Abstract
Parkinson disease (PD) is second only to Alzheimer disease as the most common neurodegenerative disorder in humans. Despite intense investigations, no effective therapy is available to halt the progression of PD. Although statins are widely used cholesterol-lowering drugs throughout the world, recent studies suggest that these drugs modulate neurodegeneration-related signaling processes and may be beneficial for PD. Simvastatin is the most potent statin in crossing the blood-brain barrier, and this particular statin drug negatively correlates with the incidence of PD and shows efficacy in animal models of PD. However, PD mainly occurs in the aging population, who are more vulnerable to cholesterol or lipid-related disorders, raising questions whether this possible beneficial effect of statins in PD patients is cholesterol dependent or cholesterol independent. This article presents data on the therapeutic efficacy of simvastatin in a chronic MPTP model of PD, reviews recent literature, and discusses the pros and cons of statin therapy in PD.
Keywords: Parkinson disease, statins, signal transduction, dopamine, neuroprotection
Discovery of statins is very much similar to that of penicillin. Penicillin was discovered from fungus Penicillium notatum, whereas statin was discovered from Penicillium citrium. In the 1970s, Dr. Endo and colleagues in Japan (Endo and others 1976; Endo and others 1977) were wondering how certain fungi had protected themselves against others. As ergosterol, a derivative of cholesterol, is an essential component of fungi membrane, they were prompted to investigate if inhibition of cholesterol biosynthesis was one of such mechanisms. As a result of their investigation, they reported the discovery of mevastatin, the first statin drug, from P. citrium in 1978. Eventually, through the laboratory of Drs. Goldstein and Brown (Bilheimer and others 1983; Brown and Goldstein 1980), these drugs emerged as the most effective means of reducing elevated levels of plasma cholesterol. In addition to cholesterol lowering, these wonder drugs are nowadays stretching their hands toward neurodegenerative disorders such as Parkinson disease (PD).
PD is one of the most devastating neurodegenerative disorders in humans. PD may appear at any age, but it is uncommon in people younger than 30 years. The actual cause of PD is not known. Although a defect in α-synuclein (PARK1), Parkin (PARK2), DJ-1 (PARK7), PTEN-induced kinase 1 (PARK6), and ubiquitin C gene terminal hydrolase L1 has recently been found in a few families with extraordinarily high incidences of PD, it is believed that a number of environmental, genetic, and immune cues are associated with the onset of PD (Olanow and Tatton 1999; Vila and Przedborski 2004). Clinically, PD is identified by tremor, bradykinesia, rigidity, and postural instability (Dauer and Przedborski 2003; Olanow and Tatton 1999). These clinical symptoms appear mainly because of a severe shortage of dopamine (DA), a chemical substance that enables people to move normally and smoothly. The deficiency of DA is caused by the gradual loss of dopaminergic neurons that, being present in the substantia nigra pars compacta (SNpc), synthesize DA from tyrosine via tyrosine hydroxylase (Dauer and Przedborski 2003; Olanow and Tatton 1999). Therefore, pathologically, it is characterized by gliosis and progressive degeneration of the dopaminergic neurons associated with the presence of intracytoplasmic inclusions (Lewy bodies) in the SNpc (Dauer and Przedborski 2003; Gao and others 2003b; Ghosh and others 2007; Hunot and others 1997; Teismann and others 2003). Interestingly, widely used statin drugs have been shown to attenuate glial activation, inhibit oxidative stress, protect dopaminergic neurons (Ghosh and others 2009), and suppress the aggregation of α-syn (Bar-On and others 2008). Although there have not been any clinical trials of statins in PD patients, analysis of a patient database and recent animal studies suggest that statins are associated with a reduced incidence of PD and may be used to protect dopaminergic neurons in the SNpc. Here we attempt to discuss these studies to find out whether statins should be clinically tried in PD patients.
Cholesterol Puzzle in PD
In humans, cholesterol is synthesized from acetyl-CoA via multiple reactions. HMG-CoA reductase is the key rate-limiting enzyme of this biosynthetic pathway (Fig. 1). Statin-CoAs are structural analogues of HMG-CoA and thereby inhibit HMG-CoA reductase competitively with an affinity of about 1000 to 10,000 times greater than that of the natural substrate. In addition to direct inhibition of cholesterol synthesis, statins have also been shown to lower plasma cholesterol levels indirectly due to an up-regulation of the low-density lipoprotein (LDL) receptor (Vaziri and Liang 2004). However, in PD, it is doubtful whether cholesterol is to blame for neurodegenerative pathology. For example, the APOE ε4 allele has been associated with higher LDL cholesterol, whereas the ε2 allele has been found to be responsible for lower plasma LDL cholesterol. Although the ε4 allele is a major susceptibility gene for Alzheimer disease (AD; Michikawa 2003), the ε2 allele is usually protective for this disease. In contrast, a study by Huang and others (2004) indicates that the ε2 allele, not the ε4 allele, is positively associated with a higher prevalence of sporadic PD.
