Abstract
New strategies for cervical cancer screening include HPV DNA testing. Using self-testing methods would increase access to testing in both developed and developing countries. The purpose of this study was to compare time-to-clearance of specific HPV types between clinician-collected-lavage (CC-L) and self-collected (SC) sampling in a single cohort. CC-L and SC samples were obtained every 4 months at alternate 2-month windows from 537 women. Eighteen high-risk (HR) HPV and 4low-risk HPV were examined. Proportional hazards model was used to compare time-to-clearance between methods for combined HR and for 13 specific HPV types. Prentice-Wilcoxon test was used for within-subject paired comparison. In the independent analysis for combined HR and LR types, no differences were found. For specific types, time-to-clearance for all HPV types examined between CC-L and SC samples was similar except for HPV 66 which showed a trend to clear slower by SC (p=0.09). When comparing methods in the same woman, time-to-clearance was similar for all types except for HPV 16 which showed a trend to clear slower by CC-L means (p=0.08). When we examined pattern of clearance among the CC-L samples, the fastest types to clear were HPV 6, 18, 66, 84 and 39 and the slowest were HPV 62, 68, 59 and 16. These patterns of fast and slow were similar for SC samples. Our findings suggest using SC vaginal swabs would observe similar natural histories of HPV compared to studies using CC-L specimens making self-testing feasible for repeated HPV DNA detection.
Keywords: HPV natural history, self-sampling, clinician-sampling
Introduction
Data from epidemiology studies describing the natural history of human papillomavirus (HPV) show that cervical infection with HPV is common and often transient with only a few individuals developing persistent infections.1–3 As HPV persistence is associated with cervical cancer risk, HPV testing has recently been introduced as a cancer-screening tool.4 The predictive value is based on the premise that detection of HPV in certain populations identifies persistent infections. The majority of these studies examining HPV testing require the aid of a speculum to adequately visualize the cervix while sampling tissue for DNA detection. Since the requirement of a speculum limits studies to clinic populations, several investigators have examined the possibility of using self-sampling from the vagina as a research and clinical tool.5–11 Its use in populations hard to reach is particularly attractive.
Cross-sectional studies comparing self-sampling and speculum-assisted sampling have shown varied correlations ranging from .24 to .96.9, 12–17 The inference from the studies with lower correlations may be that certain HPV types preferentially infect the vagina rather than the cervix or vice versa and sampling techniques reflect these differences. On the other hand, if the natural history of HPV types that infect the vagina is different than HPV types found in the cervix, then discordant findings would be expected if samples were compared at a single point in time.5, 18
In addition to clinical studies, epidemiology studies may benefit from using self-sampling since cohort studies are often restricted to groups who seek gynecologic care. No study to date has investigated whether self- and clinician-collected samples in the same cohort of women provide comparable information about aspects of the natural history of infection. The aim of this study is to compare the time-to-clearance type-specific, low and high-risk HPV infections detected by clinician-collected (CC-L) sampling and self-collected (SC) sampling in a single cohort of women.
Materials and Methods
Subject population
Women were part of a cohort study designed to examine the natural history of HPV. Women recruited between 2000 and 2006 were eligible for this analysis. Recruitment of these women has been detailed previously.19 In brief, women were recruited from a state university medical clinic and a Planned Parenthood clinic. Women were eligible if they were between the ages of 13–21 years and had less than 5 years of sexual experience. Women were excluded if they were planning to move within 3 years, immunosuppressed, currently pregnant or had a history of ablative or surgical therapy of the cervix. This study was approved by the University of California, San Francisco and San Francisco State University institutional review boards. Written consent was obtained by a trained study coordinator in a private room. At 4-month intervals, samples were obtained for HPV testing using a visually directed cervical lavage by the study nurse practicioners.19
In addition to the study visits, women were asked to obtain self-collected samples at the two month time period between the study visits. Hence the self-samples were collected every 4 months but at alternate times to the cervical lavage samples (see schematic representation in Figure 1). For self-collected samples, women used a kit that contained written instructions and supplies. They were instructed to insert a single sterile, scored Dacron swab as far into vagina as possible and move it up and down five times while keeping it in the vagina, then to remove the swab and place it into a tube containing 1mL of specimen transport medium (Quiagen, Valencia, CA). Women were instructed on the collection method with both verbal and written instructions at the initial clinic study visit. Women could choose to perform the self-collection at the study’s clinic or to receive and return the self-collection kits by mail. A pilot study to examine the integrity of samples via mail was performed and showed that mailed samples had equal integrity as clinic-collected.
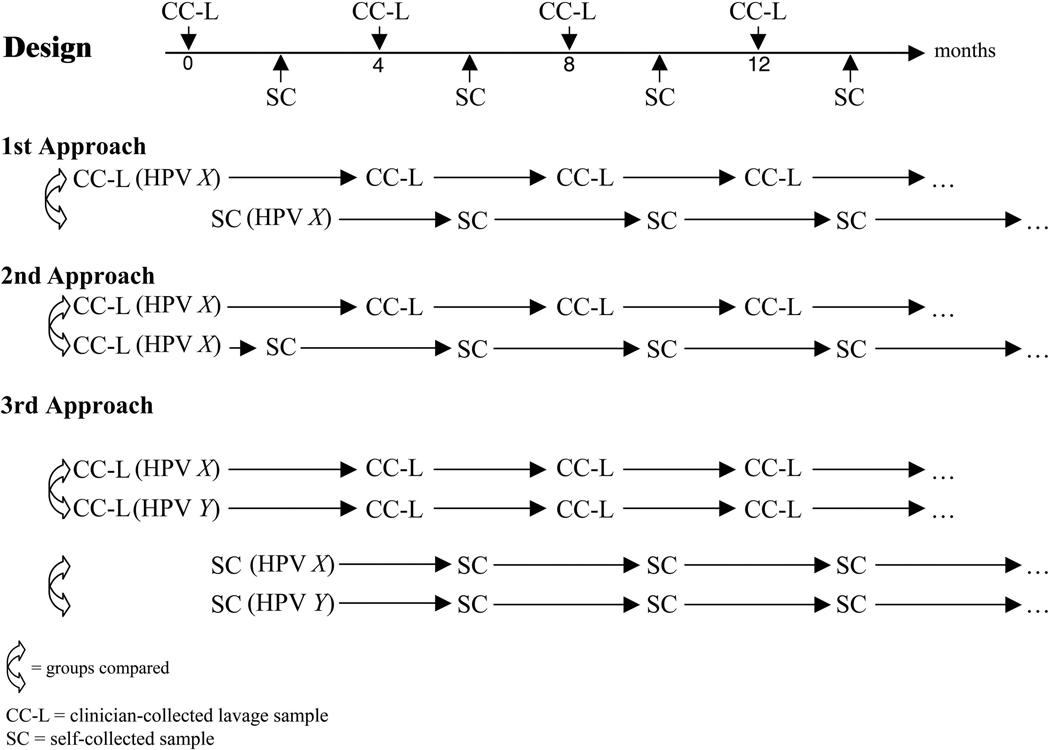
Figure 1. Cartoon shows the design of sample collection with the collection of self-swabs (SC) flanking the clinician-collected lavage (CC-L) samples at 2-month intervals.
