Abstract
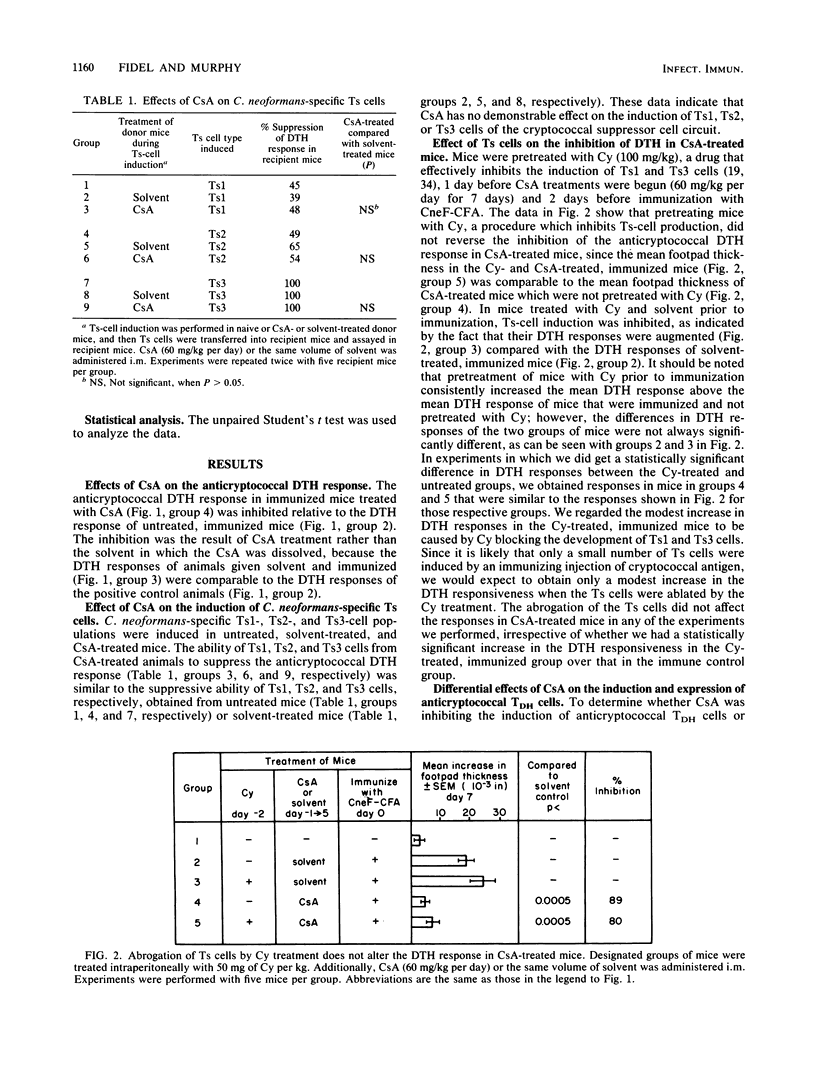
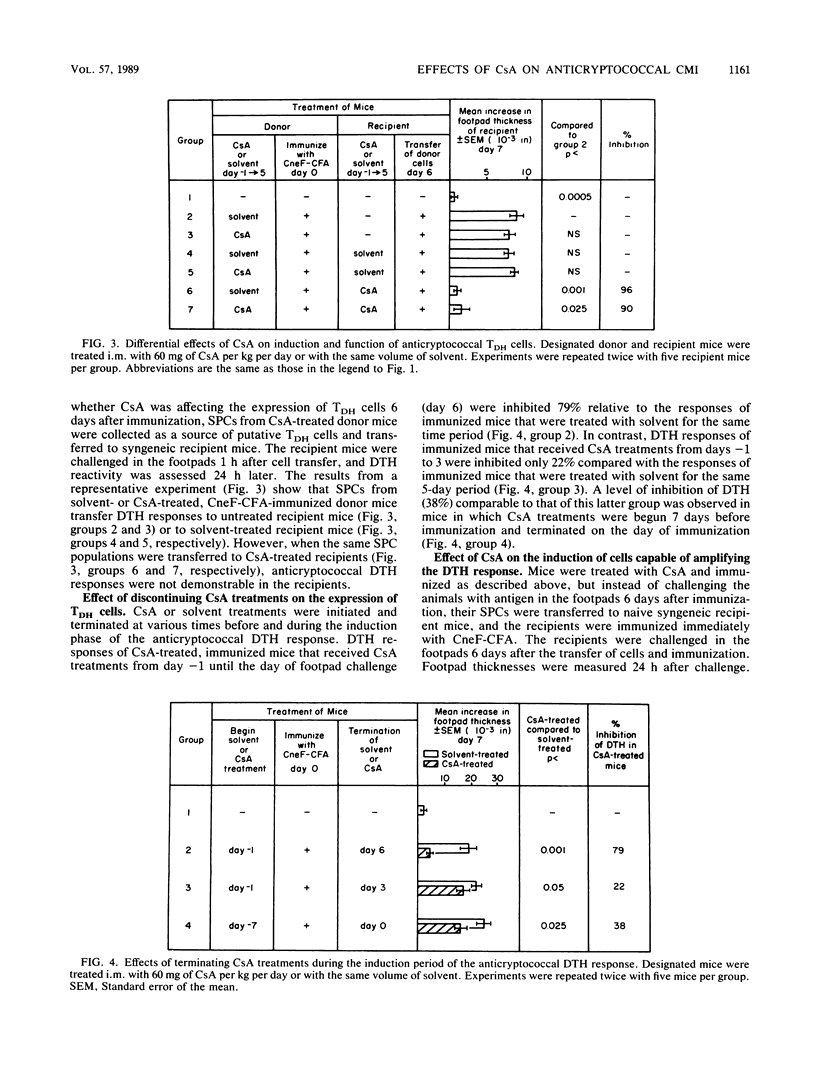
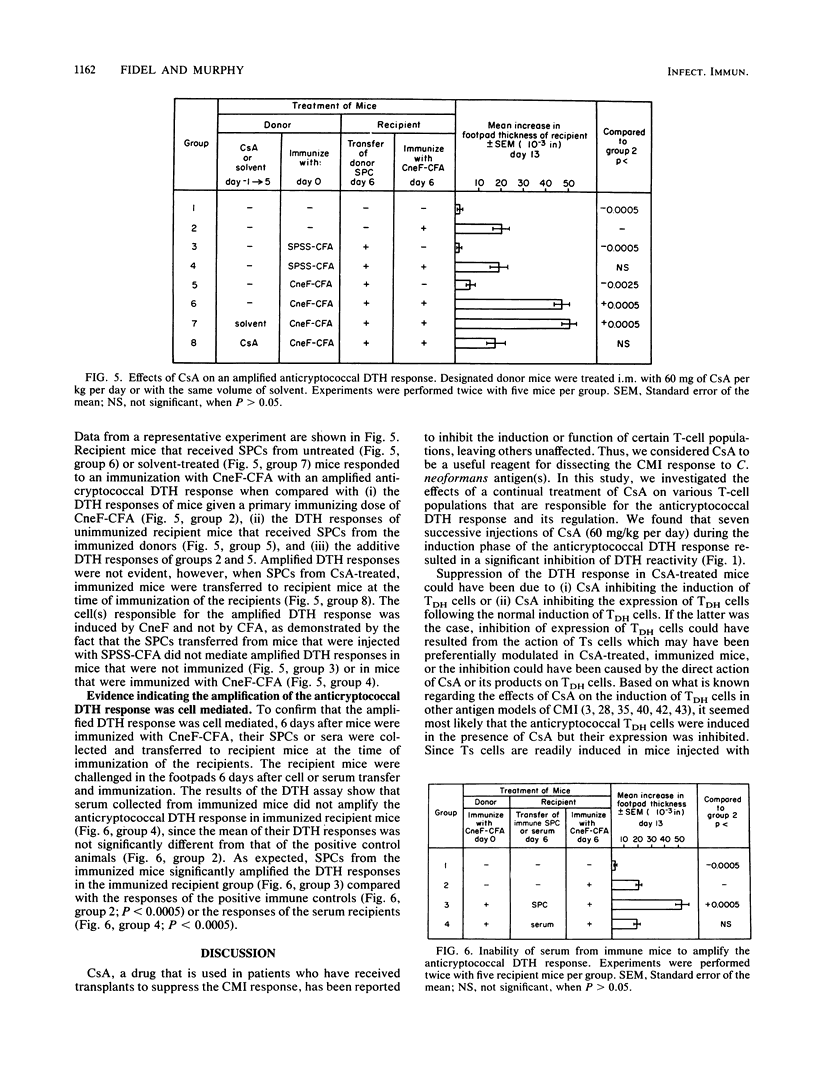
Cyclosporin A (CsA), a potent immunosuppressive drug, was used to explore further the induction, expression, and regulation of lymphoid cells involved in the delayed-type hypersensitivity (DTH) response to cryptococcal antigen(s). We found that the induction of the cells responsible for DTH (TDH cells) was not affected by CsA, but their expression was inhibited in CsA-treated mice. The inhibition of expression of the TDH cells could not be attributed to the Cryptococcus neoformans-specific suppressor T (Ts) cells, even though the Ts cells were induced in CsA-treated mice. Instead, the suppressed expression of the TDH cells in CsA-treated mice was a direct effect of CsA or its products. Our studies with CsA also resulted in the first identification of a population of cells that significantly amplify the anticryptococcal DTH response. The amplifier cells were induced in mice that were given a primary immunizing dose of cryptococcal antigen in complete Freund adjuvant, and they amplified the anticryptococcal DTH response in recipient mice when they were transferred at the time of immunization of the recipient. The amplifier cell population was distinct from the TDH cells in that CsA inhibited the production of the amplifying cells but did not affect the induction of TDH cells. Amplification of the DTH response was a cell-mediated event, since cells but not serum from immunized mice mediated the amplified response in recipient mice. Thus, CsA enabled us to characterize anticryptococcal TDH and Ts cells further and to add to the immune cell circuit of the cryptococcal system a distinct population of cells that amplifies the anticryptococcal DTH response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Blyth W. A. The effects of cyclosporin A on the induction, expression and regulation of the immune response to herpes simplex virus. Clin Exp Immunol. 