Abstract
Lysophosphatidylinositol (LPI) was recently shown to act both as an extracellular mediator binding to G protein-coupled receptor 55 (GPR55) and as an intracellular messenger directly affecting a number of ion channels including large-conductance Ca2+ and voltage-gated potassium (BKCa) channels. Here, we explored the effect of LPI on intermediate-conductance Ca2+-activated K+ (IKCa) channels using excised inside-out patches from endothelial cells. The functional expression of IKCa was confirmed by the charybdotoxin- and TRAM-34-sensitive hyperpolarization to histamine and ATP. Moreover, the presence of single IKCa channels with a slope conductance of 39 pS in symmetric K+ gradient was directly confirmed in inside-out patches. When cytosolically applied in the range of concentrations of 0.3–10 μM, which are well below the herein determined critical micelle concentration of approximately 30 μM, LPI potentiated the IKCa single-channel activity in a concentration-dependent manner, while single-channel current amplitude was not affected. In the whole-cell configuration, LPI in the pipette was found to facilitate membrane hyperpolarization in response to low (0.5 μM) histamine concentrations in a TRAM-34-sensitive manner. These results demonstrate a so far not-described receptor-independent effect of LPI on the IKCa single-channel activity of endothelial cells, thus, highlighting LPI as a potent intracellular messenger capable of modulating electrical responses in the vasculature.
Keywords: IKCa channel, Cytosolic free Ca2+ elevation, Endothelial cells, Hyperpolarization, Membrane potential, Lipid mediators, Lysophosphatidylinositol
Introduction
The discovery that lysophosphatidylinositol (LPI) acts as an endogenous agonist of the orphan receptor GPR55 [27] and subsequently triggers intracellular Ca2+ signaling [16, 27, 35], thus, influencing many physiological and pathophysiological processes [5, 8, 18], fueled great interest on the molecular mechanisms of action of this lysophospholipid (LPL). We showed recently that besides the GPR55-dependent signaling, LPI exerts a number of receptor-independent effects including activation of non-selective cation channels, inhibition of Na+–K+ ATPase [3], and bidirectional modulation of large-conductance Ca2+-activated K+ channels (BKCa) [2], pointing to LPI as a powerful diverse modulator of cell function.
In the vasculature, KCa channels are expressed both in vascular smooth muscle cells and endothelial cells. In smooth muscle cells, which predominantly express the BKCa channels, their activation counteracts depolarization during the development of myogenic tone by mediating hyperpolarizing transient outward currents. In endothelial cells, which predominantly express the IKCa and SKCa channels [15, 20, 26], K+ channel activation yields membrane hyperpolarization that increases the driving force for Ca2+ entry through non-voltage-gated Ca2+ channels and, thus, essentially contribute to endothelium-dependent relaxation by the Ca2+-dependent production of either nitric oxide or the hyperpolarizing factor(s) [17]. Furthermore, endothelial hyperpolarization is transmitted to underlying smooth muscle cells directly causing endothelium-dependent hyperpolarization and relaxation [6, 15]. Recently, we reported that LPI directly bidirectionally modulates the BKCa channel activity in endothelial cells [2]. In this study, the stimulatory effect of intracellular LPI on histamine-triggered hyperpolarization of the endothelial cell plasma membrane was not entirely inhibited by iberiotoxin, thus, pointing to an additional target for LPI, other than BKCa channels. Because IKCa channels are the most promising candidate for mediating endothelial hyperpolarization, the present study was aimed to identify whether or not IKCa channels are a target for LPI in endothelial cells. Moreover, it was tested whether or not the putative effect of LPI on the IKCa channels underpins the stimulatory effect of this LPL on membrane hyperpolarization in response to physiological cell stimulation with an inositol(1,4,5)trisphosphate (IP3)-generating agonist.
Materials and methods
Cell culture
The human umbilical vein-derived endothelial cell line, EA.hy926 [10] at passage >45 was grown in DMEM containing 10% FCS and 1% HAT (5 mM hypoxanthin, 20 μM aminopterin, 0.8 mM thimidine) and were maintained in an incubator at 37°C in 5% CO2 atmosphere. For experiments, cells were plated on glass coverslips.
Patch-clamp recordings
All patch-clamp experiments were performed at room temperature with the use of a water hydraulic micromanipulator (WR-6, Narishige, Japan). Patch pipettes were pulled from glass capillaries using a Narishige puller (Narishige Co. Ltd, Tokyo, Japan), fire polished, and had a resistance of 3–5 MΩ for whole-cell recordings and 5–7 MΩ for single-channel recordings. Currents were recorded using a patch-clamp amplifier (EPC7, List Electronics, Darmstadt, Germany) at a bandwidth of 3 kHz. The signals obtained were low pass filtered at 1 kHz using an eight-pole Bessel filter (Frequency Devices), and digitized with a sample rate of 10 kHz using a Digidata 1200A A/D converter (Axon Instruments, Foster City, CA, USA). Data collection and analysis were performed using Clampex and Clampfit software of pClamp (V9.0, Axon Instruments). Single-channel transitions were identified on the basis of the half-amplitude threshold criteria, ignoring brief transitions that lasted less than 0.3 ms. Single-channel activity was expressed as NPo, where N represents the number of functional ion channels in the membrane patch and Po represents the open channel probability determined from idealized traces. NPo was obtained from ≥20 s of continuous recording under each experimental condition and the effect of LPI was estimated after 3 min of continuous exposure to LPI-containing solution.