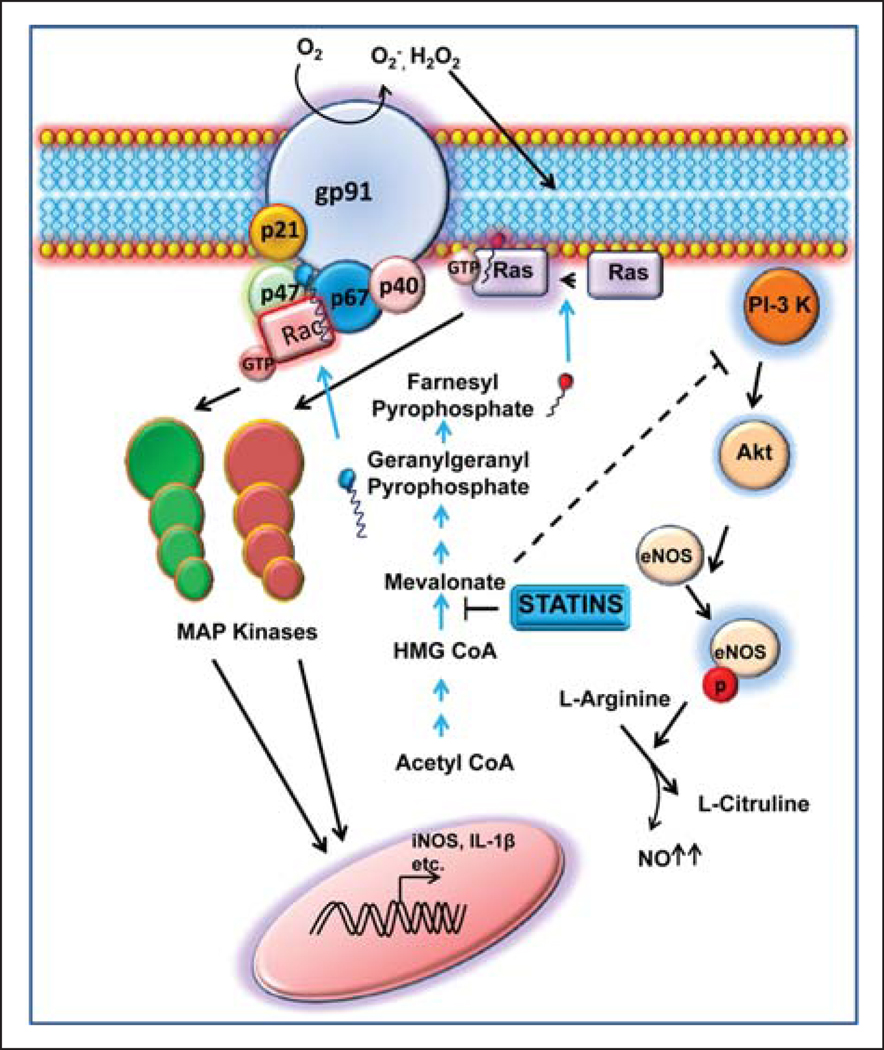
Figure 1.
Schematic representation of various anti–Parkinson disease (PD) functions (anti-inflammatory, antioxidative, and vasorelaxing) of statins. Statins inhibit the function of HMG-CoA reductase and thereby inhibit the geranylation of Rac and the farnesylation of Ras. Because both Rac and Ras are coupled to the transcription of various proinflammatory molecules via mitogen-activated protein (MAP) kinase pathways, statins reduce the expression of proinflammatory molecules. Again Rac is a component of the active nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. Therefore, by suppressing the geranylation of Rac, statins also attenuate NADPH oxidase-mediated production of reactive oxygen species (ROS). Phosphatidylinositol-3 (PI-3) kinase is known to activate Akt, the kinase that phosphorylates and stimulates endothelial nitric oxide synthase (eNOS). Interestingly, mevalonate is capable of inhibiting PI-3 kinase. Therefore, by reducing the concentration of mevalonate, statins also up-regulate eNOS-derived production of NO resulting in vasorelaxation. GTP = guanosine triphosphate; iNOS = inducible nitric oxide synthase; NO = nitric oxide.
According to Lamperti (Musanti and others 1993), plasma cholesterol concentrations in PD patients are lower than in controls. It has been shown by de Lau and others (2006) that higher serum levels of total cholesterol are associated with a significantly decreased risk of PD, particularly in women. Furthermore, the incidence of myocardial infarction and ischemic stroke is also lower in PD patients. In a study with only 124 participants, Huang and others (2007) have delineated that people with low levels of LDL cholesterol are more likely to have PD than people with high LDL levels. However, it is unclear from this study whether low LDL levels were present in study subjects before they were diagnosed with PD or if LDL levels decreased after they were diagnosed with PD. It is possible that low LDL cholesterol is a consequence rather than a cause. Statins specifically lower LDL cholesterol. However, most of the participants in this study (Huang and others 2007) did not take any statins, suggesting that the association between low LDL levels and PD exists independently from statin use. Most interestingly, the same study, when compared among statin users in control and PD groups, found a much higher percentage of statin users among controls than in PD subjects.
To add to this confusion, a more thorough Finnish study on nearly 25,000 men and women (Hu and others 2008) has demonstrated that adults younger than 55 years old may run an elevated risk of developing PD if they have been diagnosed with high cholesterol. Patients with the highest level of cholesterol were 86% more likely to develop PD than the participants with the lowest level of cholesterol (Hu and others 2008). Interestingly, this risk applies only to participants between the ages of 24 and 54 years. Therefore, the ambiguity regarding the cholesterol connection of PD moves on. It is possible that although high cholesterol facilitates the onset of the disease, once the disease sets in, cholesterol levels start decreasing because of nausea and vomiting, loss of appetite, constipation, and an overall degenerative process.
Modulation of PD-Related Signaling Events by Statins
Whether cholesterol has anything to do with PD or not, statins are fully capable of modulating many cholesterol-independent cellular signaling pathways, which are relevant to PD pathology.
