Abstract
Alpha-synuclein is implicated in the pathology of Parkinson disease (PD) and is involved in synaptic function, particularly in presynaptic events in dopamine (DA) synapses. Recently, a role for alpha-synuclein in reward and addiction, especially in alcoholism, has been reported. Since PD and alcohol dependence present a strong comorbidity with anxiety disorders, a role for alpha-synuclein in anxiety has been proposed. The aim of the present investigation was to study the involvement of alpha-synuclein in anxiety by testing alpha-synuclein knock out and wild type mice in three different emotionality tests: the open field, the elevated plus maze and the light-dark box. Alpha-synuclein knock out mice and wild type controls displayed consistently similar emotionality profiles in all the tests, suggesting a lack of involvement of alpha-synuclein in unconditioned anxiety in mice.
Keywords: Alpha-synuclein, anxiety, mice, open field, elevated plus maze, light-dark box
Introduction
The alpha-synuclein gene is highly expressed in the central nervous system and has receive increasing interest mainly because of its role in the development of the pathology associated with neurodegenerative disorders such as Parkinson’s disease (PD) (Chartier-Harlin et al., 2004; Kruger et al., 1998; Polymeropoulos et al., 1997; Singleton et al., 2003; Spillantini et al., 1998; Zarranz et al., 2004), in which certain mutations of the alpha-synuclein gene are associated with the degeneration of DA neurons (Kruger et al., 1998; Polymeropoulos et al., 1997; Zarranz et al., 2004). Alpha-synuclein down-regulates dopamine transmission by inhibiting the activity of tyrosine hydroxylase, decreasing dopamine synthesis and acting as a negative regulator of DA release (Peng et al., 2005; Perez et al., 2002). Alpha-synuclein also regulates the trafficking of the DA transporter (DAT) to the cell surface and thus regulates the rate of reuptake of DA (Fountaine and Wade-Martins, 2007; Lee et al., 2001; Wersinger and Sidhu, 2003).
Since the discovery of its involvement in DA function, there is a growing literature suggesting a role for alpha-synuclein in reward and addiction (Boyer and Dreyer, 2007). An overexpression of alpha-synuclein protein in brain reward areas of cocaine addicts (Mash et al., 2003; Qin et al., 2005) and after treatment with psychostimulants in rodents (Brenz Verca et al., 2003; Fornai et al., 2005) has been identified. Moreover, a strong association between alpha-synuclein and alcohol addiction has been suggested. For instance alpha-synuclein mRNA and protein levels were highly expressed in the hippocampus of alcohol-preferring versus non preferring rats (Liang et al., 2003), and alpha-synuclein protein and mRNA levels were elevated in alcoholic patients and were associated with craving for alcohol (Bonsch et al., 2004; Bonsch et al., 2005)
Since there is a high comorbidity of anxiety disorders in patients with PD (Marinus et al., 2002; Nuti et al., 2004; Richard, 2005; Shiba et al., 2000; Walsh and Bennett, 2001), and alcoholism (Morris et al., 2005; Terra et al., 2006) a role for alpha-synuclein in anxiety-related disorders has been proposed, and recently, an increase in the expression of alpha-synuclein in the hippocampus of rats with high levels of anxiety was reported (Chiavegatto et al., 2009). On the other hand a study using transgenic mice expressing the human alpha-synuclein with A53T mutation showed a reduction in anxiety-like behaviour, evaluated in the elevated plus maze, in comparison with wild-type and knock out mice (George et al., 2008). Nevertheless, another study in which a strain of mice with a deletion of the alpha-synuclein gene was tested, provided no conclusive differences in anxiety when the mice were tested in the light-dark box (Siegmund et al., 2005). Thus, even though some evidence seems to point out a role for alpha-synuclein on determining the anxiety phenotype, the evidence to date is inconclusive.
The optimum way to characterise the emotionality profile in rodents is by means of a test battery in which several measures of activity, exploration and anxious behaviour could be compared and a consistent picture drawn (Crawley et al., 1997; Podhorna and Brown, 2002). The open field test (Walsh and Cummins, 1976) provides information about locomotion and exploratory activity, while the presence of thigmotaxis (behaviour in close proximity to the walls) provides an anxiety measure. The elevated plus maze (Lister, 1987; Pellow and File, 1986; Stephens et al., 1986) is another well validated test specially suitable for the study of anxiety in rodents by the analysis of the behaviour in the open arms of the maze and of risk assessment behaviours, such as stretched attend postures, representing a more ethological approach to anxiety (Rodgers and Dalvi, 1997). The light-dark box also provides a measure of unconditioned anxiety by measuring the degree of avoidance of an illuminated arena towards a dark compartment, which rodents find less aversive (Costall et al., 1989; Crawley and Goodwin, 1980). In the present paper, with the aim of clarifying the role of alpha-synuclein in anxiety, we evaluated basal locomotor activity and behaviour in the open field, the elevated plus maze and the light-dark box in alpha-synuclein knock out and wild type mice.