Cartoon also depicts the 3 approaches to the time-to-clearance analyses. The first approach compared methods independently of each other—all women were included whether they did or did not have the same HPV type by SC and CC-L. Figure 2 and 3A reflect approach 1. The second approach compared methods in the same women—only women with the same HPV type in both the CC-L and flanking SC were included. Figure 3B reflects approach 2. The third approach compared specific HPV types within each method allowing the observation of which types cleared fastest or slowest within each method. Figure 4 reflects approach 3.
HPV testing
Lavage and self-collected samples were processed as previously described.19, 20 Thirty-seven HPV genotypes were detected using PCR amplification with PGMY09/11 primer system (Roche Molecular Diagnostics, Inc., Alameda, CA). The following HPV types were detected: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51–56, 58, 59, 61, 62, 64, 66–73, 81–84, 89; beta-globin detection was used as sample adequacy control.
CVL vs. cervical swab
Since the clinician collected samples in this study were by lavage and self swab, we did a small pilot study to compare the two in the same women. In 48 consecutive women in the study, a cervical swab and a CVL were collected at the same visit. The correlation was excellent (kappa coefficient = 0.87 [95% C.I. = 0.76 – 0.98]). HPV was detected from the swab in 19 women and all the HPV types were detected by CVL. In 3 women, one additional type was detected by CVL (HPV 81, 18 and 59, respectively). Twenty-seven women were HPV negative by swab and CVL. Two women were negative by swab but had one HPV type detected by CVL (HPV 62 and 53, respectively). In summary, the CVL detected all HPV types detected by swab and picked up additional types in 5 (10%) women.
Data analysis
Only the most common high-risk (HR) and low-risk (LR) HPV types detected in cervical samples were examined. Since most HPV infections in young women are extremely transient with 50% clearing within 6 months, criteria for the most common was based on their frequency among all samples, not merely prevalence or incidence. This allowed us to examine time-to-clearance among those HPV types which were more likely to persist by either collection strategy. Cutoff for most common HR types to be examined type-specifically was a frequency of >1.0% among the CC-L samples. Only the 4 most common LR types were examined since these were chosen for comparison since they have no clinical indication. Time-to-clearance was calculated for initial infections with a specific type for each collection method. If a woman had infections with more than one of the selected HPV types, she was included in analysis for each type.
Clearance of type-specific infection was defined to occur at the first instance of two consecutive HPV-negative samples. Each woman was allowed into the analysis only once for each type. HPV infections followed by a single negative sample were treated as censored at the time of the last positive test. This aim of the study was accomplished by comparing the two methods in three different ways. The first approach compared methods independently of each other. First infection of HPV type X detected by CC-L among all women were compared to the first infection of HPV type X detected by SC among all women. For the first approach, normal between-method comparisons for time-to-clearance were based on Cox proportional hazards model with SC/CC-L method representing independent variables adjusting for age and prevalence. Because the same woman can appear in both SC and CC-L data, the within-subject variance was adjusted using robust sandwich estimate.21 This analysis was performed for each of the most common types as well as combining the results for the 18 HR types (which included probable HR) and the 4 LR types separately to enhance precision. Definition of HR types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) and probable HR types (26, 53, 66, 68, 73, 82) was based on Munoz et al. 22 In the first approach, not all women had the same type infection. The second approach compared methods in the same women. So HPV X must be present in both the SC and CC-L samples from the woman. We realized our staggered testing strategies for the methods limited our ability to make direct within-individual comparisons of clearance events. To adjust for these factors, we examined time-to-clearance of a specific type starting with the initial positive CC-L specimen. In the second approach, time-to-clearance from the CC-L specimen using repeated CC-L sampling was compared to time-to-clearance using repeated SC sampling in the same women. Time-to-clearance used Prentice-Wilcoxon statistics for paired, censored event times. This analysis adjusted for the 2-month lag between CC-L and SC. We then made similar comparisons but started with the initial infection detected by SC. Because the test for comparison is not a model-based approach, only one parameter is allowed to compare time-to-clearance. Consequently, adjustments for age and prevalence was not possible. Unadjusted p values are given. A third approach compared time-to-clearance from first infection visit for each HPV type (X, Y, etc) within each methods of collection. Between-type comparisons for each of the two methods of collection was performed using Cox proportional hazard model, adjusting for within-subject variables. This allowed us to observe which HPV types among CC-L samples were fastest or slowest and compare these patterns to what we observe among the SC samples. The three approaches are schematically presented in figure 1.
When we examined the data for specific visits, we noted that more SC samples were missing than CC-L. Because this imbalance could introduce bias in between-method comparisons of distributions of time-to-clearance, we used imputation for both collection methods to provide more complete data for the figures. Random assignment based on the observed type-specific probability was given to the missing result for missing SC samples when two consecutive CC-L samples were both negative or both positive. The same was true for the missing CC-L samples. The missing results in all other scenarios were considered not imputable and considered missing (e.g. missing sample with one flanking positive and another flanking negative). Since the imputed results gave similar data as the unimputed, only imputed results are displayed.