Single-channel recordings were obtained from excised inside-out membrane patches in symmetrical solutions using the patch-clamp technique. The pipettes were filled with (in millimolar) 140 KCl, 10 HEPES, 1 MgCl2, 5 EGTA, and 4,931 CaCl2 with pH 7.2 by adding KOH (i.e., 10 μM free Ca2+, G. Droogmans, Leuven, Belgium; ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/cabuf.zip). Cells were superperfused with a bath solution containing (in millimolar) 140 NaCl, 5 KCl, 1.2 MgCl2, 10 HEPES, 10 glucose, and 2.4 CaCl2. Following gigaseal formation, bath solution was switched to the following (in millimolar) 140 KCl, 10 HEPES, 1 MgCl2, 5 EGTA, and a desired free Ca2+ concentration which was adjusted by adding different amounts of CaCl2 calculated by the program CaBuf. The pH was adjusted to 7.2 by adding KOH. In order to decrease the activity of BKCa channels, which are predominantly expressed in this cell line, the IKCa single-channel activity was recorded mainly at Vm = −80 mV.
For whole-cell recordings, the pipette solution contained (in millimolar) 100 K-aspartate, 40 KCl, 1 MgCl2, 10 HEPES, 5 EGTA, and a free [Ca2+] was adjusted to 100 nM by adding 1.924 CaCl2 calculated by the program CaBuf. Recordings were performed in high Na+ solution stated above.
Determination of critical micelle concentration
Critical micelle concentration (CMC) was determined by two methods: the capillary rise method, which is based on alterations in surface tension [32]. When capillary rise method was employed, LPI dilutions (0.1–100 μM) in the high K+ bath solution used for single-channel recordings were prepared in eppendorf vials. A clean capillary tube was immersed in each sample and the solution height in the immersed glass capillary tube was measured. A new capillary tube was used for each measurement. The measurement was repeated four to 18 times under specific condition. The values were normalized to a solution height without LPI, averaged, and plotted against LPI concentration in log scale. The CMC was estimated by the diminishing capillary height in the tube due to a loss of surface tension with increased concentrations of LPI solutions.
In addition, the critical micelle concentration value for LPI was also estimated using the fluorescence probe 1,6 diphenyl 1,3,5-hexatriene (DPH) as described previously [4, 12]. Briefly, 0.25 μM DPH dissolved in ethanol was added to a plastic cuvette containing 140 mM KCl solution used for single-channel recordings. Fluorescence measurements were carried out on a Hitachi F4500 fluorescence spectrophotometer, setting the excitation at 358 nm, and recording fluorescence emission at 430 nm. Increasing amounts of LPI (0.1–100 μM) were added during recording. The rise in fluorescence in response to LPI addition was indicative of the phase transition from monomeric to micellar state [4].
Statistics
Analysis of variance was performed, and statistical significance was evaluated using Scheffe's post hoc F test of the Prism 5 software for Windows (GraphPad Software, Avenida de la Playa, CA, USA). Level of significance was defined as P < 0.05.
Results
Identification of the IKCa as functional Ca2+-activated K+ channels in endothelial cells
To verify the functional engagement of the IKCa as well as the BKCa channels in the agonist-triggered membrane hyperpolarization in the human umbilical vein endothelial cell line used in this study, membrane hyperpolarization in response to the endothelium-dependent vasodilators histamine and ATP was tested in regard to its sensitivity to distinct inhibitors of Ca2+-activated K+ channels. Histamine (10 μM) hyperpolarized endothelial cells from −24.7 ± 4.8 mV (n = 3) to −61.0 ± 2.1 mV (n = 3). Further administration of a dual blocker of IKCa and BKCa channels charybdotoxin (100 nM) in the continued presence of histamine reversed the membrane potential values to −39.7 ± 6.5 mV (57.6 ± 8.9% inhibition; n = 3) within 3 min. (Fig. 1a). A similar inhibitory effect was observed when a selective IKCa channel inhibitor TRAM-34 (2 μM) was used (52.1 ± 6.4% inhibition; n = 5; Fig. 1b). In contrast, the selective BKCa channel blocker iberiotoxin (200 nM) only slightly affected the hyperpolarization. In this set of experiments, histamine (10 μM) hyperpolarized endothelial cells from −28.3 ± 4.1 to −58.3 ± 4.3 mV (n = 4). Further administration of 200 nM iberiotoxin in the continued presence of histamine brought the membrane potential values to −53.2 ± 4.8 mV (17.1 ± 3.3% inhibition; n = 4) within 3 min (Fig. 1c). Similar to the electrical responses to histamine, hyperpolarization to ATP (10 μM) was effectively inhibited by TRAM-34 (2 μM; 57.4 ± 9.4% inhibition; n = 3; Fig. 1d).
Fig. 1.