2. Materials and Methods
2.1. Subjects
Mice carrying targeted null mutation of the gene encoding alpha-synuclein (Abeliovich et al., 2000) on a pure genetic background were produced in Cardiff University Transgenic Animal Unit by 11 generations of backcrosses with C57BL/6J mice (Charles River). At this stage heterozygous animals were intercrossed and resulting wild type and homozygous null mutant animals were used as breeders for production of experimental cohorts. Thus, experimental mice were the offspring of homozygous knock out or wild type parents obtained by breeding heterozygous parents. Mouse genotyping was carried out as described previously (Robertson et al., 2004). A total of 36 (18 wild type and 18 knock out) male mice were obtained at 8-12 weeks of age and upon arrival at the University of Sussex, the animals were housed in groups of two or three per cage on a 12 h light/dark cycle (lights off at 7 P.M.). Mice had free access to food and water and were maintained at a temperature of 19-21°C and 50% humidity. A period of 10 days of habituation to the new environmental conditions was given before the start of the experimental procedures. All experiments were approved by the institutional ethics committee and were performed under United Kingdom legislation on animal experimentation [Animal (Scientific Procedures) Act, 1986].
2.2. Test apparatuses
2.2.1. Locomotor boxes
Locomotor activity was assessed in black perspex circular runways (internal diameter, 11 cm; external diameter, 25 cm; height, 25 cm), in which the animals were videoed from below through a translucent Perspex floor that detected the moving shadow of the animal. Images were digitised and locomotor activity determined using in-house software written in Matlab (The MathWorks, Natick, MA). Both overall distance traveled and forward locomotion (whereby animals circulate in one direction for 90° and additional counts for every 45° thereafter) were calculated.
2.2.2. Open Field
The apparatus consisted of a grey opaque floor 50cm square, entirely surrounded by grey opaque walls of 40 cm height. The floor of the open field apparatus was divided into 10 cm squares and was illuminated by a 40 W bulb located 80 cm above the apparatus. The surface of the apparatus was under an illumination of 115- 157 lux.
2.2.3. Plus maze
The Elevated Plus Maze (EPM) consisted of 2 open arms (30 cm long × 4 cm wide), 2 closed arms (30 cm long × 4 cm wide × 15 cm high) and a middle compartment (4cm long × 4 cm wide), forming the shape of a cross. The apparatus was made from black Perspex and was elevated 45 cm above the ground. The room was illuminated with dim light and the open arms of the maze were under illumination of 40-48 lux.
2.2.4. Light-dark box
The light-dark box consisted of two compartments: a large illuminated compartment (27 × 27 cm) and a small dark compartment (18 × 27 cm) connected by a shuttle door (7.5 × 7.5 cm) located in the centre of the partition at floor level. The light box was open at the top, painted white and illuminated by a 60 W bulb located 30 cm above the apparatus providing an illumination measure in the floor of the white compartment in the range of 380- 470 lux. The dark box was painted matt black and had a removable black lid at the top.
2.4. Behavioural procedures
2.4.1. Locomotor activity
Animals were handled and weighed daily for one week before the start of the experiment. Testing took place between 10:00 and 12:00 h. in 45 min sessions for four consecutive days, to investigate possible differences in locomotor activity in a novel environment as well as the within and between-sessions habituation.
2.4.2. Open field and elevated plus maze test
Mice were placed in the centre of the open field apparatus and allowed to freely explore the arena for a period of five minutes. Measures taken were entries into, total locomotor activity (number of line crossings) and time spent in the outer 16 squares (periphery zone), the middle 8 squares (intermediate zone) or the centre square (central zone). The activity, entries and time spent in the unprotected area, outside the outer 16 squares (so, not in proximity to the walls) were grouped for the analysis. Number of rearings and defecations were also measured.
Immediately after the open field testing, each animal was placed in the centre of the EPM facing one of the open arms and allowed to explore the apparatus freely for 5 minutes. Measures taken included time spent in, entries and locomotor activity into the open, closed and middle compartments and behaviours associated with the EPM; stretch-attend postures, head dipping and rearing. An entry was defined as placing all four paws within the given arm. Both apparati were cleaned with a solution containing 30% ethanol after each 5 minute run and wiped dry before the next test.