1985 Jan;59(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- Araujo J. L., Kupiec-Weglinski J. W., Araneda D., Towpik E., Heidecke C. D., Williams J. M., Tilney N. L. Phenotype, activation status, and suppressor activity of host lymphocytes during acute rejection and after cyclosporine-induced unresponsiveness of rat cardiac allografts. Transplantation. 1985 Sep;40(3):278–284. doi: 10.1097/00007890-198509000-00012. [DOI] [PubMed] [Google Scholar]
- Braida M., Knop J. Effect of cyclosporin A on the T-effector and T-suppressor cell response in contact sensitivity. Immunology. 1986 Dec;59(4):503–507. [PMC free article] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani P. M., Hess A. D. Response: calmodulin, cyclophilin, and cyclosporin a. Science. 1986 Aug 29;233(4767):988–989. doi: 10.1126/science.233.4767.988. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Colombani P. M. Mechanism of action of cyclosporine: role of calmodulin, cyclophilin, and other cyclosporine-binding proteins. Transplant Proc. 1986 Dec;18(6 Suppl 5):219–237. [PubMed] [Google Scholar]
- Hess A. D., Colombani P. N. Cyclosporin-resistant and -sensitive T-lymphocyte subsets. Ann Inst Pasteur Immunol. 1987 Jul-Aug;138(4):606–611. doi: 10.1016/s0769-2625(87)80131-9. [DOI] [PubMed] [Google Scholar]
- Hutchinson I. F., Shadur C. A., Duarte J. S., Strom T. B., Tilney N. L. Cyclosporin A spares selectively lymphocytes with donor-specific suppressor characteristics. Transplantation. 1981 Sep;32(3):210–216. doi: 10.1097/00007890-198109000-00006. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Zanker B., Zanker C., Heeg K., Wagner H. Human cytotoxic T lymphocytes. II. Frequency analysis of cyclosporin A-sensitive alloreactive cytotoxic T-lymphocyte precursors. Immunology. 1987 May;61(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- Kahan B. D., Yoshimura N., Pellis N. R., Burstain T., Van Buren C. T., Leinikki P., Schreiber R. D., Roesel T. R., Kerman R. H. Pharmacodynamics of cyclosporine. Transplant Proc. 1986 Dec;18(6 Suppl 5):238–251. [PubMed] [Google Scholar]
- Kalman V. K., Klimpel G. R. Cyclosporin A inhibits the production of gamma interferon (IFN gamma), but does not inhibit production of virus-induced IFN alpha/beta. Cell Immunol. 1983 May;78(1):122–129. doi: 10.1016/0008-8749(83)90265-4. [DOI] [PubMed] [Google Scholar]