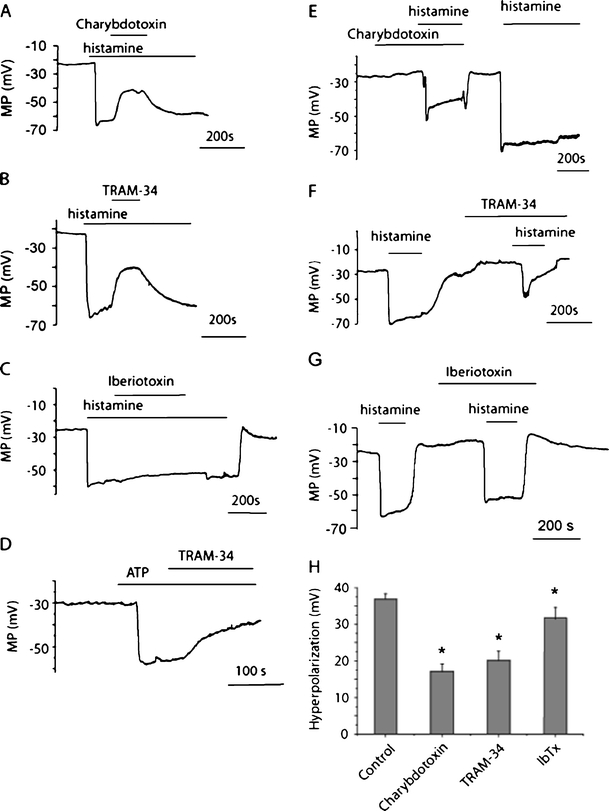
Effect of IKCa channel inhibitors on endothelial hyperpolarization. a Typical membrane potential recording showing the effect of charybdotoxin (100 nM) applied during the plateau phase of hyperpolarization to 10 μM histamine. b Typical membrane potential recording showing the effect of TRAM-34 (2 μM) applied during the plateau phase of hyperpolarization to 10 μM histamine. c Typical membrane potential recording showing the effect of iberiotoxin (200 nM) applied during the plateau phase of hyperpolarization to 10 μM histamine. d Typical membrane potential recording showing the effect of TRAM-34 (2 μM) applied during the plateau phase of hyperpolarization to 10 μM ATP. e Typical membrane potential recording showing the effect of preexposure to charybdotoxin (100 nM) on hyperpolarization to 10 μM histamine. f Typical membrane potential recording showing the effect of preexposure to TRAM-34 (2 μM) on hyperpolarization to 10 μM histamine. g Typical membrane potential recording showing the effect of preexposure to iberiotoxin (200 nM) on hyperpolarization to 10 μM histamine. h Statistical representation of the effect of charybdotoxin (100 nM; n = 4), TRAM-34 (2 μM; n = 5) and iberiotoxin (200 nM; n = 5) on peak hyperpolarization to 10 μM histamine (n = 11). *P < 0.05 vs control hyperpolarization
When endothelial cells were preexposed to charybdotoxin (100 nM) or TRAM-34 (2 μM), the peak hyperpolarization to 10 μM histamine was effectively inhibited by 48.4 ± 6.6% (n = 4) and 43.5 ± 4.3% (n = 5), respectively (Fig. 1e, f, and h), while pretreatment with iberiotoxin (200 nM) only slightly affected the peak hyperpolarization to histamine (12.9 ± 5.1% inhibition; n = 5; Fig. 1g, h). Altogether, these results clearly indicate the presence of functional IKCa channels in EA.hy926 cells and their predominant contribution to endothelial hyperpolarization to histamine and ATP.
To directly demonstrate the existence of functional IKCa channels in the endothelial cell line used in this study (EA.hy926), excised inside-out patches were exposed to micromolar concentrations of Ca2+ under symmetrical KCl solutions. Under these conditions, two types of single-channel activity could be observed in the same patch: one with high current amplitude and one with lower current amplitude (Fig. 2a). Noteworthy, the high conductance channel was predominantly observed in excised patches, while the lower conductance channel was observed in approximately 10% of recordings. Inclusion of iberiotoxin (400 nM) in the pipette inhibited the activity of channels with large amplitude, thus pointing to the BKCa channel to account for this current. In contrast, the single-channel activity with lower conductance was still observed when pipettes were filled with solution containing iberiotoxin either alone (Fig. 2b) or in combination with 100 nM apamin, an inhibitor of the small conductance Ca2+-activated K+ channel (not shown).
Fig. 2.

Functional IKCa channels are present in excised patches from EA.hy926 cells. a Representative single-channel recording out of seven independent experiments in inside-out patch exposed to 1 μM bath Ca2+ showing two types (large and intermediate conductance) of Ca2+-activated K+ single-channel activity. b Representative single-channel recording from four independent experiments in inside-out patch in the presence of bath Ca2+ as indicated. The patch was held at a holding potential of −80 mV. Pipette solution contained iberiotoxin (400 nM) and apamin (100 nM). Single-channel activity is denoted as downward deflections. Bars from the right indicate closed state of the channel
In the presence of 400 nM iberiotoxin in the pipette, a gradual increase in bath Ca2+ concentration from 0 to 10 μM increased single-channel activity in inside-out patches (Fig. 2b), thus indicating that the channel underlying the current is Ca2+ dependent. The channels were practically silent at [Ca2+] <0.3 μM. Under conditions of symmetrical K+, the channel activity showed weak voltage dependency (Fig. 3a, b), reversed at 0 mV and the respective current–voltage relationship was linear in the voltage range −80 to +40 mV with a slope conductance of 39 pS (Fig. 3c).
Fig. 3.
