Abstract
The cytoskeletal organization of detached and circulating tumor cells is currently not well-defined and may provide potential targets for new therapies to limit metastatic tumor spread. In vivo, circulating tumor cells reattach in distant tissues via a mechanism that is tubulin-dependent and suppressed by polymerized actin. The cytoskeletal mechanisms that promote reattachment of circulating tumor cells match exactly with the mechanisms supporting tubulin microtentacles, which we have recently identified in detached breast tumor cells. In this study, we aimed to investigate how microtentacle formation is affected by the microtubule-associated protein, tau, which is expressed in a subset of chemotherapy-resistant breast cancers. We demonstrate that endogenous tau protein localizes to microtentacles and is both necessary and sufficient to promote microtentacle extension in detached breast tumor cells. Tau-induced microtentacles increase reattachment of suspended cells and retention of circulating tumor cells in lung capillaries. Analysis of patient-matched primary and metastatic tumors reveals that 52% possess tau expression in metastases and 26% display significantly increased tau expression over disease progression. Tau enrichment in metastatic tumors and the ability of tau to promote tumor cell reattachment through microtentacle formation support a model in which tau-induced microtubule stabilization provides a selective advantage during tumor metastasis.
Keywords: microtentacles, tau, microtubule-associated proteins, microtubules, circulating tumor cells
Introduction
Metastatic growth is the leading cause of death among cancer patients. Unfortunately, current cancer regimens that are successful in treating primary tumors have limited success in controlling metastatic disease (Steeg, 2006). For breast cancers, metastases can develop years after primary tumor resection. While the manner by which these metastases develop remains controversial, evidence suggests that systemic spread may be an early event in breast cancer and not exclusive to the established model of late onset metastasis from large primary tumors (Husemann et al., 2008). While much remains to be understood regarding the mechanisms of metastasis, evidence indicates that a target for anti-metastatic drug development is circulating tumor cells (CTCs; Steeg and Theodorescu, 2008). Predominantly, epithelial cell dissemination into the microvasculature leads to death by anoikis or necrosis by shear forces – termed metastatic inefficiency (Weiss, 1991; Yamauchi et al., 2006). This is not true for CTCs. In addition to apoptosis resistance, metastatically-efficient CTCs possess cytoskeletal aberrations that grant tolerance to the morphological deformations that are required during dissemination (Hall, 2009).
Cytoskeletal rearrangements that affect cancer progression have been identified in each cytoskeletal system (actin, microtubules, and intermediate filaments) and their accessory factors (Hall, 2009; Mialhe et al., 2001; Thomas and Brugge, 1997; Willipinski-Stapelfeldt et al., 2005). Recently, attention has been given to microtubule-associated proteins (MAPs) as predictors of chemotherapy sensitivity and patient relapse (McGrogan et al., 2008). MAPs function throughout the cell cycle to modulate microtubule dynamics (Cassimeris and Spittle, 2001). Consequently, MAP expression can affect greatly the efficacy of microtubule-targeted therapies, such as the anti-mitotic drugs, Vinca alkaloids and taxanes (Bhat and Setaluri, 2007). To identify predictive markers of pathologic complete response to paclitaxel (Taxol) in breast cancer patients, the MAP tau was identified as the most differentially-expressed gene (Rouzier et al., 2005).
Tau is part of the MAP2 family, which promote microtubule assembly through increased nucleation, increased stabilization, and reduced catastrophes (Cassimeris and Spittle, 2001; Weingarten et al., 1975). Classically, tau is characterized as a neuronal MAP, where it is involved in neurite outgrowth and tauopathies (Goedert, 2004). In cells attached to extracellular matrix (ECM), tau promotes the extension of microtubule-based neurite-like protrusions, even in non-neuronal cell types (Knops et al., 1991). While tau expression has been studied in attached cells and in breast cancers for its contribution to chemotherapy resistance, the role of tau in detached cells, such as CTCs, is uninvestigated.
Detached human and mouse mammary epithelial and breast carcinoma cell lines produce dynamic microtubule-based extensions of the plasma membrane, termed microtentacles (McTNs), which are enhanced by actin depolymerization and reduced by microtubule depolymerization (Whipple et al., 2007). Additionally, McTNs facilitate reattachment of detached cells to ECM and to other cells (Whipple et al., 2007). In vivo studies demonstrate that CTCs bind to capillary walls by a mechanism that matches the microtubule-dependence of McTNs and is similarly increased by reducing actin polymerization (Korb et al., 2004). We have revealed that McTNs are persistent in apoptotically-resistant and highly metastatic cell lines and are enriched in detyrosinated microtubules and vimentin (Whipple et al., 2008; Whipple et al., 2007). Given cancer heterogeneity, we asked whether McTNs form by other pathways in tumor cells that lack vimentin. Tau became a strong candidate to influence McTN formation, as it affects microtubule stability and is reported in chemoresistant breast cancers (Cassimeris and Spittle, 2001; Rouzier et al., 2005). Here, we report that tau promotes McTN formation and the reattachment of detached human mammary tumor cells, resulting in greater trapping and retention in the lung microvasculature. Furthermore, in 26% of patients, tau is enriched in metastatic breast tumors compared to matched primary tumors. This supports a model in which increased McTNs and reattachment efficiency of tau-expressing tumor cells provides a selective advantage to CTCs during metastasis.
Results
Breast tumor cell lines that express tau form more McTNs
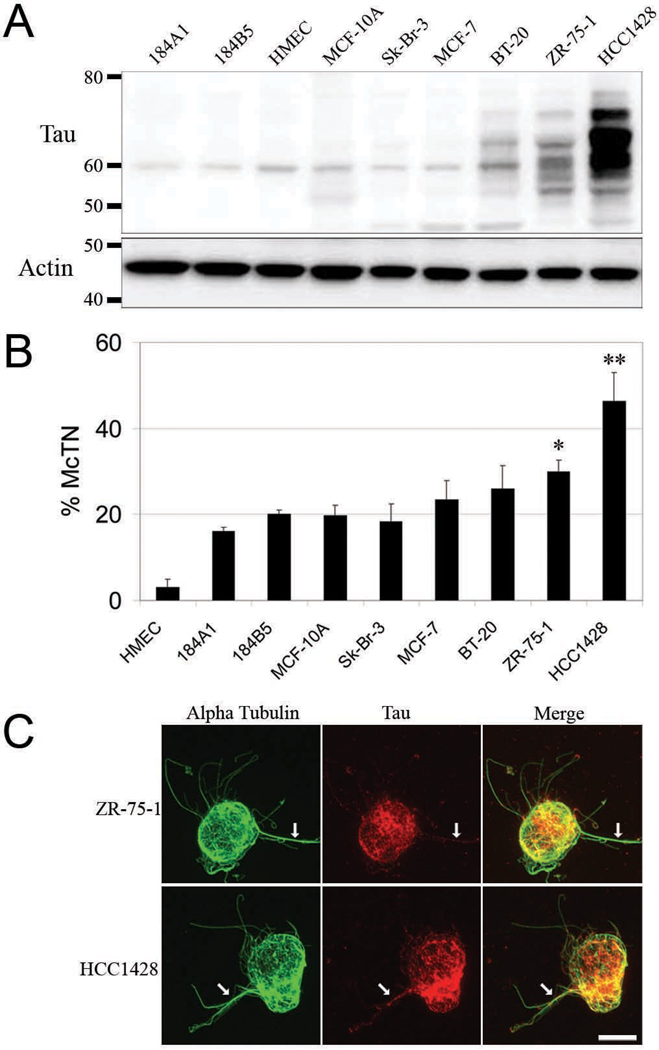
Tau was evaluated in the breast tumor cell lines Sk-Br-3, MCF-7, BT-20, ZR-75-1 and HCC1428. Expression was compared to primary human mammary epithelial cells (HMEC) and the non-tumorigenic cell lines 184A1, 184B5, and MCF-10A (Fig. 1). Tau Western blots revealed a broad molecular weight range concomitant with increasing expression level as the metastatic potential of cell lines increased. Multiple molecular weight varieties were present in BT-20, ZR-75-1, and HCC1428 (Fig. 1A). Densitometry analysis of tau western blots revealed a range of tau protein expression from a 0.3-fold change for Sk-Br-3 up to a 27-fold change for HCC1428 when normalized to MCF-10A.