Suppression of Proinflammatory Molecules
Although the disease mechanisms that cause PD are poorly understood, recent studies strongly support the role of inflammation in nigrostriatal degeneration in this disease. For example, microglial activation is evident in close proximity to damaged or dying dopaminergic neurons, and the CSF level of NO2− (nitrite), a metabolite of nitric oxide (NO), and the CNS level of inducible nitric oxide synthase (iNOS) are higher in patients with PD in comparison with a group of patients without dopaminergic dys function (Hunot and others 1996; Qureshi and others 1995). Consistently, the ablation of iNOS in mutant mice significantly attenuates MPTP neurotoxicity (Dehmer and others 2000). Similar to NO, a variety of proinflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), IL-6, eicosanoids, and other immune neurotoxins, are found in either CSF or affected brain regions in PD (Bessler and others 1999). Accordingly, transgenic mice carrying homozygous mutant alleles for both the TNF receptors, but not the individual receptors, are completely protected against MPTP (Sriram and others 2006). Recently, we (Ghosh and others 2007) have demonstrated that nuclear factor–κB (NF-κB), a transcription factor required for the transcription of most proinflammatory molecules, is activated in the SNpc of PD patients and MPTP-intoxicated mice and that selective inhibition of NF-κB in mice by NEMO-binding domain (NBD) peptides protects dopaminergic neurons from MPTP toxicity. According to Hunot and others (1997), the NF-κB-dependent apoptogenic transduction pathway in dopaminergic neurons may play a role in neuronal death in PD. Consistent with these findings, Saijo and others (2009) have demonstrated that Nurr1, a nuclear orphan receptor, suppresses glial activation by docking to NF-κB-p65 on target inflammatory gene promoters and protects dopaminergic neurons in vivo in the nigra. Taken together, controlling inflammation may be an important step to protect dopaminergic neurons in PD and its animal model.
The idea of investigating the effect of statins on the expression of iNOS and proinflammatory cytokines came to us from the fact that statins modulate isoprenoids, which are known to play an important role in the activation of small G proteins such as Ras and Rac. Accordingly, Pahan and others (1997) have shown that lovastatin inhibits the activation of NF-κB and the expression of iNOS and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in lipopolysaccharide (LPS)–stimulated rat primary astrocytes. In fact, this finding has revolutionized statin research. Nowadays, statin drugs are being widely considered as potential therapeutic agents against various neuroinflammatory and neurodegenerative disorders. Because lovastatin inhibits HMG-CoA reductase, both mevalonate and farnesyl pyrophosphate (FPP) are capable of reversing the inhibitory effect of lovastatin on the expression of iNOS and the activation of NF-κB (Pahan and others 1997). However, addition of ubiquinone and cholesterol to astrocytes does not prevent the inhibitory effect of lovastatin. These results suggest that depletion of FPP, rather than end products of the mevalonate pathway, is responsible for the observed inhibitory effect of lovastatin on the expression of iNOS.
Suppression of LPS-induced activation of NF-κB and expression of iNOS in glial cells by farnesyltransferase inhibitors (Pahan and others 2000; Pahan and others 1998) suggest an important role of farnesylation reaction in the regulation of the iNOS gene. Consistent with a role of farnesylation in the activation of p21Ras, a dominant-negative mutant of p21Ras (S17N) also attenuated activation of NF-κB and expression of iNOS in rat and human primary astrocytes (Pahan and others 2000). Recently, we have demonstrated that Parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridinium ion (MPP+) induces the activation of p21Ras in microglia within minutes of stimulation and that simvastatin suppresses MPP+-induced activation of p21Ras and NF-κB (Ghosh and others 2007). Similarly, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine MPTP intoxication also leads to the activation of p21Ras within the SNpc, and simvastatin strongly inhibits the activation of p21Ras and the expression of proinflammatory molecules in the SNpc of MPTP-intoxicated mice (Ghosh and others 2009). According to Selley (2005), simvastatin also inhibits the formation of 3-nitrotyrosine in striatal proteins in MPTP-treated mice. Although simvastatin has no effect on cholesterol concentrations in the plasma or in the striatum, simvastatin inhibits the production of TNF-α and nitric oxide in cultured rat microglia stimulated by LPS (Selley 2005). Statins also block IFN-γ-inducible (Crisby 2003) and constitutive (Muczynski and others 2003) transcription of the major histocompatibility complex (MHC) class II transactivator (CIITA), which regulates nearly all MHC class II gene expression. Together, these studies suggest that statins are capable of suppressing the expression of proinflammatory molecules in glial cells via attenuation of small G proteins and that this antiinflammatory effect of statins may be beneficial for PD patients.
Stimulation of Endothelial Nitric Oxide Synthase
With increase in age, endothelial function slowly deteriorates due to decreased synthesis of endothelium-derived NO. Although there are no data available on the role of endothelial NOS (eNOS) in PD, muscle rigidity, frozen hands, and expressionless face are common features of PD. Therefore, in general, eNOS-derived NO may be beneficial for PD via increased blood flow and endothelium relaxation (Dimmeler and Zeiher 1999). Although statins inhibit the expression of iNOS, fortunately these drugs have been found to stimulate eNOS-derived NO production (Hernandez-Perera and others 1998). Interestingly, this endothelium-favoring function of statins is independent of cholesterol lowering (Hernandez-Perera and others 1998). Reversal of statin-induced eNOS up-regulation by geranylgeranyl pyrophosphate, but not farnesyl pyrophosphate, suggests that Rac/Rho, but not Ras, plays a role in the regulation of eNOS. To understand the mechanism further, Fulton and others (1999) have shown that activated protein kinase B (Akt) phosphorylates eNOS and increases the production of NO. On the other hand, mevalonate, an intermediate of the cholesterol-biosynthetic pathway, inhibits phosphatidylinositol-3 (PI-3) kinase and thereby attenuates the activation of Akt (Skaletz-Rorowski and others 2003). Because statins are known to lower the concentration of mevalonate via inhibition of HMG-CoA reductase, statins favor the up-regulation of eNOS via suppression of mevalonate and thereby activation of the PI-3 kinase–Akt pathway. Furthermore, according to Feron and others (2001), atorvastatin increases eNOS-derived NO production by decreasing the expression of caveolin-1, a negative regulator of eNOS. Therefore, via multiple mechanisms, statins may up-regulate eNOS and improve endothelial functions in PD patients.
