Abstract
Purpose
Postoperative PSA doubling time (PSADT) may be used as a surrogate for prostate cancer (PCa)-specific mortality in patients with biochemical recurrence after radical prostatectomy. Less is known about the utility of preoperative PSA doubling time (PSADT) for the initial prediction of prostatectomy outcomes.
Materials and Methods
Preoperative PSADT was calculated in 1208 men from a large PCa screening study who were treated with radical prostatectomy. We then examined the relationship between PSADT with tumor features and biochemical progression-free survival (PFS).
Results
Overall, PSADT was associated with non-organ-confined disease (OR 0.996, 95% CI 0.992–0.999, p=0.013), but not with biochemical progression (HR 1.000, 95% CI 0.998–1.001, p=0.66). Using previously published thresholds for PSADT of 18 months and 36 months, respectively, there was no significant relationship between PSADT with specific adverse pathology features or biochemical progression. Using the concordance index, PSADT did not enhance the prediction of biochemical progression beyond that achieved with a model with PSA, clinical stage and biopsy Gleason score.
Conclusions
In our series of men with newly diagnosed clinically localized PCa, shorter preoperative PSADT was associated with non-organ confined disease, but not with biochemical progression following radical prostatectomy. All PSA kinetics calculations may not be equivalent, and caution should be exercised in using PSADT in the pretreatment setting.
Keywords: prostate-specific antigen, PSA kinetics, doubling time, biochemical progression, radical prostatectomy
INTRODUCTION
PSA doubling time (PSADT) is a method of evaluating longitudinal PSA changes, defined as the time required for the serum PSA level to double. Postoperative PSADT has been used extensively in the setting of relapse after definitive therapy. For example, Pound et al. showed that among men with biochemical recurrence after radical prostatectomy, a postoperative PSADT greater than 10 months was a significant predictor of local rather than distant recurrence.1
Although urologists are familiar with the use of PSADT in the postoperative setting, less has been published regarding its role in preoperative risk assessment. Indeed, the existing literature on pre-treatment PSADT and tumor aggressiveness is highly conflicted. While some studies have linked short PSADT with high-grade disease and adverse treatment outcomes, others have found no association between PSADT and prostate cancer (PCa) aggressiveness. For example, Nowroozi et al. recently reported on 59 men diagnosed with PCa and found a significant difference in PSADT between low-grade and high-grade tumors (median 12.7 versus 5.6 months, respectively, p=0.001). Khatami et al. reported on 70 patients who discontinued active surveillance and were treated by radical prostatectomy. In this population, the PSADT during surveillance was significantly associated with the likelihood of PSA relapse after subsequent surgical treatment (p=0.031).2 Sengupta et al. reported that a preoperative PSADT less than 18 months was associated with biochemical progression, clinical progression, and cancer-specific mortality.3 However, in unselected men from a longitudinal aging study, PSADT did not predict either high-risk (PSA ≥20 ng/ml, Gleason 8 to 10, or confirmed PCa death) or fatal PCa.4
Despite this conflicting evidence, PSADT is currently used in untreated men on active monitoring protocols to help guide the need for intervention. For example, a Canadian active surveillance program uses a threshold PSADT of 3 years to identify patients at high risk for cancer progression in their active monitoring population.5 In a recent update, Klotz et al. reported that a PSADT <3 years was significantly associated with biochemical progression among men from the surveillance program who ultimately underwent radical prostatectomy.6 The objective of the current study was to determine whether preoperative PSADT is associated with treatment outcomes in our radical prostatectomy series.
MATERIALS AND METHODS
From 1989 to 2001, approximately 36,000 men participated in a community-based PCa screening study. Participants underwent PSA testing and digital rectal examination (DRE) at 6- to 12-month intervals, as previously described.7 Prostate biopsy was recommended for a suspicious DRE or a PSA level > 4 ng/ml (before 1995) or >2.5 ng/ml (after May 1995). Of 3541 men diagnosed with PCa in this study, 2266 underwent radical prostatectomy for clinically localized disease. Men who received hormonal therapy or had clinical stage T3 disease were excluded from the analysis. Of the remaining patients, 1208 had a sufficient PSA history to enable a PSADT calculation.