2.4.3. Light-dark box
The testing took place one week after the OF and the EPM. Each animal was placed at the centre of the illuminated compartment and after 10 seconds the shuttle door was opened and the animal was allowed to explore freely both compartments for 5 minutes. Total number of crossings between the two compartments (defined the placement of all four paws in a given compartment), latency to enter into the dark compartment, latency to enter the illuminated compartment after the first entry into the dark box and time spent in both compartments were measured. Number of head entries towards both compartments (attempts to enter into the adjacent compartment), rearings in the illuminated compartment and total defecations in the apparatus were also measured.
In the Open Field, EPM and Light-dark box the movement of each animal was recorded using a video camera (Sony SPT- M108CE) connected to a videocassette recorder (Panasonic AG-5700) to allow subsequent analysis.
2.5. Statistical analysis
Statistical analysis was performed using the ‘Statistical Package for Social Sciences’ (SPSS, version 14.0). Locomotor activity data were analysed by repeated-measures analysis of variance (ANOVA) with genotype as the between-subjects factor and days (four levels) or 15-min bins (three levels) as the within-subjects factor. Paired t-tests were used for post hoc comparisons. Data from the elevated plus maze, open field and light-dark box were analysed by independent-samples t-tests.. A p<0.05 was required for results to be considered statistically significant.
3. Results
3.1. Locomotor activity
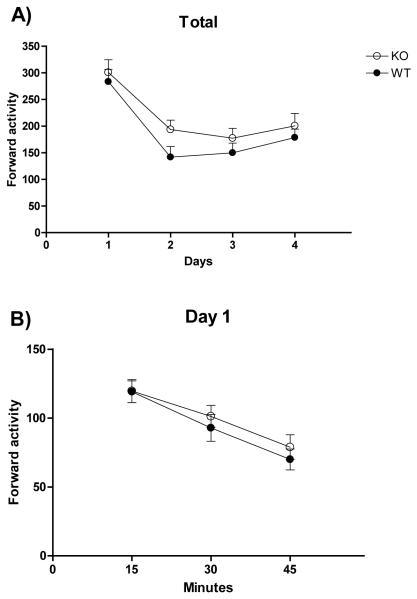
The repeated-measures ANOVA of the total locomotion showed a significant effect of ‘day’ (F(3,93)=36.442, p<0.001; Figure 1A) but groups did not differ in the amount of locomotor activity or in the habituation to the apparatus across days as indicated by the lack of a significant group × day interaction (F<0.372, n.s.). Post hoc comparisons showed that groups decreased locomotor activity in days 2, 3 and 4 when compared to day 1. To further study the response of the animals to a new environment, we analysed the locomotor activity data on the first day by splitting the session into 15-min bins. The results indicated that both groups showed similar levels of activity and habituation to the boxes during this first exposure (Time effect: F(2,66)= 64.036, p<0.001; Figure 1B). Post hoc comparisons indicated that locomotor activity decreased significantly every 15 minutes.
Figure 1. Locomotor activity.
The mean±SE of forward locomotor activity in the 45-min sessions for four consecutive days (a), and during the first session (b) for alpha-synuclein knock out (KO, open circles), and wild type (WT, filled circles) mice.
3.2. Open Field
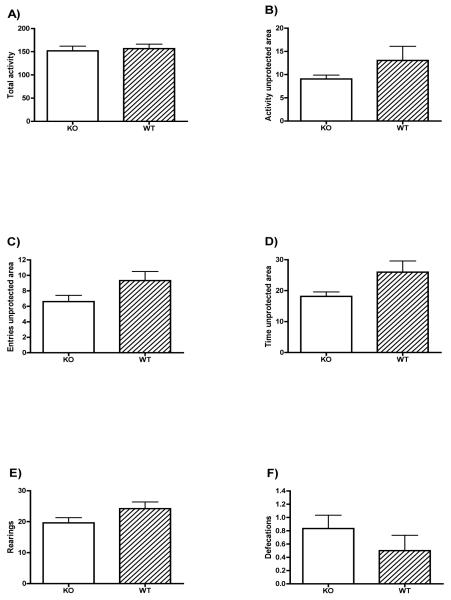
Figure 2 shows the data from the 5 min exposure to the open field apparatus. No significant differences between groups appeared in total activity or in activity in the unprotected area (t<1.26, n.s). Nevertheless, even though not reaching significance, there appeared a tendency for the WT mice to make more entries (t(34)=1.934, p=0.061) and to spend more time (t(34)=2.014, p=0.052) into the unprotected area of the apparatus than the KO, which could be interpreted as a tendency for a more anxiogenic profile in the KO mice. No significant differences between groups appeared in the number of rearings (t(34)=1.689, n.s.) or in number of defecations (t(34)=-1.083, n.s.).