Inhibition of Oxidative Stress
Oxidative stress is a hallmark of many human disorders, and PD is also not an exception. It has been found that several inflammatory and degenerative stimuli induce the production of reactive oxygen species (ROS) via the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a five-subunit protein that generates superoxide from molecular oxygen. It is composed of two membrane-bound subunits, gp91phox and p22phox, and at least two cytosolic subunits, p47phox and p67phox. Phosphorylation of p47phox results in the translocation of the p47phox–p67phox complex to the membrane, where it interacts with gp91phox and p22phox. This complex becomes complete and active after the participation of Rac, a small G protein, which associates itself with p67phox and gp91phox (Ago and others 1999; Bokoch and Knaus 2003). As shown in Figure 1 and described above, statins inhibit geranylgeranylation of Rac and thereby attenuate NADPH oxidase-mediated generation of superoxide. It has been shown that NADPH oxidase is up-regulated in the nigra of MPTP-intoxicated mice (Gao and others 2003a; Wu and others 2003). Accordingly, MPTP is unable to damage dopaminergic neurons in the SNpc of gp91phox (−/−) mice (Gao and others 2003a; Wu and others 2003), highlighting the importance of NADPH oxidase in the loss of dopaminergic neurons. Therefore, statins may reduce NADPH oxidase-mediated production of ROS in the nigra of MPTP-intoxicated mice and PD patients.
Attenuation of α-synuclein Aggregation
One of the major features of PD is the deposition of Lewy bodies in the SNpc. These intracellular deposits are very rich in one 140-amino acid protein called α-synuclein (α-syn). Several lines of evidence in recent years clearly suggest that overexpression and oligomerization of α-syn are directly related to the toxicity in the nigrostriatum. First, transgenic mice overexpressing human α-syn display Lewy body–like inclusions, dopaminergic loss, and motor deficits (Masliah and others 2000). Second, lentiviral-based expression of human α-syn in rat substantia nigra (SN) also resulted in selective dopaminergic toxicity with nonfibrillar inclusion (Lo Bianco and others 2002). Third, neuronal cell loss is directly related to increased levels of α-syn because down-regulation of α-syn expression in the SNpc in a rat model of PD, using viral vector delivery of α-syn ribozyme, rescues dopaminergic cells from cell death (Hayashita-Kinoh and others 2006). Fourth, increased expression of α-syn mRNA and protein has been observed in other models of PD, including MPTP and 1BnTIQ treatment (Purisai and others 2005; Shavali and others 2004). Fifth, parkinsonian neurotoxin MPTP fails to kill dopaminergic neurons in mice deficient in α-syn (Dauer and others 2002).
Although aggregation of α-syn is believed to play a critical role in the pathogenesis of disorders such as dementia with Lewy bodies and PD, the function of α-syn remains unclear. Recently, it has been shown that α-syn is involved in synaptic vesicle trafficking, probably via lipid binding. Accordingly, aggregation of α-syn abrogates its normal function and initiates its neurodegenerative function. Interestingly, cholesterol is linked to α-syn aggregation. For example, Bosco and others (2006) investigated if cholesterol synthesis by statins might interfere with α-syn accumulation in cultured neurons. They have delineated that concentrations of oxidized cholesterol metabolites are elevated in the brains of PD patients and that oxidized cholesterol accelerates α-syn aggregation. Interestingly, all three statins (lovastatin, simvastatin, and pravastatin) tested by Bar-On and others (2008) markedly reduce the levels of α-syn accumulation in the detergent-insoluble fraction of the transfected neuronal cell line and primary human neurons. Conversely, supplementation of the media with cholesterol increases α-syn aggregation in detergent-insoluble fractions of transfected neurons and is accompanied by reduced neurite outgrowth. In another study by Masliah and colleagues (Koob and others 2009), lovastatin has been found to ameliorate α-syn accumulation and aggregation in transgenic mouse models of α-synucleinopathies. Therefore, reducing cholesterol levels with statins may block α-syn aggregation in PD.
It has been shown that mutation of α-syn facilitates its aggregation; therefore, mutated α-syn exhibits greater toxicity than normal α-syn (Stefanis and others 2001). Accordingly, mutated α-syn is a more potent activator of glial cells and an inducer of proinflammatory molecules than wild-type α-syn (Klegeris and others 2006). As mentioned above, statins inhibit the expression of proinflammatory molecules from activated glial cells. Therefore, although it has not been tested, statins should also suppress mutated α-syn-mediated glial activation independent of the involvement of cholesterol. Taken together, in PD, statins may inhibit the aggregation of α-syn via regulation of cholesterol and attenuate mutated α-syn-mediated glial activation independent of cholesterol.
Modulation of Adaptive Immunity
Recent studies have demonstrated that T cell responses elicited during the course of MPTP intoxication lead to accelerated neurodegeneration (Benner and others 2008; Benner and others 2004). Accordingly, enhanced T cell response has also been documented in the SNpc of postmortem PD brains. Although effector T cells may aggravate the disease process, regulatory T cells (Treg) are usually protective, and it has been found that Tregs protect against MPTP-induced dopaminergic degeneration (Reynolds and others 2007). Using vasoactive intestinal peptide (VIP), a neuropeptide known to induce Treg responses (Gonzalez-Rey and others 2006), Reynolds and colleagues (Kosloski and others 2010) have shown that replacement of functional Treg within the nitrated-α-syn splenocyte mixture results in neuroprotection and that this neuroprotection is modulated through Th17 mechanisms. Recently, we have delineated that NO produced from antigen-presenting cells (APCs) during antigen priming plays an important role in the regulation of Tregs (Brahmachari and Pahan 2009, 2010). Foxp3 appears to function as the master regulator in the development and function of Tregs. Although excess NO inhibits the mRNA expression of Foxp3 via the guanylate cyclase–cGMP pathway, either scavenging NO by carboxy-PTIO or inhibiting the activity of iNOS by L-NIL increases the expression of Foxp3 (Brahmachari and Pahan 2010). We have also found that simvastatin inhibits the production of NO and thereby up-regulates Foxp3 (Brahmachari and Pahan 2010). Therefore, via enriching Tregs, simvastatin may help in the protection of dopaminergic neurons in the SNpc.