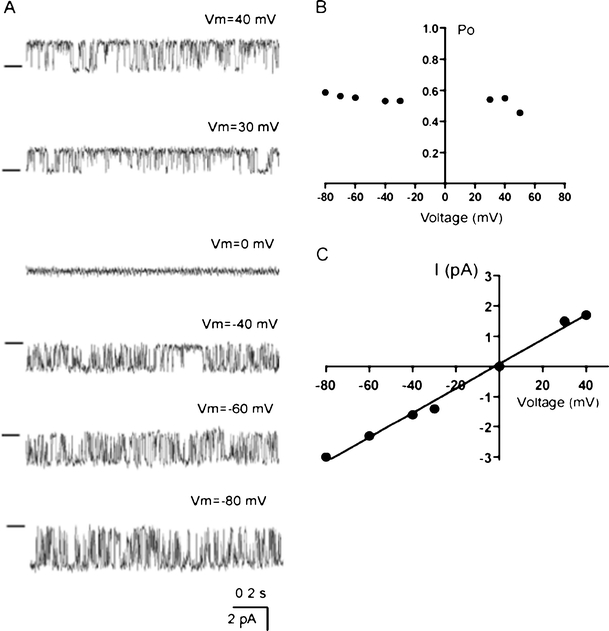
Voltage dependency of the IKCa channel. a Representative recordings out of three independent experiments showing the single IKCa channel activity at different voltages in patch containing one active channel in outside-out configuration in symmetrical K+ and free [Ca2+] in the pipette 10 μM. Bars from the left indicate the closed state of the channel. b Voltage dependency of Po presented in a. c Current–voltage relationship of IKCa in symmetrical K+ condition
The electrical properties of Ca2+-activated channels and their insensitivity to iberiotoxin and apamin, along with the sensitivity of histamine-induced cell hyperpolarization to TRAM-34 point to IKCa as the K+ channel responsible for hyperpolarization in response to histamine in this particular endothelial cell line.
Determination of CMC for LPI
At concentrations greater than their CMC, phospholipids can solubilize membrane components and disrupt the selective permeability of cell membrane. Hence, we performed experimental determination of CMC for LPI in our experimental conditions. The capillary rise method provided a CMC level for LPI about 70 μM (Fig. 4a, b). Because capillary rise method was reported to overestimate the surface tension [21], we performed CMC level determination using fluorescent methods, which provided the CMC level for LPI approximately 30 μM in our experimental conditions (Fig. 4c). Accordingly, in our further electrophysiological experiments we used LPI in concentrations below the CMC to avoid the effects of micelles on plasma membrane.
Fig. 4.
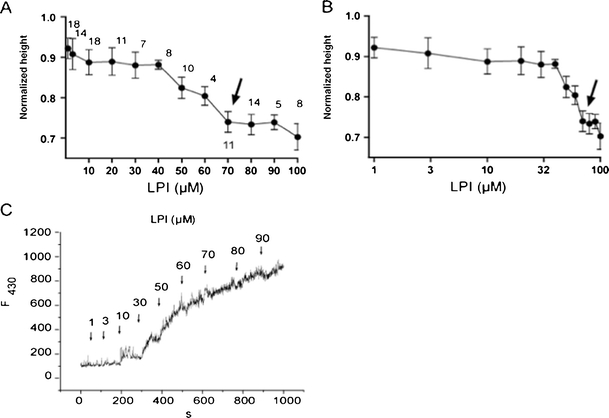
Determination of the CMC value for LPI. a and b Determination of CMC level using capillary height method. LPI-evoked changes in capillary height normalized to the mean capillary height level attained in LPI-free 140 mM K+-containing bath solution presented in linear (a) and log scale (b) to expand for clarity the data points derived from high and low LPI concentrations, respectively. Arrows indicate the point where the curve plateaued corresponding to the CMC level. The number of individual measurements is indicated above the averaged data points. c Determination of CMC level for LPI using the fluorescence method. LPI was added cumulatively to a solution containing 0.25 μM dephenylhexatriene in 140 mM K+-based bath solution and the rise in fluorescence at 30 μM LPI corresponds to transition to micelle aggregation. The excitation was 345 nm, fluorescence emission was 430 nm. Representative record out of five experiments
LPI in the bath affects the activity of single IKCa channels in the inside-out configuration
To explore whether LPI in concentration range below the CMC directly (i.e., independently of G-proteins and downstream signaling) modifies the IKCa channel activity, inside-out patches were exposed to LPI in the presence of 1 μM free Ca2+, a concentration that mimics strong cellular activation by an IP3-generating agonist such like histamine or ATP. LPI at concentrations 0.3–10 μM caused an increase in channel NPo from the basal NPo level of 0.093 ± 0.028 (n = 14) in a concentration-dependent manner (Fig. 5a, b, c). The single-channel current amplitude, however, was not affected by LPI (Fig. 5d), as determined by Gaussian fits of the amplitude histograms. A loss of gigaseal at LPI concentrations 30 μM or higher that probably reflected the loss of cell membrane integrity prevented us from testing higher LPI concentrations. The increase in NPo upon LPI addition was sustained (Fig. 5a) and reversible following a 15-min washout. The IKCa single-channel activity was potentiated by 3 μM LPI both in the presence of 1 and 10 μM bath Ca2+ and the degree of potentiation was very similar in the presence of 1 and 10 μM bath Ca2+ (Fig. 5e).
Fig. 5.