Results
Population demographics
Six hundred twenty four women were enrolled in the Natural History of HPV in Teens study. Five hundred thirty seven had provided both cervical and vaginal samples between 2000 and 2006 and were eligible for this analysis. Mean follow-up time was 996 days (range 42 to 1785 days; standard deviation (SD) 481.6). Demographic characteristics of the 537 women are given in Table 1. The median number of lifetime sexual partners is 3. The majority of initial infections were incident (80%). Thirty-eight (7.1%) women had multiple types on initial SC and 46 (8.6%) on initial CC-L visit. From these women, we had 4330 CC-L and 3080 SC samples. If we assumed all women had all visits, we would have expected 4,539 CC-L samples and 4,539 SC samples. However, there were 209 missing CC-L samples and 1,459 missing SC samples. Per the imputation criteria, we imputed 99 CC-L (2.1%) HPV results of which the majority (93%) of the imputations were negative. We imputed 913 SC samples of which the majority (96%) of the imputations were negative. The probability of being negative between two negatives was close to 100%.
Table 1.
Demographics Characteristics (N=537)
| Mean age (range) | 18 yrs (12.4 to 21.9) |
| Mean age at 1st intercourse (SD) | 16.5 yrs (1.95) |
| Mean years since menarche (SD) | 5.8 yrs (2.2) |
| Mean number of lifetime sexual partners (SD) | 4.71 (6.22) |
| Race/ethnicity | |
| White | 31% |
| Asian/Pacific Islander | 16% |
| Black | 9% |
| Latina | 6% |
| Mixed/other | 38% |
| History of sexually transmitted infection prior to entry | 8.4% |
SD = standard deviation
In the longitudinal cohort, the most common high-risk HPV infections detected among the 4,330 CC-L samples were 16, 18, 39, 51, 52, 53, 59, 66 and 68 (Table 2). The same 9 were also found to be the most common high-risk types from the 3,080 SC samples. Of the 13 types listed in Table 2, HPV 16 was found to be the most common initial (defined as first ever detected whether prevalent or incident) infection detected by cervical or vaginal sampling. The most common low risk types were 84, 6, 89, and 62 for both methods.
Table 2.
The Frequency of High-Risk and Low-Risk HPV Types Detected in Clinician Lavage- and Self-Collected Samples
| Clinician-Collected Lavage Samples (n=4330 visits) |
Self-Collected Samples (n=3080 visits) | ||||
|---|---|---|---|---|---|
| HPV type | Number of positive samples |
% of samples positive for HPV |
HPV type | Number of positive samples |
% of samples positive for HPV |
| High Risk | |||||
| 16 | 203 | 4.7% | 16 | 143 | 4.6% |
| 51 | 174 | 4.0% | 51 | 141 | 4.6% |
| 59 | 152 | 3.5% | 52 | 118 | 3.8% |
| 53* | 132 | 3.0% | 53 | 118 | 3.8% |
| 52 | 120 | 2.8% | 59 | 115 | 3.7% |
| 66* | 95 | 2.2% | 6 | 93 | 3.0% |
| 39 | 90 | 2.1% | 66 | 90 | 2.9% |
| 68* | 74 | 1.7% | 68 | 63 | 2.0% |
| 18 | 60 | 1.4% | 18 | 55 | 1.8% |
| 45 | 44 | 1.0% | 45 | 43 | 1.4% |
| 58 | 43 | 0.99% | 58 | 51 | 1.7% |
| 73* | 43 | 0.99% | 73 | 32 | 1.0% |
| 56 | 26 | 0.6% | 56 | 13 | 0.4% |
| 31 | 19 | 0.4% | 31 | 20 | 0.6% |
| 35 | 12 | 0.3% | 35 | 8 | 0.3% |
| 82* | 8 | 0.18% | 82 | 10 | 0.3% |
| 33 | 4 | 0.09% | 33 | 2 | 0.06% |
| 26* | 3 | 0.07% | 26 | 2 | 0.06% |
| Low Risk | |||||
| 84 | 132 | 3.0% | 84 | 116 | 3.8% |
| 89 | 93 | 2.1% | 89 | 111 | 3.6% |
| 6 | 89 | 2.1% | 62 | 103 | 3.3% |
| 62 | 89 | 2.0% | 6 | 93 | 3.0% |
probable HR-HPV type
Comparison of time-to-clearance for combined HR and LR HPV types and specific HPV types between CC-L and SC samples
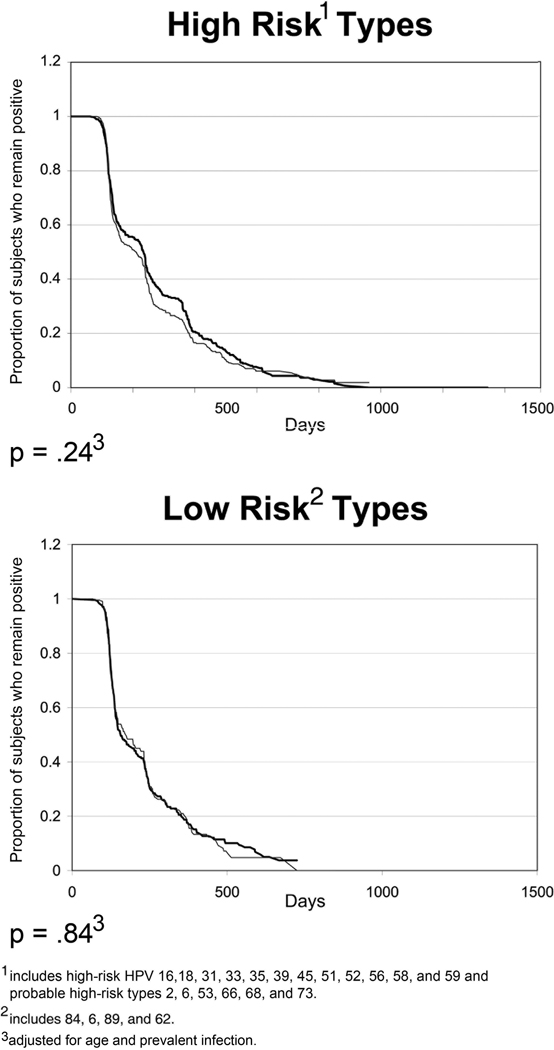
Among all samples and all women, no significant differences were found for all 18 HR HPV types (inclusive of the probable HR types) (p=0.24) or 4 LR HPV types (p=0.84) (Figure 2). When we excluded the 6 probable HR types, similar observations were made for the 12 HR HPV types (p=.22) (curve not shown). P values are adjusted for age and prevalent infection.