Figure 1. McTN formation increases with tau expression.
(A) Western blots for tau (45–75kDa) revealed an increasing expression and molecular weight range as tumorigenicity increases from the HMEC cells to HCC1428. β-actin served as loading control. (B) McTN frequency increases concomitantly with tau expression. HCC1428 forms significantly more McTNs than lower tau expressing cells lines. ZR-75-1 forms significantly more McTNs than, HMEC, 184A1, 184B5, MCF-10A and Sk-Br-3. Data (n=3) are represented as the mean ± s.d. *P<0.05. (C) ZR-75-1 and HCC1428 were subjected to indirect IF for tau followed by direct IF for α-tubulin. As tau expression increases, localization of tau within McTNs increases (white arrows). Cells and McTNs displayed are representative of those under conditions of actin depolymerization.
We then tested the hypothesis that cell lines that highly express tau also generate more McTNs. It was revealed that tau protein expression directly correlated with a greater frequency of McTNs in a population of breast tumor cells (Fig. 1B). Notably, the population of HCC1428 cells possessed a significantly higher McTN frequency compared to all of the cell lines of lower tau expression. Additionally, populations of ZR-75-1 cells displayed a significantly higher McTN frequency compared to all the nontumorigenic cell lines (HMEC, 184A1, 184B1, MCF-10A) and the poorly metastatic Sk-Br-3. Immunofluorescence revealed that endogenous tau protein localized to McTNs and colocalized with tubulin, especially in ZR-75-1 and HCC1428 cells (Fig. 1C and Fig. S1–S2). Previously, we described vimentin as a mediator of McTN formation (Whipple et al., 2008). Western blotting confirmed that tau and vimentin are not extensively co-expressed (Fig. S3).
Tau increases McTN frequency and alters McTN morphology
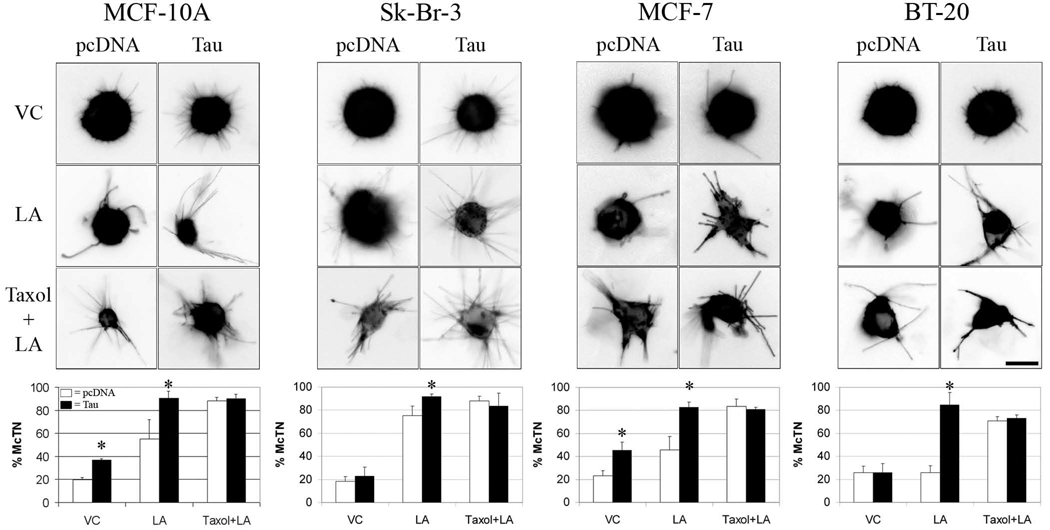
To confirm that tau expression directly promotes McTN formation, cells of lower tau expression were transiently cotransfected with membrane-targeted GFP vector and either a vector encoding a 50-kDa untagged tau protein possessing three microtubule-binding repeats (Fig. S4) or an empty vector, pcDNA. Transfected cells were assessed for McTNs in vehicle control (VC) conditions, Latrunculin-A (LA) to disrupt actin, or a combination of LA and Taxol to serve as a positive control for microtubule stabilization (Fig. 2 and Table S1). In MCF-10A, tau expression significantly increased McTN frequency in both VC and LA conditions compared to pcDNA. Tau increased McTN frequency in MCF-7 cells comparable to MCF-10A, while for Sk-Br-3 and BT-20 cells, McTN formation significantly increased only when treated with LA. In pcDNA cells, the McTN induction by LA was enhanced by Taxol; however, in tau-expressing cell lines, Taxol was unable to further increase McTNs.
Figure 2. Exogenous tau overexpression stimulates McTN formation.
McTNs were evaluated blindly in live MCF-10A, Sk-Br-3, MCF-7, and BT-20 cells exogenously overexpressing pcDNA (left micrograph panels, white bars) or tau (right micrograph panels, black bars) in VC, LA, and Taxol+LA. Tau overexpression caused distinct morphological alterations in length, thickness and ridigity of McTNs formed compared to pcDNA. Tau-induced McTNs became longer and thicker in VC conditions compared to pcDNA. Treatment of tau-transfected cells with LA increased the length, thickness, and rigidity of McTNs comparable to empty vector cells treated with Taxol+LA. Frequency of McTN formation (lower graphs) revealed that tau significantly increased McTN formation. Data (n=3) are represented as the mean ± s.d. *P<0.05, scale bars = 10µm.
Blinded live cell fluorescent microscopy images were obtained of cells in suspension and qualitatively evaluated for morphological distinctions among McTNs of tau- and pcDNA-expressing cells (Fig. 2). In VC conditions across all cell lines, pcDNA-transfected cells displayed short and thin McTNs. In tau-transfected cells, McTNs were longer and thicker than pcDNA controls. LA treatment of pcDNA cells dramatically increased the length and thickness of McTNs. LA treatment of cells overexpressing tau retained the increased length and thickness and resembled the McTNs of cells treated with Taxol+LA. Treatment of tau overexpressing cells with Taxol + LA had little effect on McTN morphology.