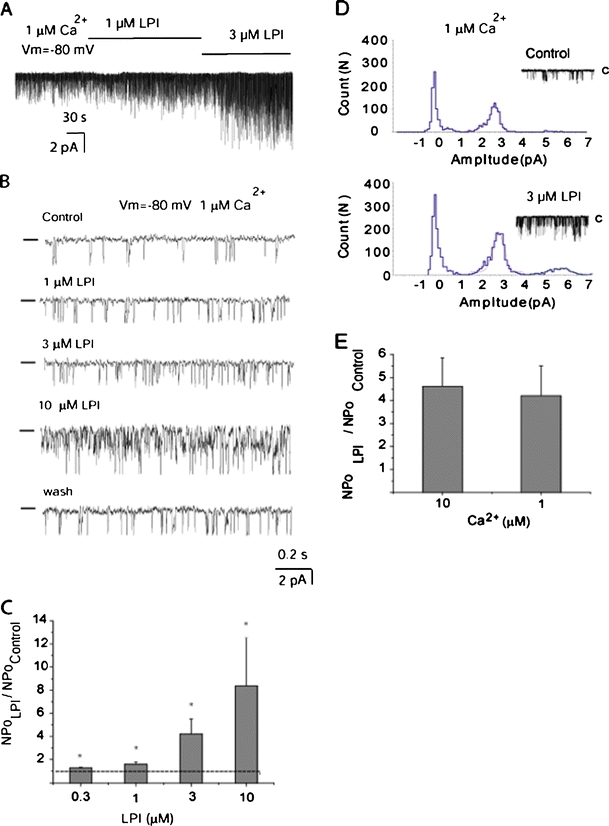
The LPI effect develops slowly, is sustained, reversible, and occurs in a concentration-dependent manner. a Representative single-channel recording in inside-out patch exposed to 1 and 3 μM LPI in the presence of 1 μM Ca2+ in the bath at a holding potential of −80 mV. Channel openings are shown as downward deflections. b Representative single-channel recording in inside-out patch exposed to 1 μM Ca2+ showing concentration-dependent activation of IKCa channel activity in response to bath application of 1, 3, and 10 μM LPI, which was reversible following 15 min of wash out of LPI. Channel openings are shown as downward deflections and bars from the left indicate the zero current level. c Statistical representation of the effect of 0.3 (n = 4), 1 (n = 5), 3 (n = 14), and 10 μM (n = 3) LPI on IKCa channel activity in the presence of 1 μM Ca2+ at a holding potential of −80 mV. *P < 0.05 vs basal NPo in the absence of LPI. d A representative amplitude histogram before (Control) and after exposure of excised patch to 3 μM LPI in the presence of 1 μM Ca2+. Data points were fitted by Gaussian fits. e Statistical representation of the effect of 3 μM LPI on IKCa channel activity in the presence of 10 μM (n = 4) and 1 μM Ca2+ (n = 14) at a holding potential of −80 mV
Potentiation in IKCa single-channel activity was observed either in patches containing multiple active channels (Fig. 5b) or only one active channel (Fig. 6a). Data from patches containing one functional channel revealed that LPI-induced increase in Po (Fig. 6a) was similar to potentiation of NPo (Fig. 5c), indicating that the stimulatory effect of LPI on NPo is due solely to modulation of Po. The action of LPI was accompanied by moderately increased mean open time (Fig. 6b). In addition, LPI strongly reduced the duration of the channel mean closed time, the latter reaching ∼26% of control (Fig. 6c). To determine which kinetic state of the channel is affected by LPI thereby resulting in the reduced closed time, we performed analysis of open and closed time distributions by constructing open and closed time event histograms. Fitting of open time distribution revealed two components (τo1 and τo2), while closed times were nicely fitted with three components (τc1, τc2, and τc3). LPI exposure was accompanied by a mild increase in average duration in long open events, while short open events remained unaffected (Fig. 6c). LPI did not affect short closed events, mildly reduced τc2 and dramatically reduced the channel long closures. Thus, dwell time distribution analysis revealed that the effect of LPI on Po results from several LPI actions, the most profound of which being the reduction of duration of long closed times.
Fig. 6.
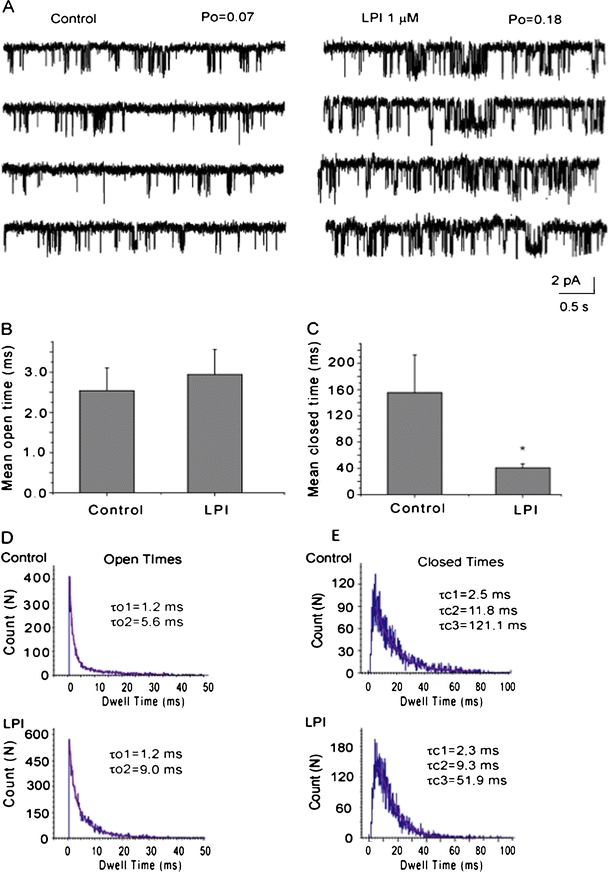
Effect of LPI on the kinetic properties of IKCa channels. a Representative single-channel recording in inside-out patch containing one active channel exposed to 1 μM Ca2+ prior (Control) and during the exposure to 1 μM LPI at the cytosolic side of the patch at a holding potential of −80 mV. Channel openings are shown as downward deflections. b The mean open time of the IKCa prior (Control) and after addition of 3 μM LPI (n = 9). c The mean closed time (frequency of openings) of the IKCa prior (Control) and after addition of LPI (n = 9). *P < 0.05 vs. control. d Open time histograms in the absence (Control) and presence of 1 μM LPI. In both cases, the open time was fitted to two exponentials (τo1 and τo2). LPI did not affect τo1 and slightly increased τo2. e Closed time histograms in absence (Control) and presence of 1 μM LPI. In both cases, the closed time was fitted to three exponentials (τc1, τc2, and τo2). LPI did not affect τc1, slightly decreased τc2, and strongly decreased τc3
Changes in the local bilayer microcurvature were proposed to explain the effects of LPLs on ion channel function [24]. According to this hypothesis, intra- and extracellular LPLs favor the formation of opposite deformations, we attempted to antagonize cup-like membrane deformations evoked by internal LPI by including low LPI concentrations into the patch pipette. In the presence 0.1 μM LPI in the patch pipette, application of 3 μM LPI to internal face of the membrane reduced the potentiation of the IKCa channel activity by LPI when compared with that obtained in the absence of the pipette LPI (pipette LPI: NPo increased by 1.4 ± 0.2 times, n = 5; control: by 5.5 ± 1.4 times, n = 17). These results indicate that membrane deformations may play a role in stimulation of the IKCa channel activity by LPI.