Attenuating Dyskinesia
To date, levodopa (L-DOPA), which is converted to dopamine in the brain, remains the gold standard for the treatment of PD. Although in many patients, L-DOPA significantly improves the quality of life for many years, chronic DA replacement therapy with L-DOPA in PD results in motor complications known as L-DOPA-induced dyskinesia, which is associated with a sequence of events, including pulsatile stimulation of DA receptors, downstream changes in proteins and genes, and abnormalities in non-DAergic transmitter systems (Berthet and Bezard 2009). All these biochemical processes combine to produce alterations in the neuronal firing patterns that signal between the basal ganglia and the cortex. Although dyskinesia is not painful, it is a very distressing side effect of L-DOPA in which rapid and repetitive motions affect the limbs, face, tongue, mouth, and neck.
Dyskinesia has been modeled in rodents as well, which has been called rodent analog abnormal involuntary movements (AIMs). AIMs have been associated consistently with an activation of the Ras-extracellular signal-regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) signaling pathway (Gerfen and others 2002; Westin and others 2007). They have also delineated that inhibitors of the MAPK signaling cascade block the aberrant supersensitive response of direct striatal pathway neurons, suggesting a possible therapeutic efficacy of MAPK inhibitors in L-DOPA-induced dyskinesia. Because statins inhibit isoprenylation of Ras, these drugs are also capable of suppressing the activation of ERK1/2 MAP kinases (Fig. 1). Consistently, Schuster and others (2008) have shown that lovastatin treatment reduces L-DOPA-induced AIM incidence and severity in the 6-OH-DA rat model of PD. Such lower AIM severity is associated with a decrease in an L-DOPA-induced increase in striatal pERK1 and ΔFosB levels and θ/α oscillations of substantia nigra pas reticulata (SNr) neurons, as well as a normalization of SNr firing frequency. Those results suggest that statins may be a treatment option for managing L-DOPA-induced dyskinesia in PD.
Reducing Depression
Although depression is not a clinical marker of PD, studies indicate that almost half of all PD patients experience depression at some point in their illness. Therefore, reducing depression may improve the lifestyle of PD patients. A couple of studies demonstrate that long-term use of statin leads to reduced risk of depression in patients with coronary artery disease (Yang and others 2003; Young- Xu and others 2003). They have demonstrated that risk of depression was 60% less in individuals using statins than in hyperlipidemic individuals not using lipid-lowering drugs. However, this beneficial effect of statins in patients with coronary heart disease could be due to the “feel-good” effect of statins through improved quality of life due to a decreased incidence of cardiovascular events. Although molecular mechanisms leading to depression are poorly understood, it is known that NO is a potent antidepressant. Although NO produced in an excessive amount from iNOS is toxic, NO produced in a physiological amount from cNOS or eNOS has many beneficial functions. As mentioned above, statins inhibit iNOS via suppression of the p21Ras–NF-κB pathway and stimulate eNOS via activation of the PI-3 kinase–Akt pathway and suppression of caveolin-1. Therefore, via increased NO production, statins may reduce depression in PD patients. On the other hand, as reported recently (Shrivastava and others 2010), due to chronic cholesterol depletion, statins may also increase depression via impairment of serotonin receptors.
Should Statins Be Tried in PD?
Although current state of knowledge goes both for and against a clinical trial of statins in PD, in our view, the evidence in favor of a clinical trial has more weight than that against it. In the following discussion, we have tried to analyze such information.
Evidence in Favor of a Clinical Trial
a) According to Hu and others (2008), adults younger than age 55 years have a greater risk of developing PD if they are diagnosed with hypercholesterolemia, suggesting that high cholesterol may be a predisposing factor for PD. Therefore, we suggest that statins may be beneficial for PD.
b) The incidence of PD is lower among statin users. Using the Decision Support System database of the US Veterans Affairs medical system, Wolozin and others (2007) have demonstrated that simvastatin is linked to reduced incidence of PD. In contrast, atorvastatin is associated with a modest, but insignificant, reduction in the incidence of PD, and lovastatin has no impact on the incidence. According to Wahner and others (2008), all statins are inversely associated with PD except for pravastatin. These are probably due to the fact that simvastatin is the most potent statin drug in terms of crossing the blood-brain barrier (BBB) and that pravastatin is unable to cross the BBB (Vuletic and others 2006).
c) Huang and others (2007) compared statin users in control and PD groups and found a much higher percentage of statin users among controls than in PD subjects. According to Mutez and others (2009), statins are also associated with delayed onset and slower course of PD. In a mixed linear model, the increase in levodopa-equivalent daily dose over two years was significantly smaller in the group taking a statin than in the matched control group.
d) At a dose of 1 mg/kg body weight/day, which is equivalent to the Food and Drug Administration–approved dose in adults, simvastatin enters into the nigra, inhibits the activation of p21Ras, suppresses the activation of NF-κB, attenuates the expression of proinflammatory molecules, protects dopaminergic neurons, restores striatal fibers and DA, and improves locomotor functions in an acute MPTP model of PD (Ghosh and others 2009). Selley (2005) has also reported that simvastatin prevents MPTP-induced striatal dopamine depletion and protein tyrosine nitration in mice.