- Kay J. E. The cyclosporin-A-sensitive event in lymphocyte activation. Ann Inst Pasteur Immunol. 1987 Jul-Aug;138(4):622–625. doi: 10.1016/s0769-2625(87)80134-4. [DOI] [PubMed] [Google Scholar]
- Kermani-Arab V., Salehmoghaddam S., Danovitch G., Hirji K., Rezai A. Mediation of the antiproliferative effect of cyclosporine on human lymphocytes by blockade of interleukin 2 biosynthesis. Transplantation. 1985 Apr;39(4):439–442. doi: 10.1097/00007890-198504000-00019. [DOI] [PubMed] [Google Scholar]
- Khakpour F. R., Murphy J. W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s). Infect Immun. 1987 Jul;55(7):1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. P., Lewis D. E. In vitro effect of cyclosporine on interleukin-2 receptor expression stimulated by Cryptococcus neoformans. J Infect Dis. 1987 Apr;155(4):799–802. doi: 10.1093/infdis/155.4.799. [DOI] [PubMed] [Google Scholar]
- Milon G., Truffa-Bachi P., Shidani B., Marchal G. Cyclosporin A inhibits the delayed-type hypersensitivity effector function of T lymphocytes without affecting their clonal expansion. Ann Immunol (Paris) 1984 Nov-Dec;135D(3):237–245. doi: 10.1016/s0769-2625(84)81188-5. [DOI] [PubMed] [Google Scholar]
- Mody C. H., Toews G. B., Lipscomb M. F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect Immun. 1988 Jan;56(1):7–12. doi: 10.1128/iai.56.1.7-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Effects of first-order Cryptococcus-specific T-suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985 May;48(2):439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- Palacios R., Möller G. Cyclosporin A blocks receptors for HLA-DR antigens on T cells. Nature. 1981 Apr 30;290(5809):792–794. doi: 10.1038/290792a0. [DOI] [PubMed] [Google Scholar]
