic plaques, consequences of weight gain may be germane to cardiovascular risk (18).
Adipose tissue macrophages largely originate from circulating monocytes (13). They can exist in at least two differentially activated states characterized as M1 macrophages that produce proinflammatory cytokines linked to insulin resistance and atherosclerosis and alternative M2 phenotypes involved in immunosuppressive functions (19). In obese animals, these cell lines show predominantly M1 characteristics (20), while human fat displays mixed phenotypes (5). Their precise role in human disease is unknown, and whether macrophage polarization influences the pathogenic profile of fat remains an open question (5). Most probably, immune changes in humans is dynamic with activation of proinflammatory “danger signals” in early phases of weight gain followed by adaptive remodeling, as the expression for many acute phase cytokines was similar in both obese groups. We demonstrated increased expression for M2 markers of CD163 and CD206 suggesting that compensatory immunosuppressive pathways may be triggered in later stages of obesity.
Our seminal finding was that obese individuals without proinflammatory adipose changes displayed more favorable clinical characteristics. This subset represented approximately 30% of our obese cohort, strikingly similar in proportion to metabolically healthier obese phenotypes in large population studies (21). Both WC and the presence of CLS in adipose tissue were independent predictors of brachial artery FMD, suggesting that both “quantity” and “quality” of fat may be germane to systemic disease (22).
Our present study has several limitations. To minimize subject discomfort, we relied on a single subcutaneous biopsy site for adipose tissue characterization; therefore, it remains possible that some individuals were miscategorized owing to sampling error. Our analyses were limited to subcutaneous fat which was readily accessible. Since visceral depots are felt to be more metabolically active and pathogenic in nature, it is possible that stronger correlations could have been observed (23, 24). Most participants in the present study were female, reflecting general clinical practice and gender differences in populations that seek weight loss treatments (25). Macronutrient intake plays an important role in adaptive immune responses. While we did not specifically record daily food logs, we acknowledge that chronic differences in dietary patterns could influence metabolic states (26, 27). These limitations are counterbalanced by the relatively large sample size for this type of invasive clinical study and novel translational information generated in severe human obesity where limited information currently exists.
In conclusion, we identified a group of obese subjects with reduced adipose inflammation that exhibit intermediate risk factor profiles. We hypothesize that individuals prone to inflammatory activation with weight gain may have increased cardiometabolic risk. Therapeutic modulation of the adipose phenotype may represent a novel target for treatment in obesity.
Acknowledgements
None.
Sources of funding and disclosures: This work was supported by National Institutes of Health grants to Dr. Gokce (R01 HL074097 and HL084213). Dr. Vita is supported by NIH grants HL083269, HL083801, HL081587, and HL75795. Dr. Apovian receives consulting fees from Novo Nordisk, Arena, Merck, Amylin, GI Dynamics, Johnson and Johnson, Sanofi-Aventis, Orexigen, and Pfizer. Dr. Apovian receives grant support from Amylin, Sanofi-Aventis, Pfizer, Orexigen, Metaproteomics, Atkins Foundation, and Arena. There are no conflicts with the current manuscript.
Abbreviations
- BMI
Body mass index
- CLS
Crown-like-structures
- FMD
Flow-mediated dilation
- HbA1C
Glycosylated hemoglobin A1C
- HOMA
Homeostasis model assessment
- hs-CRP
High-sensitivity C-reactive protein
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NMD
Nitroglycerin mediated dilation
- RT-PCR
Real-time polymerase chain reaction
- WC
Waist circumference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–75. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 2.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–9. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–57. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourlier V, Zakaroff-Girard A, Miranville A, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–15. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 6.Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–13. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–7. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 10.Freedman JE, Larson MG, Tanriverdi K, et al. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham heart study. Circulation. 2010;122:119–29. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 12.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–62. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–21. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 16.Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 17.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–85. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–23. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, van de WE, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 24.Harman-Boehm I, Bluher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–7. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 25.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33:991–7. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–7. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]