Figure 2. Comparison of type-specific time-to-clearance for 18 HR-HPV types combined and 4 LR HPV types combined between self-collected (black line) and clinician-collected lavage (gray line) samples.
X-axis is in days and Y-axis is proportion remaining positive. Figure reflects the curves inclusive of all women. P-values are comparison of all 18 HR HPV types (including probable HR types) and all 4 LR HPV types between methods using Cox Proportional Hazards Model adjusting for within-subject variance, age, and prevalence.
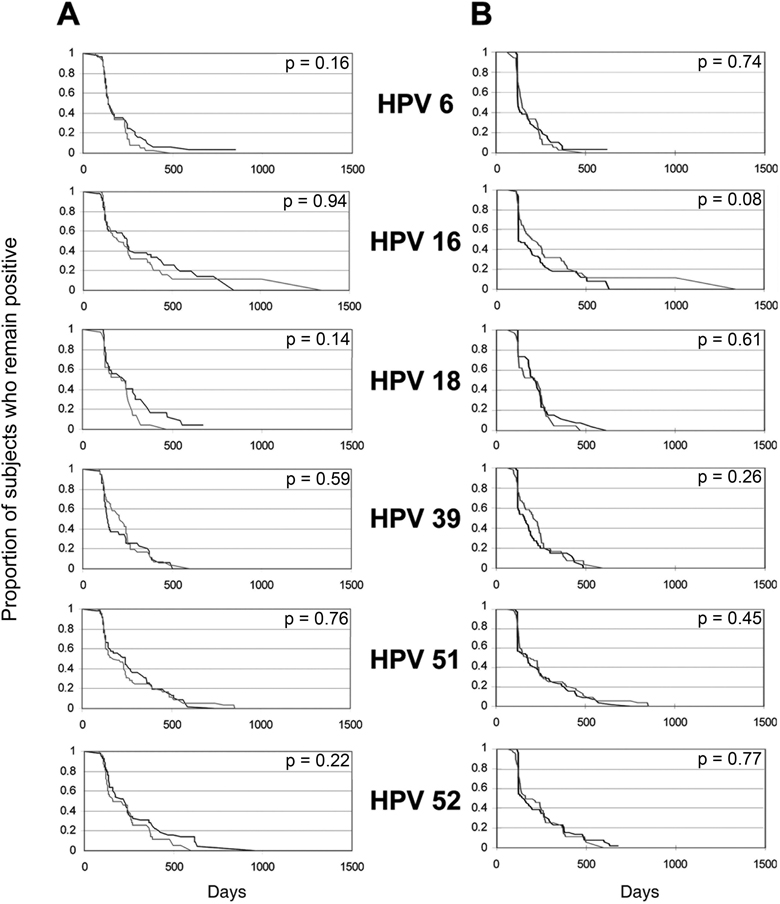
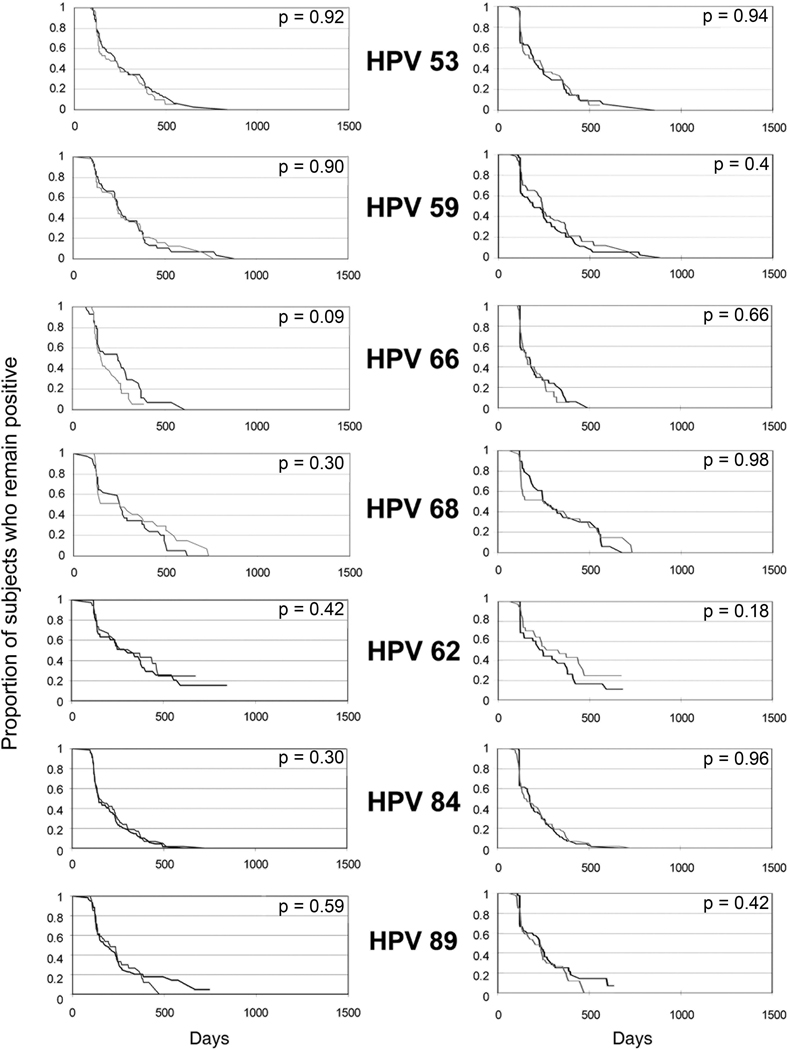
We next examined differences between 13 specific HPV types with CC-L frequencies greater than 1.0%. Among all samples and all women, no significant differences were found between clearance time distributions from CC-L and SC samples for each of the HPV types. A trend was found for HPV 66 (p=0.09) (Figure 3A). HPV 66 appeared to clear slower by SC means compared to CC-L. The above analysis was independent of sampling method results. To examine the time-to-clearance within a woman, we used the second approach which started with an initial CC-L positive test and compared time-to-clearance by sampling method. We again found no significant differences in to time-to-clearance for any HPV type (Figure 3B). A trend was found for HPV 16 (p= 0.08) which showed that CC-L samples cleared slower than SC samples. We also examined time-to-clearance between methods starting with a positive SC sample and again found no difference (p values ranged from 0.16 to 0.97) (data not shown).