Excluded men were slightly younger (65 vs. 66 years, p=0.0004) with a significantly higher preoperative PSA (5.3 vs. 4.4, p<0.0001) and clinical stage (43% vs. 32% clinical stage ≥T2, p<0.0001), compared to included men. There was also a nonsignificant trend toward a greater proportion of excluded men with a biopsy Gleason score ≥7 (17% vs. 13%, p=0.07).
The final study population included 943 men (78%) with 2 documented preoperative PSA measurements, 263 (21.8%) with 3 PSA measurements, and 2 men (0.2%) with 4 PSA measurements in the year prior to diagnosis. PSADT was calculated using PSA values from the year prior to diagnosis for all participants, according to the following formula: [log2]/[beta(slope) coefficient from regressing the log of PSA over time]. PSADT cutpoints of 18 and 36 months were selected based upon the literature for analyses involving dichotomous variables.3, 5, 6, 8 For ease of calculations, patients with a negative or 0 PSADT (no increase in PSA), as well as patients with a PSADT greater than 100 months were assigned a value of 100.
After radical prostatectomy, monitoring involved a PSA test every six months and annual DRE. Biochemical progression was defined as a postoperative PSA level > 0.2 ng/ml confirmed by a second measurement.
The chi-square and Wilcoxon tests were used to examine the relationship of preoperative PSADT with pathological features and treatment outcomes. Logistic regression was used to further examine the relationship of preoperative PSADT with non organ-confined disease. A Cox proportional hazards model was used to examine the ability of PSADT to predict biochemical progression. In addition, Kaplan-Meier survival analysis was performed to determine the relationship between preoperative PSADT and 10-year biochemical progression-free survival (PFS), and curves were compared using the log-rank test. The concordance index was also used to examine the performance of preoperative PSADT to predict biochemical progression. Subset analyses were performed excluding men with a negative PSADT, and in the subgroup with at least 3 PSA measurements for PSADT calculation. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 1208 screened men underwent radical prostatectomy for clinically localized PCa and had a calculable PSADT. The mean age was 65 years (median 66, range 43–85). Table 1 shows the clinical and pathologic characteristics of the study population. The majority of men were white, and had clinical stage T1c PCa diagnosed at a PSA level between 2.6 and 10.0 ng/ml. The biopsy Gleason score was ≤6 in 80%, and the median PSADT was 34.5 months.
Table 1.
Clinical and pathologic characteristics of the study population.
| Race White Black Other Unknown |
1102 (91%) 48 (4%) 8 (1%) 50 (4%) |
| Total PSA Mean, Median (range) <2.5 2.6–4.0 4.1–10.0 >10.0 |
5.0, 4.4, (0.2–45.3) 139 (11.5%) 335 (27.7%) 675 (55.9%) 59 (4.9%) |
| Clinical Stage T1 a/b T1c T2 Missing |
12 (1%) 805 (66.6%) 386 (32%) 5 (0.4%) |
| Biopsy Gleason Score Mean, Median (range) ≤6 7 8–10 Missing |
5.5, 6.0 (2.0 – 10) 961 (79.5%) 106 (8.8%) 40 (3.3%) 101 (8.4%) |
| PSADT Distribution Mean, Median (range) ≤0* 0–12 mos. 12–24 mos. 24–36 mos. 36–48 mos. 48–60 mos. 60–99 mos. 100 mos. |
50.5, 34.5 (1 – 100) 267 (22%) 200 (17%) 267 (22%) 154 (13%) 79 (7%) 52 (4%) 64 (5%) 125 (10%) |
| Organ-Confined | 905 (74.9%) |
| Extracapsular Extension | 129 (10.7%) |
| Positive Surgical Margins | 239 (19.8%) |
| Seminal Vesicle Invasion | 31 (2.6%) |
| Lymph Node Metastases | 5 (0.4%) |
| Prostatectomy Gleason score ≥7 | 282 (23.3%) |
Classified as 100 months in the overall analysis, excluded in subgroup analysis.