Knockdown of endogenous tau diminishes McTN formation in ZR-75-1 cells
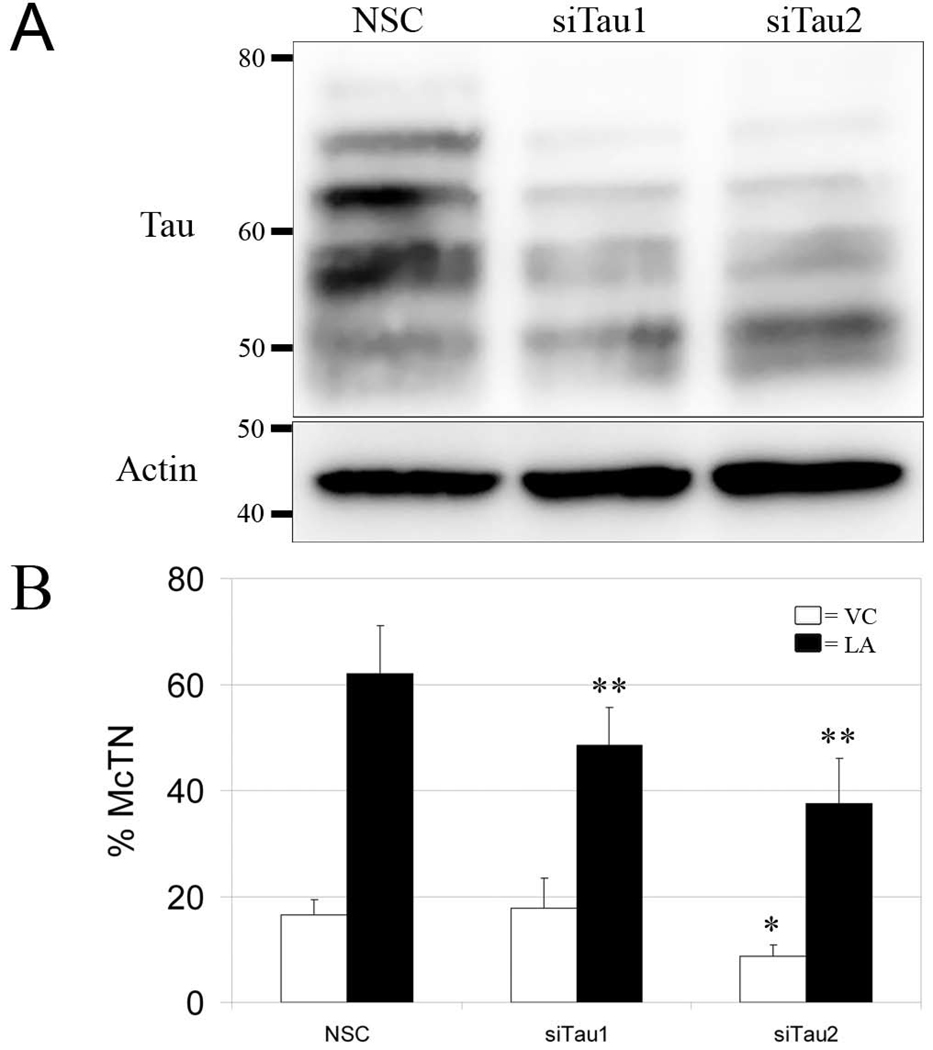
To address if reducing tau expression represses McTN formation, we chose a stably-transfectable shRNA plasmid system to knockdown tau expression in ZR-75-1 cells. Two clones, designated siTau1 and siTau2, possessed ~65% less tau expression compared to the non-silencing control (NSC) cells (Fig. 3A). These clones were evaluated for McTN formation. siTau2 displayed significantly lower McTN formation compared to the NSC when suspended in the vehicle only (Fig. 3C, white bars); however, upon depolymerization of actin with LA, both siTau1 and siTau2 formed significantly less McTNs than the NSC (Fig. 3C, black bars).
Figure 3. shRNA-mediated knockdown of tau in ZR-75-1 cells significantly reduces McTN formation.
(A) siTau1 and siTau2 express less tau than the NSC parent line on Western blots. β-actin served as a loading control. (B) Under conditions of actin depolymerization with LA (black bars), siTau1 and siTau2 possess significantly less McTNs compared to NSC. When left untreated in VC (white bars), siTau2 form significantly less McTNs than NSC. Data (n=3) represents the mean ± s.d. *P<0.05; **P<0.001
Tau expression significantly affects initial reattachment of suspended cells
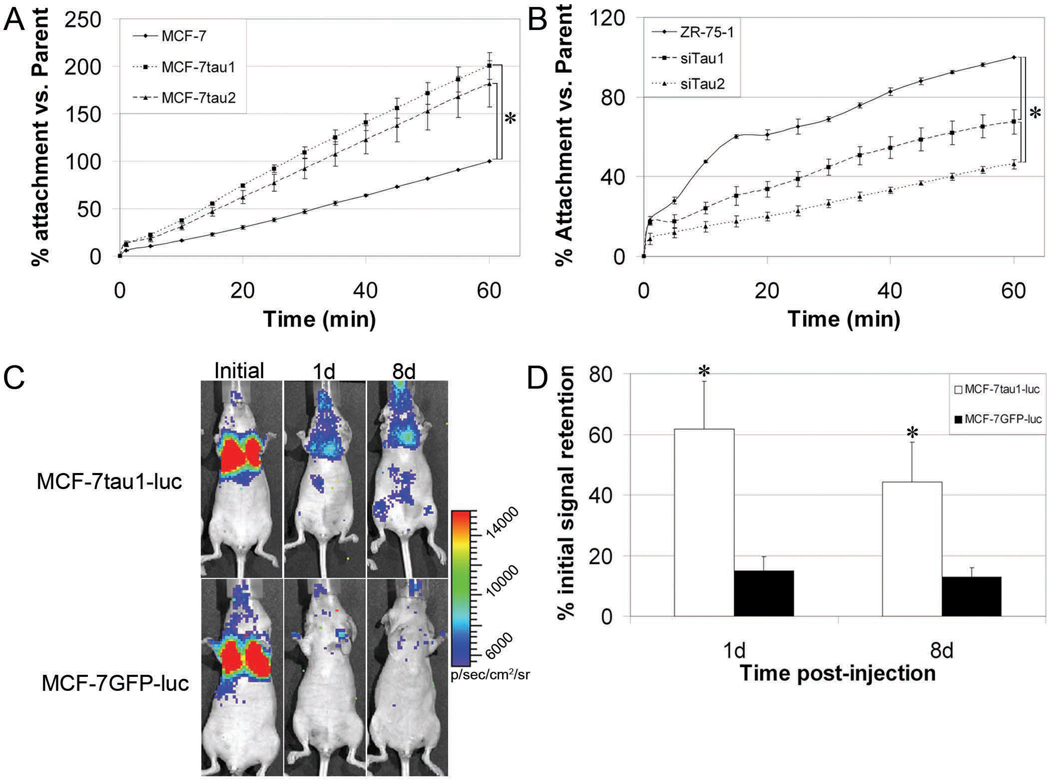
As McTN formation has been shown to greatly influence reattachment of suspended cells (Whipple et al., 2008; Whipple et al., 2007), we evaluated the efficiency of suspended cell reattachment as a consequence of tau expression. MCF-7 cells stably expressing GFP-tau, designated MCF-7tau1 and MCF-7tau2 (Fig. S5), and siTau1 and siTau2, were assessed for cell-substratum reattachment and compared to the parental control cells. MCF-7tau1 and MCF-7tau2 cells attached more efficiently than the parental MCF7 cells. By one hour, 200% and 180% more MCF-7tau1 and MCF-7tau2 cells attached, respectively (Fig. 4A). For tau knockdown cells, siTau1 and siTau2, cell attachment was significantly impaired compared to parental control ZR-75-1 cells (Fig. 4B). At one hour, 68% and 42% less siTau1 and siTau2 cells attached compared to control, respectively. Additionally, attachment efficiency of siTau2 was significantly less than siTau1, matching the differences observed in McTN formation (Fig. 3C).