- Palay D. A., Cluff C. W., Wentworth P. A., Ziegler H. K. Cyclosporine inhibits macrophage-mediated antigen presentation. J Immunol. 1986 Jun 15;136(12):4348–4353. [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect Immun. 1985 Oct;50(1):22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Ferguson R., Fryd D. S., Balfour H. H., Jr, Rynasiewicz J., Simmons R. L. Infectious diseases in hospitalized renal transplant recipients: a prospective study of a complex and evolving problem. Medicine (Baltimore) 1982 Nov;61(6):360–372. doi: 10.1097/00005792-198211000-00002. [DOI] [PubMed] [Google Scholar]
- Schiltknecht E., Ada G. L. In vivo effects of cyclosporine in the generation and expression of effector cell function. Transplant Proc. 1986 Apr;18(2):357–359. [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Shidani B., Motta I., Truffa-Bachi P. Cyclosporin A does not affect the in vitro induction of antigen-specific delayed-type hypersensitivity-mediating T cells. Eur J Immunol. 1987 Feb;17(2):291–294. doi: 10.1002/eji.1830170222. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Inoue Y., Geczy C. L., Nelson D. S. Modification of delayed-type hypersensitivity reactions to ovalbumin in cyclosporin A-treated guinea-pigs. Immunology. 1983 Feb;48(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Wang B. S., Heacock E. H., Chang-Xue Z., Tilney N. L., Strom T. B., Mannick J. A. Evidence for the presence of suppressor T lymphocytes in animals treated with cyclosporin A. J Immunol. 1982 Mar;128(3):1382–1385. [PubMed] [Google Scholar]
- Webster L. M., Thomson A. W. Augmentation of delayed-type hypersensitivity to high dose sheep erythrocytes by cyclosporin A in the mouse: influence of drug dosage and route of administration and analysis of spleen cell populations. Clin Exp Immunol. 1988 Jan;71(1):149–154. [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N., Kahan B. D. Nature of the suppressor cells mediating prolonged graft survival after administration of extracted histocompatibility antigen and cyclosporine. Transplantation. 1985 Feb;39(2):162–168. doi: 10.1097/00007890-198502000-00011. [DOI] [PubMed] [Google Scholar]
- Yoshimura N., Kahan B. D. The immunosuppressive action of suppressor cells from antigen-cyclosporine-treated hosts on renal allograft survival. Transplantation. 1985 Oct;40(4):384–389. doi: 10.1097/00007890-198510000-00008. [DOI] [PubMed] [Google Scholar]