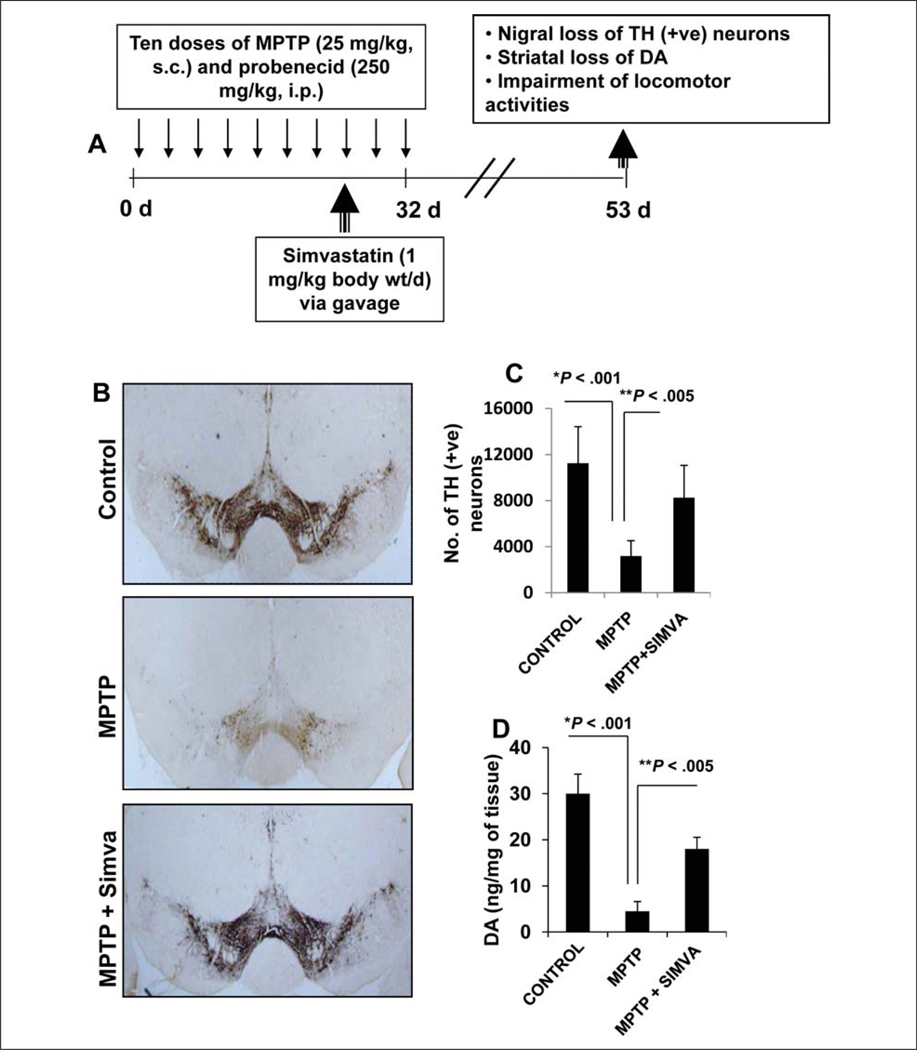
e) Although the acute MPTP model is helpful for quick drug screening, elucidating molecular mechanisms, and determining the interaction between drug and MPTP, effects of acute intoxication reverse over time. Therefore, recently, a chronic intoxicated model has been described (Meredith and others 2008) in which significant loss of dopaminergic neurons is seen even six months after MPTP intoxication. We examined if simvastatin was capable of protecting neurons in a chronic model. As expected, chronic MPTP intoxication led to marked loss of tyrosine hydroxylase (TH)–positive neurons and striatal neurotransmitters (Fig. 2) and a decrease in rotorod performance, total distance, horizontal activity, stereotypy, rearing, stereotypy time, clockwise revolutions, and anticlockwise revolutions (Fig. 3). On the other hand, MPTP increased the rest time in a chronic MPTP model of PD (Fig. 3). We initiated simvastatin treatment from the late chronic phase and continued for three weeks after the last injection of MPTP (Fig. 2A). Consistent with that observed in an acute model (Ghosh and others 2009), simvastatin, in this instance, also significantly protected nigral dopaminergic neurons and striatal dopamine (Fig. 2) and improved locomotor activities (Fig. 3). Although mice results are not always translated to humans, these results from acute and chronic MPTP models suggest that simvastatin may be tried for therapeutic intervention in PD.
Figure 2.
Simvastatin protects nigral dopaminergic neurons in chronic MPTP mouse model of Parkinson disease (PD). (A) In this model, six- to eight-week-old male C57BL/6 mice received 10 injections of MPTP (subcutaneously [s.c.]; 25 mg/kg body weight) together with 10 injections of probenecid (intraperitoneally [i.p.]; 250 mg/kg body weight) for five weeks. Animal maintaining and experiments were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Rush University Medical Center. Control group of mice received only saline. One group of mice was treated with simvastatin (1 mg/kg body weight) daily via gavage from the eighth injection of MPTP and continued for 4 weeks thereafter. (B) Animals were sacrificed and nigral sections were immunostained for tyrosine hydroxylase (TH), and (C) TH-positive neurons in the SNpc were counted by the STEREO INVESTIGATOR software (MicroBrightfield, Williston, VT) by using an optical fractionator. (D) The level of dopamine (DA) in striatum was measured by high-performance liquid chromatography (HPLC). Data are means ± SEM of five mice per group.
Figure 3.
Simvastatin improves motor functions in the chronic MPTP mouse model of Parkinson disease (PD). Mice receiving saline (n = 5), MPTP (n = 5), and simvastatin (n = 5) (1 mg/kg body weight per day) were tested for different motor tasks—(A) rotarod, (B) total distance, (C) horizontal activity, (D) stereotypy counts, (E) rearing, (F) stereotypy time, (G) rest time, (H) clockwise revolutions, and (I) anticlockwise revolutions—three weeks after the last injection of MPTP. Data are means ± SEM of five mice per group. *P < .05 vs. MPTP.