Figure 4. Initial cell attachment is dependent on McTN formation as affected by tau.
(A) Over 1h, MCF7 cells stably overexpressing GFP-tau (MCF-7tau1 and MCF-7tau2) attach more efficiently (200% and 180%, respectively) compared to MCF-7 controls. Data (n=4) represents the mean percentage of attachment ± s.d. *P<0.01. (B) Over 1h, siTau1 and siTau2 attach less efficiently (67% and 46%, respectively) compared to ZR-75-1 controls. Data (n=3) is represented as the mean percentage of attachment ± s.d. *P<0.01. (C) Glowscale of luciferase-expressing cells injected into the tail vein of nude mice demonstrate equal initial signal retention in regions of interest (ROI) emcompassing the lungs. Over a period of 8d post-injection, MCF-7tau1-luc cells are more retained than control MCF-7GFP-luc cells. (D). On average over all injections, 61.8% and 44.4% of MCF-7tau1-luc cells (n=6) were retained in lung capillaries 1d and 8d post-injection. In comparison, 15.9% and 12.9% of control MCF-7GFP-luc cells (n=5) were retained at the same time points. This difference was significantly different for both 1d (*P<0.02) and 8d (**P<0.05) post-injection by a non-parametric t-test.
Tau-expressing cells are more efficiently trapped and retained in the microvasculature
Given that tau greatly enhances the reattachment efficiency of detached cells, we tested whether CTCs that express more tau would more efficiently trapped and retained in the first capillary bed that they encounter. To test this, MCF-7tau1 and MCF-7 cells stably expressing GFP alone (MCF-7GFP) were stably infected with a retroviral luciferase vector. Resultant clones, MCF-7tau1-luc and MCF-7GFP-luc, were intravenously injected into the tail vein of athymic nude mice and tracked for bioluminescence signal in the lungs using a Xenogen IVIS-200 whole-animal optical imager. At 1d and 8d, retention of bioluminescent signals were measured and expressed as percent of initial peak signal intensities (Fig. 4C). At 1d post-tail vein injection, 61.8% of MCF-7tau1-luc cells were retained in the lungs while only 15.1% of MCF-7GFP-luc were retained (p<0.02). One week later, 44.4% of the MCF-7tau1-luc cells remained trapped in the lungs compared to 12.9% of the MCF-7GFP-luc cells (p<0.05) (Fig. 4D).
Tau is enriched in patient metastases
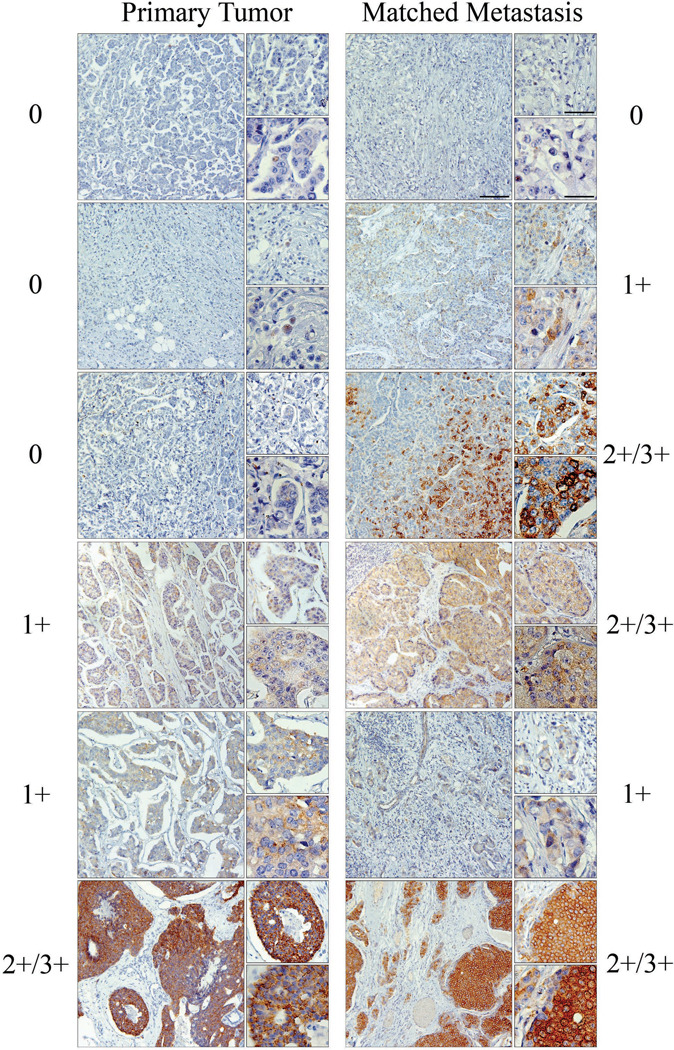
Given our in vivo data, we examined whether tau was differentially expressed in the matched metastases of primary breast tumors. Immunohistochemistry for tau was performed on tissue microarrays (TMAs) comprised of 102 primary breast carcinomas and matched metastases. 41% of primary breast tumors showed tau staining with a pathological score 1+ or higher, while 52% of the matched metastatic tumors had tau staining of 1+ or higher. Tau staining was distinguishable between pathological scores of 0 and 1; therefore, tissues were categorized as 0, 1+ or 2+/3+ (Fig. 5). Primary tumors were matched to metastases and assessed by pathological score for gain or loss of tau expression (Table 1). 26% (27 patients) showed an increase in tau between their primary to metastatic tumors, with 22 of these patients displaying metastatic expression of tau despite none in their primary tumor. It was noteworthy that none of the 10 patients with strong tau staining (2+/3+) in primary tumors showed a reduction of tau in their metastatic tumors. Statistical comparisons of tau expression in the matched patient pairs indicated that tau was significantly increased in the lymph node metastases compared to the primary lesions (generalized McNemar’s test; p=0.0169). The increase in proportion of patients with heavy staining (2+/3+) is most pronounced (p=0.0027). In addition, such increase continues to be significant (p=0.001) after adjusting for the tumor grade and diagnosis. These 102 patients were also evaluated for vimentin expression within their primary and metastatic tumors. In contrast to tau, which stained within the tumor, vimentin expression was localized to the surrounding stroma of these tumors and almost exclusively separate from tau expression (Fig. S6). Statisitcal comparison of these tumor microarrays revealed that tau and vimentin are not co-expressed significantly (Table S2, P<0.002, McNemar’s test).