Overall, 905 (75%) men had organ-confined disease at radical prostatectomy. Preoperative PSADT was significantly higher in men with organ-confined than non organ-confined disease (52.1 (mean) and 37.0 (median), versus 45.7 (mean) and 26.8 (median) months, respectively, p=0.038 by Wilcoxon Rank Sum test). Univariate logistic regression showed a significant association between preoperative PSADT (continuous variable) and non-organ-confined disease (OR 0.996, 95% CI 0.992–0.999, p=0.013). However, preoperative PSADT was similar between those with a prostatectomy Gleason score of 6 versus 7 to 10 (51.4 (mean) and 36.0 (median), versus 48.8 (mean) and 29.7 (median) months, respectively, p=0.25). As shown in Table 2, a preoperative PSADT cutpoint of 18 months was not associated with pathologic features; whereas, men with a PSADT >36 months were significantly more likely to have organ-confined disease than those with a PSADT ≤36 months (79% vs. 73%, p=0.018).
Table 2.
The relationship between PSADT with adverse pathology and biochemical progression.
| PSADT ≤ 18 mos |
PSADT > 18 mos |
p-value | PSADT ≤36 mos |
PSADT >36 mos |
p-value | |
|---|---|---|---|---|---|---|
| Organ- confined |
254 (75%) | 651 (76%) | 0.71 | 446 (73%) | 459 (79%) | 0.018 |
| Extracapsular extension |
38 (11%) | 91 (11%) | 0.76 | 72 (12%) | 57 (10%) | 0.26 |
| Positive margins |
70 (21%) | 169 (20%) | 0.81 | 135 (22%) | 104 (18%) | 0.083 |
| Seminal vesicle invasion |
12 (4%) | 19 (2%) | 0.23 | 21 (3%) | 10 (2%) | 0.07 |
| Lymph node metastases |
2 (0.6%) | 3 (0.4%) | 0.63 | 3 (0.5%) | 2 (0.4%) | 0.99 |
| Prostatectomy Gleason ≥7 |
85 (26%) | 197 (24%) | 0.49 | 153 (26%) | 129 (23%) | 0.22 |
| Biochemical progression |
58 (17%) | 133 (15%) | 0.54 | 96 (16%) | 95 (16%) | 0.75 |
| 10-Year Progression- free survival |
80% | 82% | 0.54 | 82% | 80% | 0.66 |
Comparisons of pathologic features made using the chi-square tests. 10-year progression-free survival was calculated using the Kaplan-Meier method and compared by the log-rank test.
A total of 191 (16%) men had biochemical progression at a median follow-up of 114 months (range 5–209). The mean PSADT was similar among men with and those without biochemical progression (51.4 (mean) 35.2 (median), versus 50.4 (mean) median (34.4) months, respectively, p=0.89). Preoperative PSADT (continuous variable) was not associated with time to biochemical progression in a univariate Cox proportional hazards model (HR 1.000, 95% CI 0.998–1.001, p=0.66). The results of Cox proportional hazards models were similar in the subset excluding men with negative PSADT (HR 0.999, 95% CI 0.997–1.001, p=0.85), and in men with at least 3 PSA measurements (HR 1.000, 95% CI 0.997–1.004, p=0.89).
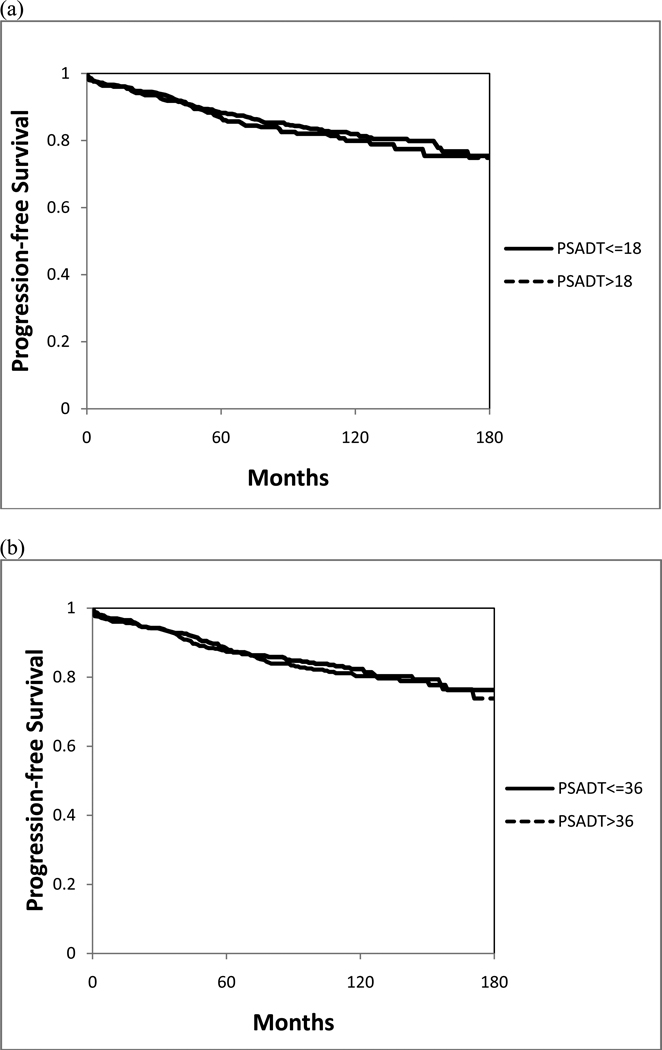
Figure 1 shows the Kaplan-Meier curves for progression-free survival, stratified by PSADT. The 10-year PFS was similar among men with a PSADT ≤18 and >18 months (80% vs. 82%, p=0.54), or with a PSADT ≤36 months and >36 months (82% vs. 80%, p=0.66).
Figure 1.
Kaplan-Meier biochemical progression-free survival curves, stratified by a PSADT of (a) 18 months and (b) 36 months.
The C-index was 0.505 to predict biochemical progression using preoperative PSADT alone (continuous variable). A separate model including PSA, clinical stage, and biopsy Gleason score had a C-index of 0.648 for predicting biochemical progression; the addition of PSADT to this model was associated with no change in C-index (0.648, p=0.95). Excluding men with negative PSADT, the C-index for biochemical progression was 0.495 using PSADT. Among men with at least 3 PSA measurements, the C-index for biochemical progression was 0.513 with PSADT.