Figure 3. Comparison of type-specific time-to-clearance between self-collected (black line) and clinician-collected lavage (gray line) samples.
X-axis is in days and Y-axis is proportion remaining positive. Column A reflects curves inclusive of all women. P values are comparison of type-specific time-to-clearance using Cox Proportional Hazards Model adjusting for within-subject variance, age, and prevalence. Column B reflects curves for women who were initially positive by CC-L. Comparison in time-to-clearance is between sampling methods: SC vs CC-L for the same woman. P values are comparison of type-specific time-to-clearance using paired Prentice-Wilcoxon. Number of subjects for each HPV type and rates of clearance are given in Table 3.
Time-to-clearance of specific HPV types among CC-L samples and among SC samples
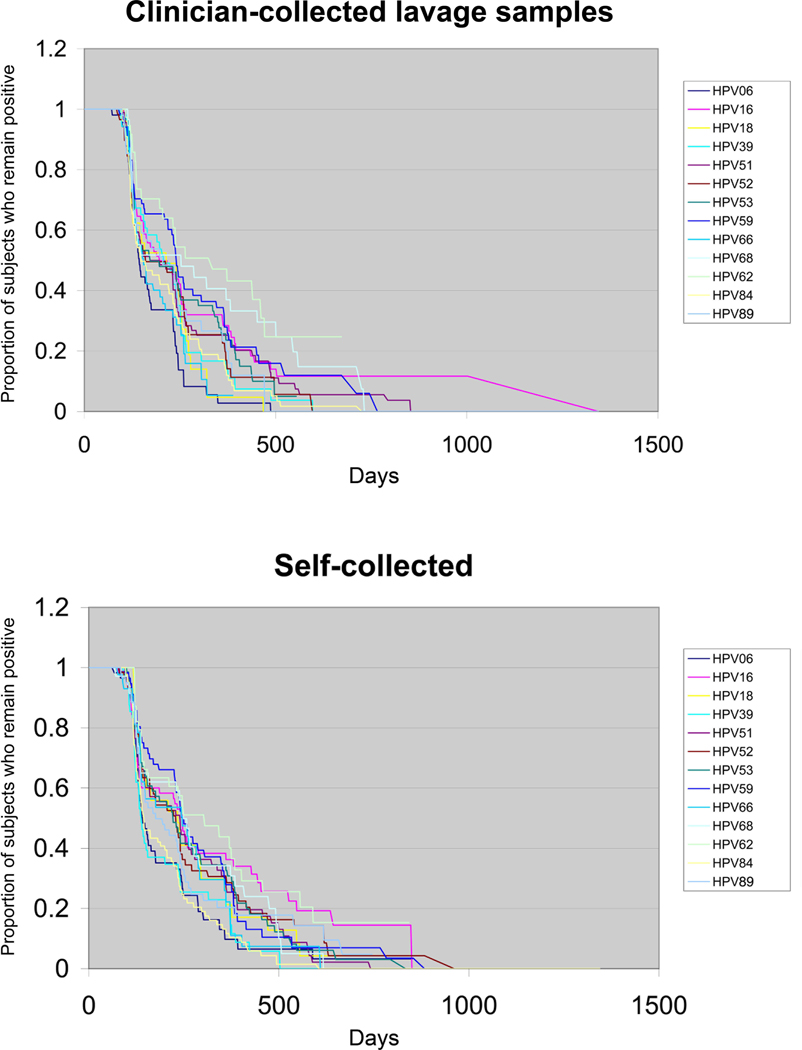
The third approach to compare sampling method was to examine patterns of HPV types (faster, slower) within a sampling method and see if those patterns were also present in the other method. Among the CC-L samples, HPV 6, 66 and 18 had the shortest time-to-clearance with 97%, 95% and 95%, respectively, showing clearance by 1 year. Following these 3 types, HPV 84, 39 and 89 were the second fastest group to clear. The slowest to clear by one year were HPV 62, 68, 59 and 16. HPV 6 was statistically significantly faster to clear than HPV 16, 39, 51, 52, 53, 59, and 68. Among HR HPV types, HPV 66 was faster to clear than HPV 16, 53, 59 and 68 and HPV 18 was faster than 59 and 68 with a trend for HPV 16. HPV 39 was also found to clear faster than HPV 59 and 68. HPV 16 was similar to most other oncogenic HPV types except 66 as noted. Among the LR types, HPV6 was also faster to clear than HPV 62 and 89, but not HPV 84. Curves for time-to-clearance of CC-L samples are shown in Figure 4; times-to-clearance and Hazard ratios for CC-L samples are summarized in Tables 3 and 4, respectively.
Figure 4. Comparison of HPV type-specific clearance among clinician-collected lavage samples and self-collected samples.
See Table 3 for clearance rates and Table 4 for statistical comparison.
Table 3.
Clearance rate of 13 specific HPV types among 537 women using clinician-collected lavage and self-collected samples
| Clinician-collected lavage samples | Self-collected samples | |||||||
|---|---|---|---|---|---|---|---|---|
| HPV type |
N | Median Days to 50% clearance |
Median Days to 75% clearance |
% Clearance at 1 year |
N | Median Days to 50% clearance |
Median Days to 75% clearance |
% Clearance at 1 year |
| 6 | 65 | 143 | 235 | 97 | 69 | 141 | 247 | 90 |
| 16 | 109 | 196 | 384 | 72 | 86 | 245 | 490 | 64 |
| 18 | 41 | 187 | 261 | 95 | 40 | 222 | 329 | 79 |
| 39 | 60 | 206 | 253 | 83 | 63 | 137 | 241 | 80 |
| 51 | 108 | 172 | 294 | 75 | 94 | 235 | 363 | 75 |
| 52 | 75 | 160 | 272 | 77 | 77 | 226 | 379 | 71 |
| 53 | 80 | 172 | 364 | 74 | 79 | 221 | 386 | 69 |
| 59 | 80 | 240 | 376 | 68 | 74 | 244 | 379 | 72 |
| 62 | 48 | 300 | 471 | 57 | 54 | 276 | 458 | 60 |
| 66 | 63 | 154 | 253 | 95 | 56 | 245 | 359 | 78 |
| 68 | 37 | 200 | 477 | 59 | 41 | 249 | 398 | 66 |
| 84 | 91 | 148 | 268 | 85 | 101 | 144 | 246 | 90 |
| 89 | 58 | 201 | 333 | 80 | 74 | 176 | 269 | 82 |
Table 4.