Figure 5. Tau is expressed in primary breast tumors and matched lymph node metastases.
Primary tumors and patient matched lymph node metastases displayed variable levels of tau expression. 0 = No expression, 1+ = low expression, and 2+–3+ = high expression. A number of lymph node metastases displayed tau expression despite no expression in matched primary tumors, while other patients displayed stable or increased expression in lymph node deposits compared to primary tumor. Large panels, scale bar = 125µm; Top small panels, scale bar = 63µm; Bottom small panels, scale bar = 31µm. Tau expression increases significantly in metastatic tumors (Table 1, p=0.0169).
Table 1. Tau expression is significantly increased in patients’ lymph node metastases.
Primary tumors and matched lymph node metastases from 102 patients were assessed for concordance and discordance of tau expression. The generalized McNemar’s test statistic is 10.2 with 3 degrees of freedom (P=0.0169), indicating a significant difference in tau score between the primary tumor and the metastases at significance level 0.05. Dark shaded region emphasizes the number of patients (n=27, 26%) that displayed increased tau expression in the lymph node metastases.
| 102 patients total | Met Score | |||
|---|---|---|---|---|
| 0 | 1 | 2–3 | ||
| Primary Score | 0 | 37 | 18 | 4 |
| 1 | 12 | 16 | 5 | |
| 2–3 | 0 | 0 | 10* | |
indicates 10 patients with sustained strong tau staining in both primary and metastatic lesions.
Discussion
CTCs undergo cytoskeletal rearrangements that enhance their metastatic efficiency, increasing patients’ risk of metastatic disease. Previously, we reported that detached mammary epithelial and breast carcinoma cells generate McTNs that facilitate homotypic aggregation and cell reattachment to ECM (Whipple et al., 2007). McTNs arise from the coordination of detyrosinated-tubulin and vimentin and occur more frequently in cells of high metastatic potential (Whipple et al., 2008). Here, we investigated how tau, a MAP expressed in a subset of chemotherapy-resistant breast cancers, influences McTNs in detached breast carcinoma cells and contributes to the metastatic efficiency of CTCs. We report that tau enhances the reattachment and microvascular trapping of detached CTCs by stimulating McTNs. Additionally, tau is enriched in the metastatic breast tumors of 26% of patients in our cohort. These data support a molecular mechanism of McTN formation and tumor cell metastasis by which tau stabilizes and strengthens microtubules, overcoming the constraining actin cortex, to protrude the plasma membrane for more efficient cell reattachment (Fig. 6). Numerous lines of evidence support this proposed mechanism.
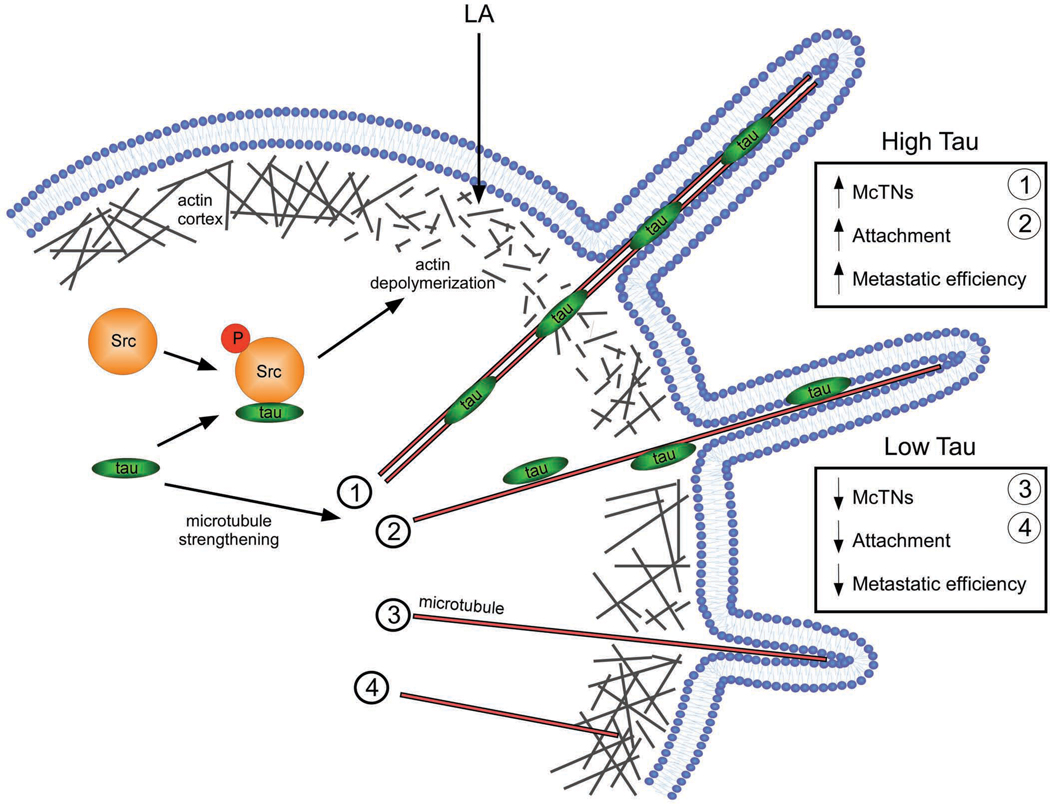
Figure 6. Model of tau-induced McTN formation.
In detached CTCs expressing tau, tau binds to and stabilizes microtubules, inducing microtubule bundling [1] and/or increasing individual polymer strength [2]. These microtubules are more able to penetrate the actin cortex due to preexisting defects in actin bundling or tau-induced actin depolymerization via priming of Src phosphorylation (Sharma et al., 2007). Consequently, microtubules easily deform the plasma membrane into McTNs that increase the ability of the cell to reattach to an ECM and improve metastatic efficiency. In detached CTCs lacking tau, microtubules are more dynamic and weaker; therefore, they are only able to form short McTNs [3] on none at all [4] due to the constriction of the actin cortex, even in the presence of preexisting cortical weaknesses. The lack of tau expression results in decreased efficiency of attachment and metastasis.