Evidence against a Clinical Trial
a) Huang and others (2007) have compared the incidence of PD in 236 patients with different levels of LDL cholesterol. Those with the lowest levels were three and a half times more likely to have the disease than patients with higher levels. However, this report on a small number of patients with PD does no more than suggest there might be a statistical association between low levels of LDL cholesterol and PD.
b) According to de Lau and others (2006), cholesterol concentration is low in PD patients. They have indicated that higher serum levels of total cholesterol are associated with decreased risk of PD, particularly in women. From this angle, simvastatin may lower cholesterol further and thereby aggravate PD symptoms.
c) Kreisler and others (2007) have shown that both simvastatin and atorvastatin have deleterious effects on the nigrostriatum in MPTP-intoxicated mice. However, this study used a concentration of simvastatin that is 10 to 40 times higher than a regular human dose, and treatment began five or seven days before MPTP intoxication. When we repeated this experiment, we (Ghosh and others 2009) also found that simvastatin at a high dose (40 mg/kg body weight/day) was deleterious for the nigrostriatum.
Conclusion
Over the past decade, scientists have accomplished significant progress in unfolding newer aspects of statins. Biological functions of statins are no longer restricted to only lipid lowering; these wonder drugs function to modulate intracellular signaling pathways, inhibit inflammation, suppress the production of ROS, and modulate adaptive immunity. Therefore, statins are currently being considered as possible therapeutics for several neurodegenerative disorders, including PD (Wang and others 2010; Wood and others 2010). For PD, statins can protect dopaminergic neurons, inhibit α-syn aggregation, and attenuate L-DOPA-induced dyskinesia. Accordingly, although many PD patients take simvastatin for cholesterol and other problems, some unresolved issues raise doubts over the widespread use of statins in PD. It has not been shown that higher neuronal cholesterol increases Lewy body formation or alters the expression of PD-related genes. It is also not known whether neurons in PD have more cholesterol than control neurons. Cholesterol is a major component of neuronal cell membranes and synapses and is essential for maintaining their structure and function.
Therefore, the real challenge is to maintain cholesterol homeostasis after the use of statins to minimize side effects in PD patients who do not suffer from cholesterol-related problems. In these conditions, targeting of simvastatin-sensitive biological processes that are responsible for the possible amelioration of PD pathology by a specific drug or molecule rather than statin itself may be a better option. For example, inhibitors of farnesyltransferase or geranylgeranyltransferase, which do not alter cholesterol levels while attenuating inflammation and oxidative stress, hallmarks of PD pathogenesis, may be considered for the treatment of PD patients who do not suffer from cholesterolrelated problems. In fact, recently, we have demonstrated that farnesyltransferase inhibitor II alone is capable of restoring dopamine in MPTP-intoxicated mice (Ghosh and others 2007). While considering a replacement for statins, we must remember that statins are multifunctional and that these drugs exhibit many functions independent of cholesterol. At this point, we do not know whether farnesyltransferase inhibitors mimic most of the cholesterol-independent effects of statins.
Acknowledgments
Financial Disclosure/Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by grants from the National Institutes of Health (NS39940, NS064564, and NS071479) and the Michael J. Fox Foundation for Parkinson Research.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox): triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274(47):33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- Bar-On P, Crews L, Koob AO, Mizuno H, Adame A, Spencer B, et al. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem. 2008;105(5):1656–1667. doi: 10.1111/j.1471-4159.2008.05254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3(1):e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101(25):9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A, Bezard E. Dopamine receptors and L-dopa-induced dyskinesia. Parkinsonism Relat Disord. 2009;15 suppl 4:S8–S12. doi: 10.1016/S1353-8020(09)70827-2. [DOI] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson’s disease. Biomed Pharmacother. 1999;53(3):141–145. doi: 10.1016/S0753-3322(99)80079-1. [DOI] [PubMed] [Google Scholar]
- Bilheimer DW, Grundy SM, Brown MS, Goldstein JL. Mevinolin stimulates receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Trans Assoc Am Physicians. 1983;96:1–9. [PubMed] [Google Scholar]
- Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28(9):502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P, Jr, et al. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol. 2006;2(5):249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol. 2010;184(4):1799–1809. doi: 10.4049/jimmunol.0804394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J Immunol. 2009;183(3):2045–2058. doi: 10.4049/jimmunol.0800276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21(5):505–517. [PubMed] [Google Scholar]
- Crisby M. Modulation of the inflammatory process by statins. Drugs Today (Barc) 2003;39(2):137–143. doi: 10.1358/dot.2003.39.2.740209. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, et al. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99(22):14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson’s disease. Am J Epidemiol. 2006;164(10):998–1002. doi: 10.1093/aje/kwj283. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74(5):2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Nitric oxide: an endothelial cell survival factor. Cell Death Differ. 1999;6(10):964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo) 1976;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- Endo A, Tsujita Y, Kuroda M, Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur J Biochem. 1977;77(1):31–36. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103(1):113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003a;17(13):1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003b;24(8):395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22(12):5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104(47):18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2009;29(43):13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107(9):3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashita-Kinoh H, Yamada M, Yokota T, Mizuno Y, Mochizuki H. Down-regulation of alpha-synuclein expression can rescue dopaminergic cells from cell death in the substantia nigra of Parkinson’s disease rat model. Biochem Biophys Res Commun. 