Hazard ratio* for time-to-clearance between HPV types for clinician-collected lavage samples and self-collected samples
| Clinician-collected lavage samples | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 16 | 18 | 39 | 51 | 52 | 53 | 59 | 62 | 66 | 68 | 84 | 89 | |
| 1.89*** | 1.43 | 1.60*** | 1.63*** | 1.64** | 1.80*** | 2.28*** | 3.15*** | 1.22 | 2.49*** | 1.36 | 1.62** | 6 | |
| 0.70* | 0.76 | 0.85 | 0.79 | 0.89 | 1.06 | 1.62* | 0.66** | 1.14 | 0.66** | 0.81 | 16 | ||
| 1.09 | 1.25 | 1.22 | 1.42 | 1.68*** | 2.48*** | 0.85 | 2.04** | 1.03 | 1.26 | 18 | |||
| 1.14 | 1.04 | 1.20 | 1.50** | 2.20*** | 0.80 | 1.77** | 0.90 | 1.07 | 39 | ||||
| 0.91 | 0.99 | 1.21 | 1.83** | 0.76 | 1.31 | 0.75* | 0.91 | 51 | |||||
| 1.12 | 1.38 | 2.04*** | 0.78 | 1.66* | 0.87 | 1.02 | 52 | ||||||
| 1.23 | 1.85** | 0.66** | 1.52 | 0.75 | 0.88 | 53 | |||||||
| 1.48 | 0.55*** | 1.14 | 0.61*** | 0.74 | 59 | ||||||||
| 0.38*** | 0.77 | 0.41*** | 0.49*** | 62 | |||||||||
| 1.93** | 1.08 | 1.36 | 66 | ||||||||||
| 0.52*** | 0.62* | 68 | |||||||||||
| 1.21 | 84 | ||||||||||||
| 89 | |||||||||||||
| Self-collected samples | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 16 | 18 | 39 | 51 | 52 | 53 | 59 | 62 | 66 | 68 | 84 | 89 | |
| 1.63** | 1.44 | 0.97 | 1.38 | 1.49** | 1.44* | 1.70*** | 2.12*** | 1.43 | 1.57* | 0.96 | 1.28 | 6 | |
| 0.83 | 0.55*** | 0.77 | 0.84 | 0.78 | 0.88 | 1.77 | 0.76 | 0.83 | 0.52*** | 0.77 | 16 | ||
| 0.65* | 1.00 | 1.07 | 1.00 | 1.12 | 1.55 | 0.92 | 1.05 | 0.63** | 0.94 | 18 | |||
| 1.57** | 1.62** | 1.65*** | 1.70** | 2.28*** | 1.40 | 1.75** | 0.97 | 1.41* | 39 | ||||
| 1.13 | 1.04 | 1.13 | 1.60** | 0.95 | 1.07 | 0.63*** | 0.98 | 51 | |||||
| 0.94 | 1.04 | 1.38 | 0.86 | 0.98 | 0.59*** | 0.90 | 52 | ||||||
| 1.08 | 1.53* | 0.86 | 1.03 | 0.61*** | 0.94 | 53 | |||||||
| 1.36 | 0.81 | 1.02 | 0.56*** | 0.79 | 59 | ||||||||
| 0.61* | 0.71 | 0.42*** | 0.65* | 62 | |||||||||
| 1.23 | 0.66 | 0.97 | 66 | ||||||||||
| 0.55 | 0.90 | 68 | |||||||||||
| 1.46** | 84 | ||||||||||||
| 89 | |||||||||||||
p<0.01;
p<0.05;
p<0.10
Hazard ratio is defined as the hazard of HPV types listed in right column compared to HPV listed on top row.
Similar patterns were seen among the SC samples. The top 6 fastest types to clear by 1 year were the same as seen among the CC-L samples (in order starting with fastest: HPV 6, 84, 89, 39, 18 and 66). The slowest to clear were also similar (in order starting with the slowest: HPV 62, 16 and 68). Statistical comparisons found that HPV 6 was significantly faster than HPV 16, 52 and 59 with trends for HPV 53 and 68. Among the high risk types, HPV 39 was faster to clear than HPV 16, 51, 52, 53, 59, and 68 with a trend for HPV 18. Among the high-risk HPV types, no statistically significant differences were seen for HPV 18 or HPV 66, as seen with the CC-L samples. HPV 16 again was similar to the other high-risk types except HPV 39. Among the low-risk types, HPV 6 was faster than HPV 62, but no difference was seen for HPV 84 and 89. Curves for time-to-clearance for SC samples are shown in Figure 4; times-to-clearance and Hazard ratios for SC samples are summarized in Tables 3 and 4, respectively. In a separate analysis, we compared HPV 16 time-to-clearance to all the other oncogenic types combined and found no statistical difference for either the SC or CC-L samples (data not shown).
Comparison of flanking CC-L and SC samples
The previous analysis focused on the natural history with repeated measures. We were also interested in examining how often CC-L and SC samples correlated in proximity to time. This analysis focused on high-risk types only and no imputed data were used for this analysis.
First, we examined the probability that the intervening SC sample was positive if 2 consecutive CC-L samples were both positive for the same type. If 2 consecutive CC-L samples were positive for the same type, 69% (range 53% − 88%) of intervening SC samples were also positive for the same type (+++). HPV 18 had the highest probability (88%) and HPV 53 had the lowest (53%). In comparison, if 2 consecutive SC samples were positive for the same type, 61% (range 30% – 77%) of the intervening CC-L samples were positive (p = 0.02). HPV 59 had the highest (77%), and HPV 39 had the lowest (30%). Results for specific HPV types are shown in Table 5; columns A and B. If 2 consecutive samples were negative, whether CC-L or SC, the intervening sample was almost always likely to be negative. The probability was over 99% for all types (data not shown).
Table 5.