LPI facilitates agonist-induced hyperpolarization via its effect on IKCa channels
Because endothelial hyperpolarization to histamine and ATP is primarily mediated by IKCa channels and considering that LPI directly stimulates the IKCa channel activity, we next explored whether intracellular LPI potentates the hyperpolarization to histamine via its stimulatory effect on IKCa channels. In accordance with our previous observation [2], inclusion of 1 μM LPI into the patch pipette solution resulted in an amplification of endothelial hyperpolarization in response to 0.5 μM histamine from 17.9 ± 2.5 mV (n = 12) to 27.2 ± 1.9 mV (n = 14; Fig. 7a). Preexposure to the IKCa channel inhibitor TRAM-34 (2 μM) blunted the stimulatory effect of LPI on histamine-induced peak hyperpolarization by 54.0 ± 6.7% (n = 4) and eliminated the sustained membrane hyperpolarization (Fig. 7b).
Fig. 7.
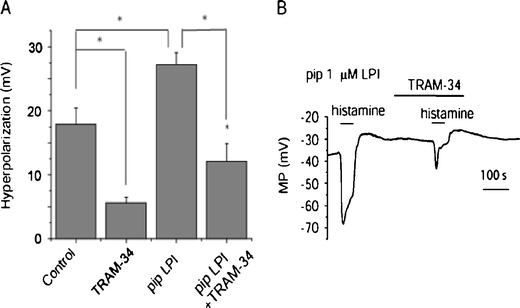
Intracellular LPI potentiates endothelial cell hyperpolarization to histamine via activation of IKCa channels. a Statistical analysis of the effect of 2 μM TRAM-34 on histamine- (0.5 μM) induced hyperpolarization in the absence (control, n = 12; TRAM-34, n = 5) or presence of 1 μM LPI in the pipette (control, n = 14; TRAM-34, n = 4). b Representative traces in membrane potential out of 14 independent experiments showing endothelial cell hyperpolarization to bath application of 0.5 μM histamine in the presence of 1 μM LPI in the patch pipette and its sensitivity to TRAM-34 (2 μM)
Discussion
In the vasculature, KCa channels are widely recognized to serve as crucial effectors controlling vascular tone by their important contribution to plasma membrane potential of the cells that, in turn, affect other ion channels such as the smooth muscle L-type Ca2+ channels as well as endothelial store-operated Ca2+ entry channels. In endothelial cells, this function is achieved by mediating hyperpolarization in response to physical and chemical stimuli. Notably, membrane hyperpolarization increases the driving force and sustains Ca2+ influx that is required for nitric oxide synthesis [23] and directly controls endothelium-derived hyperpolarizing factor-mediated response [13].
Cultured endothelial cells are known for their abnormal high expression of BKCa channels, which are not typically detected in healthy intact vessels [20, 26]. However, in the present study we report that histamine- and ATP-induced membrane hyperpolarization of cells from the human umbilical vein endothelial cell-derived cell line EA.hy926 cells is considerable more sensitive to charybdotoxin and TRAM-34 than to iberiotoxin. The present data demonstrate a functional expression of IKCa and point to the primary role of IKCa channels in hyperpolarizing responses to these agents in this particular cell line. Notably, this finding is similar to that obtained in in situ endothelial cells from excised rat aorta, where hyperpolarization to acetylcholine was shown to be mediated by IKCa channels [26]. Previously, in EA.hy926 cells, TRAM-34 plus apamin in combination were reported to inhibit Ca2+ signaling, hyperpolarization, and nitric oxide production in response to histamine and ATP [30]. In this particular study, however, the relative contributions of IKCa and SKCa channels to these responses have not been dissected and the underlying single-channel activity was not characterized. In the present work, in inside-out patches a K+ channel with a slope conductance of 39 pS under symmetrical K+ conditions was detected with biophysical properties (i.e., weak voltage dependency, Ca2+ sensitivity, and unitary conductance) similar to IKCa channel.