We demonstrate that tau expression directly regulates the formation and incidence of McTNs in populations of detached breast epithelial and carcinoma cell lines (Figures 1 – 3). The microtubule-stabilizing effect of tau is concentration dependent. In vitro polymerization assays reveal that tau decreases the tubulin critical concentration required for microtubule assembly; thus, the microtubule growth rate increases as the tau concentration increases (Levy et al., 2005; Panda et al., 2003). Studies of differentiating PC12 cells also reveal that the tau:tubulin ratio increases 4-fold during neurite outgrowth (Drubin et al., 1985). For tau-overexpressing tumor cells, a higher tau:tubulin ratio would yield a larger pool of polymerizing microtubules. Our results that tau overexpression and downregulation directly affect McTN formation and reattachment support our model for tau-induced effects on detached tumor cells. In vivo data also support this model, indicating that McTN-mediated CTC reattachment is dependent upon microtubule stabilization. Additionally, increased tau expression is concomitant with an increased range of molecular weight isoforms on tau Western blots (Figure 1A). These bands most likely represent phosphorylated and/or alternatively spliced isoforms. Phosphorylation and splicing regulate the tau-tubulin association, the latter by altering the number of microtubule binding domains (4R or 3R) in the C-terminus. Here, a 3R tau isoform (Figure S5 and S6) produced robust alterations in McTN frequency and morphology (Figure 2) as well as suspended cell reattachment (Figure 4). Future investigations will address how individual splice or phophorylation variants affect McTN formation.
In addition to stabilization, tau strengthens microtubules (Schaap et al., 2007). Naked microtubules are the most easily deformed among the three cytoskeletal polymers, withstanding 0.35 nano-Newtons of deforming force before irreversible collapse occurs (Janmey et al., 1991; Schaap et al., 2007). When tau-bound, individual microtubules can withstand an additional 0.1 nano-Newtons (Schaap et al., 2007). Tau can also strengthen the microtubule network by bundling microtubule polymers via the N-terminal projection domain (Chen et al., 1992). While the mechanism remains controversial, reports suggest that microtubule bundling occurs via antiparallel dimerization of tau projection domains, functionally tethering adjacent microtubules (Rosenberg et al., 2008). With added stability and strength, tau-decorated microtubules and microtubule bundles can deform the plasma membrane more efficiently, evidenced by morphological distinctions of longer and thicker tau-induced McTNs (Fig. 2). For attached cells overexpressing tau, plasma membrane deformation is independent of increased microtubule stability, as artificial microtubule stabilization with Taxol fails to induce such projections (Knops et al., 1991). This is true for suspended cells, as Taxol alone does not increase McTN formation (Balzer et al., 2009). In contrast, tau alone can increase McTN formation in MCF-10A and MCF-7 cells (Fig. 2). In combination with LA, Taxol increases McTN frequency equivalent to that of tau-expressing cells treated with LA alone. Clearly, our data elucidate a genetic mechanism by which tau overexpression in CTCs stimulates McTNs through microtubule stabilization, strengthening, and bundling (Figs. 2 and 6). Increased lung microvascular trapping of tau-overexpressing MCF-7 cells, enrichment of tau in 26% of matched metastatic tumors, and the dependence of ZR-75-1 cells on endogenous tau for attachment all support a significant biological role for tau upregulation in CTC biology (Figs. 3 – 5).
Actin cortex integrity influences tau-stimulated McTN formation. In normal cells, the contractility of the actin cortex counteracts the expansion of growing microtubules (Ingber, 2003). Alterations to either force can drastically change cellular mechanics. Actin cortex weakening increases the plasma membrane deformability of CTCs, which is linked to both tumor progression and metastasis by in vivo observations of CTCs within capillaries and by optical stretcher technology (Remmerbach et al., 2009; Weiss, 1991). Here, we artificially weakened the actin cortex using LA; however, mutations that alter actin dynamics are frequent among cancers. For example, Src-family kinase mutations lead to defects in actin polymerization and crosslinking that weaken the cortex (Thomas and Brugge, 1997). Interestingly, evidence suggests that tau induces actin remodeling by priming Src activation, leading to sustained actin breakdown and membrane ruffling (Fig. 6, Sharma et al., 2007). Here, tau increases McTN formation, but also that the actin cortex can restrict tau-induced McTNs. Only in tau-overexpressing MCF-10A and MCF-7 cells did McTNs significantly increase when left untreated, but tau significantly increased McTNs in all cell lines when actin was disrupted with LA (Fig. 2). One can deduce that the actin cortices of Sk-Br-3 and BT-20 cells are more capable of counteracting the increased stability and strength of tau-decorated microtubules. Surprisingly, in pcDNA experiments, LA treatment of BT-20 cells was insufficient to increase McTNs. Only with tau expression and LA treatment did McTNs increase, indicating that microtubules within BT-20 cells are not resilient enough to deform a compromised actin cortex. These data support a role for both microtubule stabilization and weakened actin cortical integrity for McTN formation, indicating that the metastatic efficiency of CTCs would increase if both the actin cortex is disrupted and the microtubule network is stabilized, as with either tau or vimentin expression (Whipple et al., 2008). Considering the separation of tau and vimentin expression within the cell lines utilized and within our patient cohort, each may represent independent mechanisms by which CTCs can form McTNs (Fig. S6 and Table S2).
CTC reattachment is microtubule-dependent. Suspended cell reattachment is stimulated by increased McTNs resultant from tau expression in MCF-7, while downregulation of endogenous tau in ZR-75-1 reduced attachment. Remarkably, our results from attachment studies in siTau1 and siTau2 correlate identically with the frequency of McTNs in these cells (Figs. 3 and 4). These data are mimicked in vivo, as MCF-7 cells that overexpress tau are more efficiently trapped and retained in lung capillaries, whereas the tau-deficient counterparts are not retained and likely die by shear-induced fragmentation or anoikis. This and previous studies have demonstrated that microtubules and their stability are crucially involved in CTC attachment to the microvasculature of host organs. Korb et al. observed that HT-29 colon carcinoma cells adhere to blood vessel walls in a microtubule-dependent manner that is enhanced by actin disruption. We have established an identical mechanism by which actin disruption significantly enhances microtubule-dependent McTNs, which facilitate reattachment of suspended cells (Whipple et al., 2007). Cells with McTNs possess greater surface area; thus, McTNs increase the probability of CTC-ECM attachment by providing a mechanical structure that can breach an endothelial barrier. Interestingly, under flow conditions, it has been shown that selectin-mediated interactions with endothelial cells are insufficient to arrest CTCs and that integrin-mediated contact with the ECM beneath the endothelial cells is required (Haier and Nicolson, 2001). Tau-expressing CTCs would be more efficient in this process, as they form more McTNs and are more able to be retained within and to reattach to the microvasculature. Two lines of evidence support for this conclusion. First, the high tau-expressing cell lines used in this study, ZR-75-1 and HCC1428, are derived from patient metastatic tumors (Engel et al., 1978; Gazdar et al., 1998). Second, tau expression was significantly increased in 26% (27 patients) of the matched lymph node metastases from primary breast tumors (Fig. 5 and Table 1). Remarkably, 22 patients displayed tau expression in their metastases despite a lack of it in their primary tumors. An additional 10 patients retained equivalently intense tau staining (2+/3+) over the course of their disease.