Comparison between flanking clinician-collected lavage and self-collected samples for 9 high-risk HPV types
| A | B | C | D | |
|---|---|---|---|---|
| HPV type | Probability of intervening SC sample being positive between 2 consecutive positive CC-L samples1 |
Probability of intervening CC- L sample being positive between 2 consecutive positive SC samples1 |
Probability of intervening or flanking SC sample being positive when 2 consecutive CC- L samples are positive2 |
Probability of intervening or flanking CC-L samples being positie when 2 consecutive SC samples are positive2 |
| 16 | .67 | .67 | .79 | .90 |
| 18 | .88 | .50 | 1.0 | .57 |
| 39 | .80 | .30 | 1.0 | .56 |
| 51 | .78 | .74 | .98 | .95 |
| 52 | .68 | .60 | .91 | .79 |
| 53 | .53 | .67 | .87 | .91 |
| 59 | .70 | .77 | .97 | .95 |
| 66 | .60 | .54 | .75 | .71 |
| 68 | .60 | .72 | .87 | .89 |
compares +⊕+ vs +⊖+, where ○ reflects the intervening test.
compares +⊕+ or +-+⊕or ⊕+-+ vs⊖+⊖+⊖, where ○ reflects the intervening or flanking test.
We next examined the rate of SC positive samples if we used repeated SC sampling. Among 2 consecutive positive CC-L samples, the rate of matching positive tests was higher if two consecutive SC samples were examined; 90.4% (range 75%–100%) of positive CC-L specimens had at least one flanking SC sample [either sandwiched (+⊕+) or before (⊕+-+-) or after (-+-+⊕) positive for the same type. The rate of matching positive tests was also higher when examining the inverse relationship. Among 2 consecutive positive SC samples, 80.3% (range 56% to 95%) of positive SC samples had at least one flanking CC-L sample (either sandwiched or before or after) positive for the same type. Results for specific HPV types are shown in Table 5; columns C and D.
Discussion
This is the first study to compare estimates of specific features of the natural history of HPV based on both SC and CC-L samples in a single cohort. We showed that both sampling methods lead to similar estimated clearance rates for high-risk and low-risk HPV types, suggesting that both likely obtain HPV-infected cells in the same vicinity. In addition, “patterns” of HPV types which were fastest or slowest among the SC samples were extremely similar to the CC-L samples. HPV 6, 18, 39, 66, 84 and 89 were found to be the fastest to clear by both methods and HPV 68, 16 and 62 were the slowest to clear. All of these findings show that the natural history defined by SC will be similar for most HPV types to that observed by CC-L samples. This conclusion was underscored by the fact that the natural history of women with an initial positive cervical test was similar if followed by CC-L samples or if followed by SC means. We believe self-sampling will be an excellent tool for following women with HPV infections. Its clinical use has yet to be defined, however, one example would include follow-up of women who are HR-HPV positive but have normal cytology. Rather than require a repeat clinical visit as currently recommended 4, women could mail in an SC sample.
Although most HPV types display similar clearance patterns, HPV 66 had a trend association with faster clearance in the CC-L samples when directly compared to SC samples in the independent analysis and HPV 16 had a trend for the within-woman analysis with CC-L samples clearing somewhat slower. This finding was consistent for HPV 66 with the observation that within the CC-L samples 66 cleared faster (statistically) than some of the other high-risk types whereas within the SC samples this difference was not observed. Little to nothing is known about the natural history of vaginal HPV and its relationship to vaginal cancers. However, the slower clearance in the SC swabs has likely no clinical implications since HPV 66 is equally rare in vaginal as well as cervical cancers.23, 24 The slightly slower clearance seen in the CC-L samples for HPV 16 may imply that a negative test by SC for HPV 16 must be interpreted with some caution.
Of interest, HPV 16 did not vary significantly from most of the other HR-HPV types in its natural history, whether measured by CC-L or SC means. This finding is consistent with several studies which show that incident HPV 16 infections do not clear at a substantially different rate than other high-risk HPV types.25–28 Others have shown that the longer HPV persists, the more likely it is to persist.28, 29 Hence, studies in older women are more likely to represent HPV 16 persistent infection at entry. As seen in other studies using cervical sampling, low-risk HPV 6 was much faster to clear than the high-risk types by either method.29 Interestingly, the other low-risk types behaved much closer to the other high-risk types.
In contrast to the comparisons over time, the cross-sectional analysis pointed to discrepancies between methods. Because of our 2-month sampling design, we were unable to examine test results at the same moment. However, we were able to look at results for one method sandwiched in time between results for the other method. In this analysis, we selected women with HPV persistence of 4 months. We showed that the probability of having a positive SC sample obtained between two positive CC-L samples was around 70%. These results suggest that a single SC swab may miss a type detected by CC-L, as found in other studies.30 However, these discrepancies appear to become mute over time. If we looked at 2 consecutive SC samples (i.e., the one sandwiched or the ones before or after the two consecutive positive CC-L samples), the chance of at least one of them matching the CC-L was much higher – closer to 90%. This observation is similar to cytology where the sensitivity of CIN detection increases with the frequency of the testing. In the case of self-sampling which could be mailed in, there may be cost savings that offset the need for repeated sampling. Of the specific HPV types examined in this study, HPV 16 had one of the greater chances of missing a cervical infection (20%) even with repeated sampling.
One of the limitations of our study was that we employed cervical lavages as used in other natural history studies31 which may have enhanced the correlation as compared to clinician-collected swabs which are more directed to the cervix. The large number of missing SC samples compared to CC-L samples may have also influenced our results. We feel that this did not significantly impact our results since the unimputed data looked similar and the majority of imputations were negative samples sandwiched between other negative samples. Using the raw values, the probability for this occurrence for most types was over .95. These studies should also be performed in older women since the similar findings in the natural history in our study may have been influenced by the fact that most HPV infections in young women are transient. Clearly, the clinical utility of our findings will rely on further work focusing on detection of high-grade squamous intra-epithelial lesions (HSIL) in women, specifically using HPV 16 and 18 specific detection tests. We had too few cases to examine HSIL as an outcome in this young cohort.
In conclusion, clinician-directed lavages and self-obtained sampling show little difference in describing the natural history of HPV making self-sampling an attractive and feasible method of following women with HPV.
Novelty and Impact of Paper.
Self-collected samples are equivalent to clinician-collected samples in defining the natural history of HPV.
Acknowledgements
This work was supported by the National Institutes of Health/National Cancer Institute [2 R37 CA51323] and Maternal and Child Health Bureau/Health Resources and Services Administration [T71MC00003]. This work was carried out in part in the Pediatric Clinical Research Center, Moffitt Hospital, University of California San Francisco with funds provided by the National Center for Research Resources [5 M01 RR-01271]. Roche Molecular Diagnostics provided training and supplies to support the HPV DNA testing. We would like to acknowledge Anthony Kung for his support in preparation of the manuscript.