Recently, we showed that in excised patches of the same cell type, LPI in physiological relevant concentrations [8, 36] directly modulates BKCa channel activity in a bidirectional manner [2]. Notably, the stimulatory effect of LPI on cell membrane hyperpolarization in response to low histamine concentration was only partially inhibited by iberiotoxin, thus, pointing to ion channel(s) other than BKCa being mainly responsible for the agonist-triggered hyperpolarization in endothelial cells. In the present work, we demonstrate that LPI directly (i.e., without interaction with G-proteins) stimulates IKCa channel activity without affecting the single-channel amplitude, thus, indicating that LPI unlikely interacts with the pore-forming domain of the channel. Because the stimulatory effect of LPI on IKCa channels was found both in multi- and single-channel patches, our data indicate that the rise in Po rather than the increase in the number of active channels most probably underpins the stimulatory effect of LPI on these ion channels. LPI marginally affected the mean open time and caused a profound decreased in the mean closed time. However, analysis of the kinetics of channel opening indicates that during control recordings, the channel opening is minimally described by two open and three closed states, which is consistent with previous reports [9, 33]. LPI decreased long closures, had no effect on fast closed events and moderately increased long channel openings, indicating that the effect of LPI on Po results from several LPI actions.
To ensure that micelle formation is not involved in the molecular effect of LPI on the IKCa channel activity, we performed CMC measurements under our experimental conditions. Two methods gave CMC values of about 30–70 μM LPI. The discrepancy between two methods may be explained by the observation that capillary rise method tends to overestimate the surface tension, and hence, the CMC value, possibly due to intermolecular forces between the sample surface and the capillary walls [21].
LPLs are known to modify the activities of ion channels including BKCa channels [2, 19], cold-activated TRPM8 channels [1, 34], TRPC5 [14], and TREK-1 and TRAAK channels [25]. One of the mechanisms which was proposed to underlie the changes in channel function by LPLs is mechanical membrane deformation [24]. Because in the presence of small concentration of LPI in the pipette, the degree of potentiation of the IKCa channel activity by “internally” applied LPI was decreased, the changes in cellular membrane deformations induced by LPI may indeed play a role in alterations in IKCa channel function. Although the precise mechanism of LPI action on IKCa channel function is unclear, direct interaction of LPI with the channel protein structures seems unlikely, since LPI does not affect single-channel conductance.
Similar to the effect of LPI on BKCa channels, its stimulatory effect on IKCa channel was sustained, concentration dependent, and reversible. However, a remarkable difference between the effects of LPI on these two Ca2+-activated K+ channels is that the BKCa channel activity was found to be dually affected by LPI [2], while in the case of IKCa channels, LPI exhibited only stimulatory but not inhibitory properties. In fact, in the presence of 1 and 10 μM free Ca2+, which represent conditions under which LPI predominantly inhibits BKCa channel activity [2], the IKCa channel activity was still equally increased by LPI.
Endothelial hyperpolarization that is predominantly mediated via IKCa and SKCa channels in the vast majority of vascular beds is required for endothelium-dependent relaxation via the production of nitric oxide as well as the endothelium-derived hyperpolarizing factor(s) [17]. Furthermore, endothelial cell hyperpolarization passively spreads to the underlying smooth muscle cells via gap junctions reducing the Cav1.2 channel activity and directly contributing to vasodilation [7]. Accordingly, compounds that facilitate IKCa and SKCa channel activity have great therapeutic interest in order to rescue dysfunctional blood vessel relaxation that occurs in many diseases and aging [13]. Furthermore, activators of IKCa and SKCa channels enhance agonist-triggered endothelial hyperpolarization [31] and subsequently evoke endothelium-dependent relaxation [7, 11, 22]. In agreement with these reports, histamine-induced hyperpolarization was augmented by LPI and this effect was blunted by TRAM-34, a selective IKCa channel inhibitor. These data indicate that the direct stimulatory effect of LPI on IKCa channels reported herein subsequently results in augmentation of histamine-evoked membrane hyperpolarization.
It remains to be investigated whether the direct modulation of IKCa channel activity is a common feature of lysophospholipids or unique for LPI. Nevertheless, it was previously shown that other lysophospholipids, namely sphingosine phosphate, lysophosphatidic acid (LPA) and lysophosphatidylcholine (LPC) stimulate migration of microglial cells via stimulation of IKCa channels [28, 29]. However, due to the lack of single-channel recordings, it remains uncertain, whether these reported effects of lysophospholipids on IKCa activity require the binding of the lipid to specific G protein-coupled receptor(s) or occurs receptor-independently.
In conclusion, in the present study we provide the first evidence that LPI directly stimulates IKCa channel activity in endothelial cells leading to enhanced IKCa channel-mediated hyperpolarization to histamine. Considering that LPI is produced by endothelial cells in response to various stimuli including cytosolic Ca2+ elevation, the formation of LPI might establish an intrinsic magnifying pathway that facilitates and prolongs membrane hyperpolarization and thus Ca2+ entry in order to improve Ca2+-dependent endothelial functions. Although the stimulatory properties of LPI on IKCa channels appear interesting to be utilized for an improvement of endothelial function, the therapeutic potential of this approach requires further investigations.