Given these data, it is important to consider if tau is predictive of tumor metastasis. While 26% of our patient population possesses enriched tau expression in their metastases, this is not directly indicative of the metastatic potential of the primary tumor. Due to tumor heterogeneity, metastasis is dependent upon a number of factors within individual tumor cells, not just tau expression, that allow them to be more capable of dissemination. In this study, we address how tau affects the metastatic efficiency in a model of experimentally disseminated CTCs. Intravenously injected CTCs that express tau are more capable of retention and reattachment in the microvasculature through increased McTN formation. Recently, a randomized clinical trial of breast cancer patients treated with doxorubicin, cyclophosphamide and paclitaxel revealed that tau expression in primary tumors was associated with better disease-free and overall survival (Pusztai et al., 2009) While this contradicts previous reports of tau-associated Taxol resistance, only primary tumors were assessed for tau expression. This contrasts with our study that analyzed chemotherapeutic naïve patients matched for primary and metastatic tumors. This removes the complicating factor of different therapeutic interventions. While overall tau expression in primary tumors could indicate a greater risk of metastasis, expanded clinical studies comparing tau expression in early tumors to metastatic tumors after long-term follow-up would be required to address this question. By focusing on matched primary and metastatic lesions, our clinical data currently support a model where, in a tumor heterogeneous for tau expression, the tumor cells which express tau would have an increased ability to reattach, evidenced by increased lung retention of tau-expressing MCF-7 cells. Collectively, these data indicate that tau may confer a selective advantage to CTCs, allowing for more efficient trapping in the microvasculature than cells that lack tau.
In summary, MAPs have powerful implications for chemotherapy efficacy and metastatic progression. Tau provides taxane-resistance through binding-competition on microtubules (Wagner et al., 2005). Furthermore, taxanes have been determined to increase CTCs by 10 000-fold among breast cancer patients (Camara et al., 2007). As CTC persistence is indicative of an increased risk of disease recurrence (Janni et al., 2005), and tau increases the trapping and reattachment of detached breast tumor cells, pathological evaluation of tau may benefit patients by minimizing metastasis through CTC mobilization. Elucidating the role of tau and other MAPs in cancer may lead to new therapeutics that target MAP expression or the regulation of MAP-tubulin interactions. It has been suggested that the expression of neuronal MAPs in non-neuronally-derived cancers speak to their origin from stem cell-like precursors (Bhat and Setaluri, 2007). This raises the potential for collaboration between the neurodegenerative and cancer drug fields. Aberrant regulation of tau leads to neurodegenerative tauopathies. Consequently, numerous tau-targeted compounds have been characterized, some of which are current therapeutics (Bulic et al., 2009). Promising are two drugs, aziridinylbenzoquinone and albendazole, which can reduce tau expression by 38% and 52%, respectively, without detectable cytotoxicity (Dickey et al., 2006). As cancer therapeutics, these and other anti-tau drugs may hold potential to inhibit tumor metastasis by reducing tau-induced McTNs.
Materials and Methods
Plasmids
Tau vectors were obtained commercially (Accession #BC000558; Genecopoeia, Germantown, MD, USA). Tau or pcDNA 3.1 was transiently cotransfected with a membrane-targeted AcGFP1 (GFP-mem, Clontech, Mountain View, CA, USA). For initial McTN evaluation, cells were transfected with GFP-mem only. MAPT shRNA plasmids (Accession #NM_005910) and a NSC plasmid were obtained commercially (SA Biosciences, Frederick, MD, USA). MCF-7-GFPtau1-luc and MCF-7GFP-luc clones were created using pMSCV-Luciferase PGK-hygro expression vector (Addgene).
Immunofluorescence
Suspended cells were centrifuged (300rpm, 5min) onto 1% poly(ethyleneimine)-coated coverslips (Sigma-Aldrich). Fixation/permeabilization was performed as described (Lee and Rook, 1992). Tau was stained in indirectly utilizing anti-tau antibody (Tau46, 1:1000; Cell Signaling, Danvers, MA, USA) followed by AlexaFluor555-conjugated secondary antibody (1:500; Invitrogen). After extensive washing, microtubules were stained in directly using AlexaFluor488-conjugated anti-α-tubulin (DM1A, 1:1000; eBioscience, San Diego, CA, USA). AlexaFluor488-conjugated normal mouse IgG served as negative control. Z-stacks were obtained using an Olympus (Center Valley, PA, USA) FV1000 confocal microscope. Stacks were split for maximum intensity projections using ImageJ (NIH, Bethesda, MD, USA) and adjusted for brightness and contrast using Adobe Photoshop (San Jose, CA, USA).
Western blotting
Western blots were performed as described (Whipple et al., 2008; Whipple et al., 2007). Antibodies were polyclonal anti-human tau (1:2000, Dako, Glostrup, Denmark), monoclonal anti-vimentin (V9; Invitrogen, 1:1000), and monoclonal anti-β-actin (AC-15; Sigma-Aldrich; 1:5000) in 2.5% milk in TBS, 0.5% Tween-20.
McTN evaluation
McTNs were evaluated within a cell population as described (Whipple et al., 2008). Briefly, cells were detached using Hank’s based Enzyme-free Cell Dissociation Solution (Millipore, Billerica, MA, USA) and resuspended in ultra-low attachment plates (Corning, Corning, NY, USA) with 5 µM LA (BioMol, Plymouth Meeting, PA, USA), 1.17 µM Taxol (Invitrogen) or a combination of both. VCs were phenol-red-free growth media alone or that supplemented with 0.1% ethanol, 0.1% DMSO, or in combination. For McTN evaluation of ZR-75-1 siTau clones and NSC cells, cells were washed with HBSS and stained with CellMask Orange (5µg/ml in HBSS; Invitrogen) for 15min at 37°C and 5% CO2. Cells were washed with HBSS, detached, suspended, and evaluated for McTNs as described above in LA or VC.
Attachment Assays
An xCelligence RTCA SP real-time cell sensing device (Roche) assessed attachment. MCF-7, MCF-7tau1, MCF-7tau2, ZR-75-1, siTau1, and siTau2 cells were suspended in media lacking selection antibiotics. 1.0×104 cells were transferred in triplicate to 96-well electronic microtiter plates (E-plates). Attachment was measured as the relative impedance change, cell index (CI), across microelectronic sensors integrated into the uncoated wells of flat-bottom E-plates (Atienza et al., 2006). MCF-7 and ZR-75-1 served as positive controls. CI from each time point was normalized to the maximum CI attained for the control cell lines at 1h. Test cell lines were represented as percentage attachment of control.
Experimental Metastasis Assays
MCF-7-GFPtau1-luc and MCF-7-GFP-luc cells were detached in Enzyme-free Cell Dissociation buffer and resuspended at 1×106 cells/ml in 1× PBS. Prior to initial injection and imaging, athymic nude-Foxn1nu mice were injected intraperitoneally with Luciferin (150mg/kg, Xenogen) and returned to their cages for 5min to allow for biodistribution. 3×105 cells were then injected intravenously into the tail vein of mice restrained by mouse tail illuminator tail vein injection apparatus (Braintree Scientific). Mice were anesthetized with 2% isoflurane gas (integrated within the Xenogn IVIS-200 system) and imaged 5mins for a single-photon emission. Total photon flux (photons/sec) was calculated and corrected for tissue depth by spectral imaging using Living Image 3.0 software (Xenogen).