There were 7 (0.6%) deaths from PCa in the study population. The median PSADT was 58.4 months among men who died from PCa, and 34.4 months in those who did not (p=0.80).
DISCUSSION
In the early 1990’s, it was first suggested that changes in PSA over time may help distinguish between benign conditions and PCa.9 One way to express these longitudinal PSA changes is PSA velocity (PSAV), or the change in PSA units per year (ng/ml/year). In a hallmark study, Carter et al. demonstrated that the median PSAV measured over more than a decade was significantly higher in men with PCa than those with BPH or controls (p<0.01).10 Among men with PCa in their study, the pre-diagnostic PSAV was significantly higher in men with metastases than in those with local or regional disease, suggesting that PSAV is not only useful for PCa detection, but may also be linked to outcomes. Recently, Carter et al. reported that men with a PSAV >0.35 ng/ml/year had a 5-fold greater risk of PCa death 15 or more years later.11
Other research groups have further explored the relationship between pre-treatment PSA velocity and treatment outcomes. Several studies have demonstrated significant associations between pre-treatment PSAV and cancer-specific survival after radical prostatectomy or radiotherapy.8, 12 However, other studies reported that the preoperative PSAV does not predict either the pathological outcome or biochemical progression after radical prostatectomy.13
Changes in PSA over time can also be expressed as PSA doubling time (PSADT), or the time required for the serum PSA level to double. Although post-operative PSADT predicts PCa mortality among men with biochemical progression after treatment, there is conflicting evidence on whether it also has any role in pre-treatment prognostication. For example, Schmid et al. found a significantly shorter preoperative PSADT in men with Gleason scores of 7 or greater.14 Goluboff et al. examined preoperative PSADT in 56 patients undergoing radical prostatectomy and found that it correlated with final pathological stage but not with Gleason score or biochemical progression at 17.3 months.15 In 86 men undergoing radical prostatectomy, Freedland et al. reported that PSADT was not associated with either pathological stage or short-term biochemical progression.13 By contrast, Sengupta et al. reported a PSADT shorter than 18 months was a significant independent predictor of biochemical progression, clinical progression, and cancer-specific mortality.3 In men undergoing external beam radiation therapy, Hanks et al. reported that men with a pretreatment PSADT less than 12 months had a 50% progression-free survival at 18 months follow-up compared to 89% in men with a slower pretreatment PSADT, and 97% in men whose PSADT was not increasing.16, 17
Vickers and colleagues recently performed a systematic review of PSA kinetics to predict endpoints ranging from PCa diagnosis to biochemical progression or mortality after treatment.18 The analysis included 64 studies using PSAV, 17 studies using PSADT, and 6 studies with both types of measurement. The authors concluded that there was little evidence to suggest that PSA dynamics are of incremental value for early-stage PCa.