Acknowledgments
The authors thank Mrs. Anna Schreilechner, BSc for her excellent technical assistance and Dr. C.J.S. Edgell (University of North Carolina, Chapel Hill, NC) for the EA.hy926 cells. We also thank Dr. Maud Frieden (University Geneva, CH) for fruitful discussions and a critical reading of the manuscript. This work was supported by the Austrian Science Fund, FWF (F21857-B18). The Institute of Molecular Biology and Biochemistry was supported by the infrastructure program of the Austrian ministry of education, science, and culture.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondarenko A, Malli R, Graier WF. The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+-activated large-conductance K+ channels in endothelial cells. Pflugers Arch. 2011;461:177–189. doi: 10.1007/s00424-010-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondarenko A, Waldeck-Weiermair M, Naghdi S, Poteser M, Malli R, Graier WF. GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells. Br J Pharmacol. 2010;161:308–320. doi: 10.1111/j.1476-5381.2010.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chattopadhyay A, London E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal Biochem. 1984;139:408–412. doi: 10.1016/0003-2697(84)90026-5. [DOI] [PubMed] [Google Scholar]
- 5.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008;1781:531–539. doi: 10.1016/j.bbalip.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- 7.Coleman HA, Tare M, Parkington HC. Myoendothelial electrical coupling in arteries and arterioles and its implications for endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2002;29:630–637. doi: 10.1046/j.1440-1681.1999.03701.x. [DOI] [PubMed] [Google Scholar]
- 8.Corda D, Iurisci C, Berrie CP. Biological activities and metabolism of the lysophosphoinositides and glycerophosphoinositols. Biochim Biophys Acta. 2002;1582:52–69. doi: 10.1016/s1388-1981(02)00137-3. [DOI] [PubMed] [Google Scholar]
- 9.Dunn PM. The action of blocking agents applied to the inner face of Ca2+-activated K+ channels from human erythrocytes. J Membr Biol. 1998;165:133–143. doi: 10.1007/s002329900427. [DOI] [PubMed] [Google Scholar]
- 10.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards G, Gardener MJ, Feletou M, Brady G, Vanhoutte PM, Weston AH. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br J Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falasca M, Corda D. Elevated levels and mitogenic activity of lysophosphatidylinositol in k-ras-transformed epithelial cells. Eur J Biochem. 1994;221:383–389. doi: 10.1111/j.1432-1033.1994.tb18750.x. [DOI] [PubMed] [Google Scholar]
- 13.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- 15.Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Feletou M. Role of SKCa and IKCa in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- 17.Holzmann S, Kukovetz WR, Windischhofer W, Paschke E, Graier WF. Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-L-arginine in coronary arteries. J Cardiovasc Pharmacol. 1994;23:747–756. doi: 10.1097/00005344-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Karliner JS. Lysophospholipids and the cardiovascular system. Biochim Biophys Acta. 2002;1582:216–221. doi: 10.1016/s1388-1981(02)00174-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim MY, Liang GH, Kim JA, Kim YJ, Oh S, Suh SH. Sphingosine-1-phosphate activates BKCa channels independently of G protein-coupled receptor in human endothelial cells. Am J Physiol Cell Physiol. 2006;290:C1000–C1008. doi: 10.1152/ajpcell.00353.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery: A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- 21.Koskinen J, Hyvarinen A-P, Prisle N, Virtanen AN, Lihavainen H (2010) Surface tension measurements of carboxylic acids with 4 to 12 carbon molecules. Differences between measurement methods. International Aerosol Conference, Helsinki http://www.atm.helsinki.fi/IAC2010/abstracts/pdf/19.pdf
- 22.Leuranguer V, Gluais P, Vanhoutte PM, Verbeuren TJ, Feletou M. Openers of calcium-activated potassium channels and endothelium-dependent hyperpolarizations in the guinea pig carotid artery. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:101–109. doi: 10.1007/s00210-008-0267-x. [DOI] [PubMed] [Google Scholar]
- 23.Luckhoff A, Pohl U, Mulsch A, Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988;95:189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundbaek JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol. 1994;104:645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 26.Marchenko SM, Sage SO. Calcium-activated potassium channels in the endothelium of intact rat aorta. J Physiol. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 28.Schilling T, Eder C. Lysophosphatidylcholine- and MCP-1-induced chemotaxis of monocytes requires potassium channel activity. Pflugers Arch. 2009;459:71–77. doi: 10.1007/s00424-009-0710-y. [DOI] [PubMed] [Google Scholar]
- 29.Schilling T, Repp H, Richter H, Koschinski A, Heinemann U, Dreyer F, Eder C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca2+-dependent K+ channels. Neuroscience. 2002;109:827–835. doi: 10.1016/S0306-4522(01)00534-6. [DOI] [PubMed] [Google Scholar]
- 30.Sheng JZ, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- 31.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith GD, Violet GP. An inexpensive glass capillary 'tensiometer' for determining the critical micelle concentration of surfactants. Chem Educ. 2009;14:239–242. [Google Scholar]
- 33.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K + channels. Am J Physiol Cell Physiol. 2000;278:C570–C581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- 34.Vanden Abeele F, Zholos A, Bidaux G, Shuba Y, Thebault S, Beck B, Flourakis M, Panchin Y, Skryma R, Prevarskaya N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J Biol Chem. 2006;281:40174–40182. doi: 10.1074/jbc.M605779200. [DOI] [PubMed] [Google Scholar]
- 35.Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, Malli R, Graier WF. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao YJ, Schwartz B, Washington M, Kennedy A, Webster K, Belinson J, Xu Y. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem. 2001;290:302–313. doi: 10.1006/abio.2001.5000. [DOI] [PubMed] [Google Scholar]