Abbreviations used
- CC
clinician-collected
- DNA
Deoxyribonucleic acid
- HPV
Human Papillomavirus
- HR
High-risk
- LR
Low-risk
- PCR
polymerase chain reaction
- SC
self-collected
- CVL
Cervical vaginal lavage
REFERENCES
- 1.Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 2.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, Darragh T, Palefsky J. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 3.Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Agorastos T, Dinas K, Lloveras B, Font R, Kornegay JR, Bontis J, de Sanjose S. Self-sampling versus physician-sampling for human papillomavirus testing. Int J STD AIDS. 2005;16:727–729. doi: 10.1258/095646205774763225. [DOI] [PubMed] [Google Scholar]
- 6.Holanda F, Jr, Castelo A, Veras TM, de Almeida FM, Lins MZ, Dores GB. Primary screening for cervical cancer through self sampling. Int J Gynaecol Obstet. 2006;95:179–184. doi: 10.1016/j.ijgo.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Longatto-Filho A, Roteli-Martins C, Hammes L, Etlinger D, Pereira SM, Erzen M, Branca M, Naud P, Derchain SF, Sarian LO, Matos J, Gontijo R, et al. Self-sampling for human papillomavirus (HPV) testing as cervical cancer screening option. Experience from the LAMS study. Eur J Gynaecol Oncol. 2008;29:327–332. [PubMed] [Google Scholar]
- 8.Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, Rozendaal L, Jaspars LH, Voorhorst FJ, Verheijen RH, Meijer CJ. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J Clin Pathol. 2002;55:435–439. doi: 10.1136/jcp.55.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellors JW, Lorincz AT, Mahony JB, Mielzynska I, Lytwyn A, Roth P, Howard M, Chong S, Daya D, Chapman W, Chernesky M. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ. 2000;163:513–518. [PMC free article] [PubMed] [Google Scholar]
- 10.Stenvall H, Wikstrom I, Wilander E. High prevalence of oncogenic human papilloma virus in women not attending organized cytological screening. Acta Derm Venereol. 2007;87:243–245. doi: 10.2340/00015555-0205. [DOI] [PubMed] [Google Scholar]
- 11.Szarewski A, Cadman L, Mallett S, Austin J, Londesborough P, Waller J, Wardle J, Altman DG, Cuzick J. Human papillomavirus testing by self-sampling: assessment of accuracy in an unsupervised clinical setting. J Med Screen. 2007;14:34–42. doi: 10.1258/096914107780154486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baay M, Verhoeven V, Wouters K, Lardon F, Van Damme P, Avonts D, Van Marck E, Van Royen P, Vermorken JB. The prevalence of the human papillomavirus in cervix and vagina in low-risk and high-risk populations. Scand J Infect Dis. 2004;36:456–459. doi: 10.1080/00365540410020677. [DOI] [PubMed] [Google Scholar]
- 13.Karwalajtys T, Howard M, Sellors JW, Kaczorowski J. Vaginal self sampling versus physician cervical sampling for HPV among younger and older women. Sex Transm Infect. 2006;82:337–339. doi: 10.1136/sti.2005.019430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmisano ME, Gaffga AM, Daigle J, Brinkman J, Mire K, Lenczyk K, Martin DH, Hagensee ME. Detection of human papillomavirus DNA in self-administered vaginal swabs as compared to cervical swabs. Int J STD AIDS. 2003;14:560–567. doi: 10.1258/095646203767869183. [DOI] [PubMed] [Google Scholar]
- 15.Petignat P, Faltin DL, Bruchim I, Tramer MR, Franco EL, Coutlee F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105:530–535. doi: 10.1016/j.ygyno.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Seo SS, Song YS, Kim JW, Park NH, Kang SB, Lee HP. Good correlation of HPV DNA test between self-collected vaginal and clinician-collected cervical samples by the oligonucleotide microarray. Gynecol Oncol. 2006;102:67–73. doi: 10.1016/j.ygyno.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Stewart DE, Gagliardi A, Johnston M, Howlett R, Barata P, Lewis N, Oliver T, Mai V. Self-collected samples for testing of oncogenic human papillomavirus: a systematic review. J Obstet Gynaecol Can. 2007;29:817–828. doi: 10.1016/s1701-2163(16)32636-6. [DOI] [PubMed] [Google Scholar]
- 18.Baldwin S, Santos C, Mendez Brown E, Nuno T, Giuliano A, Davis J, Garcia F. Comparison of type-specific human papillomavirus data from self and clinician directed sampling. Gynecol Oncol. 2005;97:612–617. doi: 10.1016/j.ygyno.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–232. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 20.Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, Miller S, Canjura-Clayton L, Farhat S, Broering JM, Darragh TM. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–1683. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ. The Robust Inference for the Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 22.Munoz N, Castellsague X, de Gonzalez AB, Gissman L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24:S3-1–S3-11. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz N, Bosch FX, De Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiological Classification of Human Papillomavirus Types Associated with Cervical Cancer. NEJM. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 24.Daling JR, Madeleine MM, Schwartz SM, Shera KA, Carter JJ, McKnight B, Porter PL, Galloway DA, McDougall JK, Tamimi H. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol. 2002;84:263–270. doi: 10.1006/gyno.2001.6502. [DOI] [PubMed] [Google Scholar]
- 25.Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, Coutlee F, Franco EL. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–490. [PubMed] [Google Scholar]
- 26.Munoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Munoz A. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 27.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68:8813–8824. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trottier H, Mahmud S, Prado JC, Sobrinho JS, Costa MC, Rohan TE, Villa LL, Franco EL. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. J Infect Dis. 2008;197:1436–1447. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Progression and regression of incident cervical HPV 6, 11, 16 and 18 infections in young women. Infect Agent Cancer. 2007;2:15. doi: 10.1186/1750-9378-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castle PE, Rodriguez AC, Porras C, Herrero R, Schiffman M, Gonzalez P, Hildesheim A, Burk RD. A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis. 2007;34:849–855. doi: 10.1097/OLQ.0b013e318064c8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural History of cervicovaginal papillomavirus infection in young women. NEJM. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]