2006;341(4):1088–1095. doi: 10.1016/j.bbrc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101(12):2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Antikainen R, Jousilahti P, Kivipelto M, Tuomilehto J. Total cholesterol and the risk of Parkinson disease. Neurology. 2008;70(21):1972–1979. doi: 10.1212/01.wnl.0000312511.62699.a8. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen H, Miller WC, Mailman RB, Woodard JL, Chen PC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov Disord. 2007;22(3):377–381. doi: 10.1002/mds.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Chen PC, Poole C. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62(12):2198–2202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, et al. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience. 1996;72(2):355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci U S A. 1997;94(14):7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20(12):2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- Koob AO, Ubhi K, Paulsson JF, Kelly J, Rockenstein E, Mante M, et al. Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Exp Neurol. 2009;221(2):267–274. doi: 10.1016/j.expneurol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosloski LM, Ha DM, Hutter JA, Stone DK, Pichler MR, Reynolds AD, et al. Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases. J Neurochem. 2010;114(5):1261–1276. doi: 10.1111/j.1471-4159.2010.06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler A, Gele P, Wiart JF, Lhermitte M, Destee A, Bordet R. Lipid-lowering drugs in the MPTP mouse model of Parkinson’s disease: fenofibrate has a neuroprotective effect, whereas bezafibrate and HMG-CoA reductase inhibitors do not. Brain Res. 2007;1135(1):77–84. doi: 10.1016/j.brainres.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. Alpha-synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2002;99(16):10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord. 2008;14 suppl 2:S112–S115. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J Neurosci Res. 2003;72(2):141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- Muczynski KA, Ekle DM, Coder DM, Anderson SK. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14(5):1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- Musanti R, Parati E, Lamperti E, Ghiselli G. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem Med Metab Biol. 1993;49(2):133–142. doi: 10.1006/bmmb.1993.1016. [DOI] [PubMed] [Google Scholar]
- Mutez E, Duhamel A, Defebvre L, Bordet R, Destee A, Kreisler A. Lipid-lowering drugs are associated with delayed onset and slower course of Parkinson’s disease. Pharmacol Res. 2009;60(1):41–45. doi: 10.1016/j.phrs.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Pahan K, Liu X, McKinney MJ, Wood C, Sheikh FG, Raymond JR. Expression of a dominant-negative mutant of p21(ras) inhibits induction of nitric oxide synthase and activation of nuclear factor-kappaB in primary astrocytes. J Neurochem. 2000;74(6):2288–2295. doi: 10.1046/j.1471-4159.2000.0742288.x. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Khan M, Namboodiri AM, Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1998;273(5):2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100(11):2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purisai MG, McCormack AL, Langston WJ, Johnston LC, Di Monte DA. Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis. 2005;20(3):898–906. doi: 10.1016/j.nbd.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Qureshi GA, Baig S, Bednar I, Sodersten P, Forsberg G, Siden A. Increased cerebrospinal fluid concentration of nitrite in Parkinson’s disease. Neuroreport. 1995;6(12):1642–1644. doi: 10.1097/00001756-199508000-00013. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007;82(5):1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S, Nadjar A, Guo JT, Li Q, Ittrich C, Hengerer B, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson’s disease. J Neurosci. 2008;28(17):4311–4316. doi: 10.1523/JNEUROSCI.4720-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley ML. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005;1037(1–2):1–6. doi: 10.1016/j.brainres.2004.02.083. [DOI] [PubMed] [Google Scholar]
- Shavali S, Carlson EC, Swinscoe JC, Ebadi M. 1-Benzyl-1,2,3,4-tetrahydroisoquinoline, a Parkinsonism-inducing endogenous toxin, increases alpha-synuclein expression and causes nuclear damage in human dopaminergic cells. J Neurosci Res. 2004;76(4):563–571. doi: 10.1002/jnr.20082. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin(1A) receptors. Biochemistry. 2010;49(26):5426–5435. doi: 10.1021/bi100276b. [DOI] [PubMed] [Google Scholar]
- Skaletz-Rorowski A, Lutchman M, Kureishi Y, Lefer DJ, Faust JR, Walsh K. HMG-CoA reductase inhibitors promote cholesterol-dependent Akt/PKB translocation to membrane domains in endothelial cells. Cardiovasc Res. 2003;57(1):253–264. doi: 10.1016/s0008-6363(02)00618-1. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J. 2006;20(6):670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21(24):9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, et al. Pathogenic role of glial cells in Parkinson’s disease. Mov Disord. 2003;18(2):121–129. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Liang K. Effects of HMG-CoA reductase inhibition on hepatic expression of key cholesterol-regulatory enzymes and receptors in nephrotic syndrome. Am J Nephrol. 2004;24(6):606–613. doi: 10.1159/000082510. [DOI] [PubMed] [Google Scholar]
- Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson’s disease. Nat Med. 2004;10 suppl:S58–S62. doi: 10.1038/nm1068. [DOI] [PubMed] [Google Scholar]
- Vuletic S, Riekse RG, Marcovina SM, Peskind ER, Hazzard WR, Albers JJ. Statins of different brain penetrability differentially affect CSF PLTP activity. Dement Geriatr Cogn Disord. 2006;22(5–6):392–398. doi: 10.1159/000095679. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Statin use and the risk of Parkinson disease. Neurology. 2008;70(16, pt 2):1418–1422. doi: 10.1212/01.wnl.0000286942.14552.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yan J, Chen X, Li J, Yang Y, Weng J, et al. Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol Apr. 2010;18 doi: 10.1016/j.expneurol.2010.04.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62(7):800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WG, Eckert GP, Igbavboa U, Muller WE. Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci. 2010;1199:69–76. doi: 10.1111/j.1749-6632.2009.05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100(10):6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926–1932. doi: 10.1001/archinte.163.16.1926. [DOI] [PubMed] [Google Scholar]
- Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol. 2003;42(4):690–697. doi: 10.1016/S0735-1097(03)00785-X. [DOI] [PMC free article] [PubMed] [Google Scholar]