Our research group has previously reported that a preoperative PSAV >2 ng/ml/year was associated with a 10-fold increased risk of PCa death following radical prostatectomy.8 Another recent study compared PSAV and PSADT for the prediction of life-threatening PCa in 681 men from an aging study.4 The addition of PSAV to a base model including age, date of diagnosis, and PSA to predict high-risk PCa increased the concordance index from 0.85 to 0.88 (p<0.001); whereas, PSADT did not improve the concordance index (c = 0.85). Of note, precise data on treatment type were not available in that study. In the current study, we found an association between preoperative PSADT with non-organ confined disease but not with biochemical progression after radical prostatectomy.
Several differences between our study and other reports on pretreatment PSADT warrant discussion. Our sample size was intermediate between those in the studies by Freedland et al and Sengupta et al; however, the median follow-up was longer in our study (9 years compared to 2 and 7.1 years in Freedland et al and Sengupta et al, respectively). Another important issue is that different PSA assays were used for the serial measurements in some studies but not in others. For example, we used the same PSA assay on an annual basis and did not find PSADT to be useful for predicting biochemical progression; whereas, Sengupta based the PSA kinetics calculations on uncorrected PSA values from multiple sources and nevertheless found PSADT to significantly predict treatment outcomes in their population. 3 The reasons for this discrepancy are unclear, since interassay variability is a known confounder of PSA kinetics.19 Of note, Sengupta et al also examined PSAV in the same population, and concluded that although “PSADT may be biologically more accurate and stronger on multivariate analysis, PSAV is clinically easier to use and a good approximation in the short term.”
Finally, the time interval used in the PSADT calculation differed between studies. Freedland used PSA values from an unrestricted period of time prior to diagnosis, while Sengupta only used PSA measurements to two years prior to diagnosis. In the current study, we restricted PSA measurements to the year prior to diagnosis, since we have previously demonstrated a strong association between the PSAV in this time interval with mortality after treatment;8 thus, we used the same interval for the present analysis to maintain consistency. Although some have suggested that PSADT should instead be calculated using as many as 9 serial measurements over 2 to 3 years,5 it remains of great interest to determine whether PSA kinetics calculations involving a few measurements within one year would still predict outcomes. We performed subset analysis including only men with at least 3 PSA measurements and the results were similar. Further analyses are warranted to determine the optimal method of calculation for PSA kinetic parameters.
One hypothesis to explain our generally negative findings using PSADT in the pretreatment setting is that men are diagnosed with PCa at highly variable baseline serum PSA levels, making a preoperative PSADT examination more complex. This differs from the scenario after radical prostatectomy, in which the serum PSA level usually becomes undetectable, thereby making subsequent rises more straightforward to compare using postoperative PSADT. Overall, preoperative PSADT may be more heavily dependent upon the baseline PSA level, making it potentially more difficult to apply in the heterogeneous pretreatment population.
A limitation of our study is that PSA kinetics calculations may be confounded by prostatitis. Moreover, approximately one third of men had a preoperative PSADT that was zero, extremely long, or negative, making it difficult to select an alternate cutpoint for use in the analysis. However, this finding is consistent with other reports involving men undergoing serial screening. For example, Thompson et al. reported the initial results of PSAV in the placebo group of the Prostate Cancer Prevention Trial and stated that they did not even calculate PSA doubling time “because the PSA values for many participants declined over time.”20
In addition, because the focus of this study was the relationship between PSADT and treatment outcomes, the study population was limited to men from our screening study who underwent radical prostatectomy. Also, a PSADT calculation was not possible for men who were diagnosed with PCa on the initial screening visit, or who did not follow the study protocol with annual screening. Accordingly, excluded men had higher risk disease features, illustrating the selection bias associated with the requirement for multiple serial PSA values.