Patients
Formalin fixed and paraffin embedded TMAs were obtained commercially (US Biomax, Rockville, MD, USA). Tissues were chemotherapeutic-naïve. 102 female breast cancer patients were included (age range 27–80 years; mean = 49.5 years). TMAs underwent antigen retrieval before immunohistochemistry using DakoCytomation Autostainer kit (Dako). Antibodies were mouse anti-tau (T46; 1:800; Cell Signaling) and mouse IgG (1:800; Jackson Labs, Bar Harbor, ME) as a negative control. A breast pathologist blindly scored TMAs. Images were obtained on an Olympus BX50 upright microscope equipped with a ProgResC5 Color CCD camera (Jenoptik, Jena, Germany).
Statistics
Significance was measured by t-test (Excel, Microsoft, Redmond, WA, USA). A generalized McNemar’s test assessed the association of tau staining with tumor site (primary vs. metastases) while adjusting for tumor grade. (Splus, Tibco, Palo Alto, CA, USA).
Supplementary Material
Acknowledgements
Grant support: R01-CA124704 from National Cancer Institute, Breast Cancer Idea Award from USA Medical Research and Materiel Command (BC061047) and a Clinical Innovator Award from Flight Attendants Medical Research Institute (CIA-062497). We thank Dr. I-Chu Tseng and the Dr. Chen-Yong Lin laboratory for immunohistochemistry assistance, Dr. Larry Changwan Lu for his assistance with the statistical analyses, Aric Colunga and the Dr. Laure Aurelian laboratory for imaging assistance, Dr. William Twaddell for assistance with pathological scoring of vimentin staining, and Dr. Michele Weiss for Fig. 6 assistance.
Abbreviations
- CTC
circulating tumor cell
- ECM
extracellular matrix
- LA
Latrunculin-A
- MAP
microtubule-associated protein
- McTN
microtentacle
- NSC
non-silencing control
- Taxol
paclitaxel
- TMA
tissue microarray
- VC
vehicle control
Footnotes
Conflict of Interest. The authors declare no competing financial interests.
Supplementary information available at Oncogene’s website.
References
- Atienza JM, Yu N, Kirstein SL, Xi B, Wang X, Xu X, et al. Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol. 2006;4:597–607. doi: 10.1089/adt.2006.4.597. [DOI] [PubMed] [Google Scholar]
- Balzer EM, Whipple RA, Cho EH, Matrone MA, Martin SS. Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM, Setaluri V. Microtubule-associated proteins as targets in cancer chemotherapy. Clin Cancer Res. 2007;13:2849–2854. doi: 10.1158/1078-0432.CCR-06-3040. [DOI] [PubMed] [Google Scholar]
- Bulic B, Pickhardt M, Schmidt B, Mandelkow EM, Waldmann H, Mandelkow E. Development of tau aggregation inhibitors for Alzheimer's disease. Angew Chem Int Ed Engl. 2009;48:1740–1752. doi: 10.1002/anie.200802621. [DOI] [PubMed] [Google Scholar]
- Camara O, Rengsberger M, Egbe A, Koch A, Gajda M, Hammer U, et al. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol. 2007;18:1484–1492. doi: 10.1093/annonc/mdm206. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Spittle C. Regulation of microtubule-associated proteins. Int Rev Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Ash P, Klosak N, Lee WC, Petrucelli L, Hutton M, et al. Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol Neurodegener. 2006;1:6. doi: 10.1186/1750-1326-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LW, Young NA, Tralka TS, Lippman ME, O'Brien SJ, Joyce MJ. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res. 1978;38:3352–3364. [PubMed] [Google Scholar]
- Gazdar AF, Kurvari V, Virmani A, Gollahon L, Sakaguchi M, Westerfield M, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Goedert M. Tau protein and neurodegeneration. Semin Cell Dev Biol. 2004;15:45–49. doi: 10.1016/j.semcdb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Haier J, Nicolson GL. Tumor cell adhesion under hydrodynamic conditions of fluid flow. Apmis. 2001;109:241–262. doi: 10.1034/j.1600-0463.2001.d01-118.x. [DOI] [PubMed] [Google Scholar]
- Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janni W, Rack B, Schindlbeck C, Strobl B, Rjosk D, Braun S, et al. The persistence of isolated tumor cells in bone marrow from patients with breast carcinoma predicts an increased risk for recurrence. Cancer. 2005;103:884–891. doi: 10.1002/cncr.20834. [DOI] [PubMed] [Google Scholar]
- Knops J, Kosik KS, Lee G, Pardee JD, Cohen-Gould L, McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991;114:725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb T, Schluter K, Enns A, Spiegel HU, Senninger N, Nicolson GL, et al. Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–247. doi: 10.1016/j.yexcr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Lee G, Rook SL. Expression of tau protein in non-neuronal cells: microtubule binding and stabilization. J Cell Sci. 1992;102(Pt 2):227–237. doi: 10.1242/jcs.102.2.227. [DOI] [PubMed] [Google Scholar]
- Levy SF, Leboeuf AC, Massie MR, Jordan MA, Wilson L, Feinstein SC. Three- and four-repeat tau regulate the dynamic instability of two distinct microtubule subpopulations in qualitatively different manners. Implications for neurodegeneration. J Biol Chem. 2005;280:13520–13528. doi: 10.1074/jbc.M413490200. [DOI] [PubMed] [Google Scholar]
- McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–5027. [PubMed] [Google Scholar]
- Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc Natl Acad Sci U S A. 2003;100:9548–9553. doi: 10.1073/pnas.1633508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, et al. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol. 2009;27:4287–4292. doi: 10.1200/JCO.2008.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmerbach TW, Wottawah F, Dietrich J, Lincoln B, Wittekind C, Guck J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009;69:1728–1732. doi: 10.1158/0008-5472.CAN-08-4073. [DOI] [PubMed] [Google Scholar]
- Rosenberg KJ, Ross JL, Feinstein HE, Feinstein SC, Israelachvili J. Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7445–7450. doi: 10.1073/pnas.0802036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap IA, Hoffmann B, Carrasco C, Merkel R, Schmidt CF. Tau protein binding forms a 1 nm thick layer along protofilaments without affecting the radial elasticity of microtubules. J Struct Biol. 2007;158:282–292. doi: 10.1016/j.jsb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Sharma VM, Litersky JM, Bhaskar K, Lee G. Tau impacts on growth-factor-stimulated actin remodeling. J Cell Sci. 2007;120:748–757. doi: 10.1242/jcs.03378. [DOI] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Wagner P, Wang B, Clark E, Lee H, Rouzier R, Pusztai L. Microtubule Associated Protein (MAP)-Tau: a novel mediator of paclitaxel sensitivity in vitro and in vivo. Cell Cycle. 2005;4:1149–1152. doi: 10.4161/cc.4.9.2038. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. Deformation-driven, lethal damage to cancer cells. Its contribution to metastatic inefficiency. Cell Biophys. 1991;18:73–79. doi: 10.1007/BF02989807. [DOI] [PubMed] [Google Scholar]
- Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68:5678–5688. doi: 10.1158/0008-5472.CAN-07-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple RA, Cheung AM, Martin SS. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp Cell Res. 2007;313:1326–1336. doi: 10.1016/j.yexcr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, et al. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.