A final limitation is that the primary endpoint in this study was biochemical progression, rather than clinical progression or mortality. In addition, due to the association between short PSADT and non organ-confined disease, a relationship with biochemical progression may become apparent with additional follow-up. Furthermore, because this represented a serially screened population, very few men in this cohort died of PCa, limiting our statistical power. Overall, future prospective studies are needed to determine the optimal PSA kinetic calculation to predict long-term treatment outcomes.
CONCLUSIONS
Preoperative PSADT is more complicated to calculate than other PSA kinetics metrics and may be dependent upon the baseline PSA level, which is highly variable among men with diagnosed PCa. Moreover, we did not find preoperative PSADT to be a useful predictor of biochemical progression following radical prostatectomy.
Acknowledgments
This study was supported in part by the Beckman Coulter, Inc., Fullerton, CA., the Urological Research Foundation, Prostate SPORE grant (P50 CA90386-05S2) and the Robert H. Lurie Comprehensive Cancer Center grant (P30 CA60553).
REFERENCES
- 1.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Khatami A, Aus G, Damber JE, et al. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int J Cancer. 2007;120:170. doi: 10.1002/ijc.22161. [DOI] [PubMed] [Google Scholar]
- 3.Sengupta S, Myers RP, Slezak JM, et al. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol. 2005;174:2191. doi: 10.1097/01.ju.0000181209.37013.99. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Kettermann A, Ferrucci L, et al. PSA doubling time versus PSA velocity to predict high-risk prostate cancer: data from the Baltimore Longitudinal Study of Aging. Eur Urol. 2008;54:1073. doi: 10.1016/j.eururo.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Loblaw A, Klotz L. Modeling prostate specific antigen kinetics in patients on active surveillance. J Urol. 2006;176:1392. doi: 10.1016/j.juro.2006.06.103. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L, Zhang L, Lam A, et al. Clinical Results of Long-Term Follow-Up of a Large, Active Surveillance Cohort With Localized Prostate Cancer. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 7.Smith DS, Catalona WJ. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol. 1994;152:1732. doi: 10.1016/s0022-5347(17)32372-8. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 9.Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 10.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. Jama. 1992;267:2215. [PMC free article] [PubMed] [Google Scholar]
- 11.Carter HB, Ferrucci L, Kettermann A, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amico AV, Renshaw AA, Sussman B, et al. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. Jama. 2005;294:440. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Dorey F, Aronson WJ. Preoperative PSA velocity and doubling time do not predict adverse pathologic features or biochemical recurrence after radical prostatectomy. Urology. 2001;57:476. doi: 10.1016/s0090-4295(00)01016-5. [DOI] [PubMed] [Google Scholar]
- 14.Schmid HP, McNeal JE, Stamey TA. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer. 1993;71:2031. doi: 10.1002/1097-0142(19930315)71:6<2031::aid-cncr2820710618>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Goluboff ET, Heitjan DF, DeVries GM, et al. Pretreatment prostate specific antigen doubling times: use in patients before radical prostatectomy. J Urol. 1997;158:1876. doi: 10.1016/s0022-5347(01)64154-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee WR, Hanks GE, Corn BW, et al. Observations of pretreatment prostate-specific antigen doubling time in 107 patients referred for definitive radiotherapy. Int J Radiat Oncol Biol Phys. 1995;31:21. doi: 10.1016/0360-3016(94)00339-M. [DOI] [PubMed] [Google Scholar]
- 17.Hanks GE, Hanlon AL, Lee WR, et al. Pretreatment prostate-specific antigen doubling times: clinical utility of this predictor of prostate cancer behavior. Int J Radiat Oncol Biol Phys. 1996;34:549. doi: 10.1016/0360-3016(95)02154-x. [DOI] [PubMed] [Google Scholar]
- 18.Vickers AJ, Savage C, O'Brien MF, et al. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27:398. doi: 10.1200/JCO.2008.18.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Link RE, Shariat SF, Nguyen CV, et al. Variation in prostate specific antigen results from 2 different assay platforms: clinical impact on 2304 patients undergoing prostate cancer screening. J Urol. 2004;171:2234. doi: 10.1097/01.ju.0000127736.86597.e7. [DOI] [PubMed] [Google Scholar]
- 20.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]