Figure 2. Open field.
The mean±SE of total activity (a) activity in the unprotected area of the apparatus (intermediate + central zone) (b), entries in the unprotected area (c), time in the unprotected area (d), rearings (e) and defecations (f) for alpha-synuclein knock out (KO, open circles), and wild type (WT, filled circles) mice.
3.3. Elevated plus maze
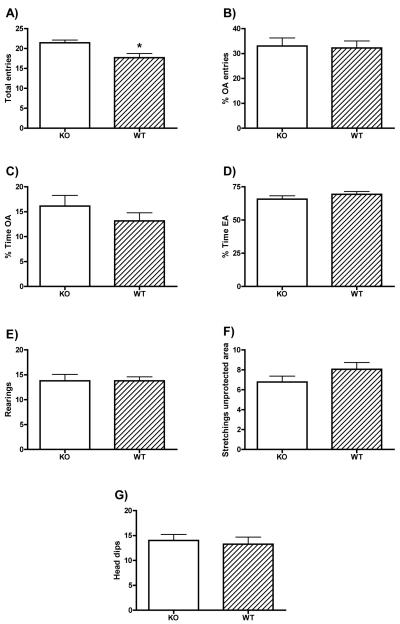
Data from the Elevated plus maze are shown in figure 3. Statistical differences between groups appeared in the number of total entries into the arms of the apparatus (open arms + closed arms entries) (t(34)=−3.010, p=0.005; Fig. 3A). KO mice made more entries than WT mice. As differences in total entries were found, the data of entries and time spent in the open and enclosed arms were analysed as percentages. Groups did not differ in percentage of open arm entries nor in percentage of time spent in the open or the enclosed arms, showing similar levels of emotionality (all t<1.1, n.s.). Apart from these classical measures, ethological variables were also measured. Nevertheless, we failed to find any statistical differences in the number of rearings, stretch attend postures in the unprotected areas of the maze (centre + open arms) or in the number of head dips (all t<1.4, n.s.), suggesting again similar levels of emotionality in both groups of mice in the elevated plus maze.
Figure 3. Elevated plus maze.
The mean±SE of total entries (a), percentage of open arm entries (b), percentage of time spent in the open arms (c), of time spent in the enclosed arms (d) rearings (e), stretchings in the unprotected area (f) and total number of head dips (g) for alpha-synuclein knock out (KO, open circles), and wild type (WT, filled circles) mice. * p<0.05.
3.4. Light-dark box
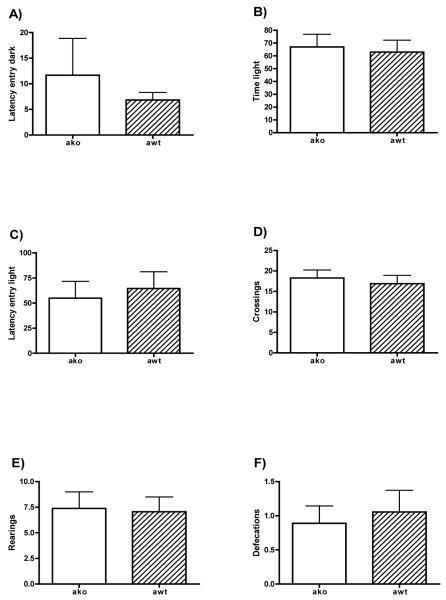
Figure 4 shows the data from the Light-dark box test. No statistical differences between groups appeared in the latency to enter into the dark compartment nor in total time spent in the illuminated compartment of the apparatus (both t<0.66, n.s.). Nevertheless, we also measured the latency to enter into the illuminated compartment after the first entry into the dark, but the ANOVA failed to show statistical differences between groups in this measure (Figure 4C). The fact that the animal is initially allocated into the illuminated compartment makes the interpretation of the latency to enter into the dark difficult, because high values on this measure can be due to a more intense freezing response rather than to a reduction in emotionality. Nevertheless groups showed similar values in both variables, showing similar levels of emotionality. Groups did not differ in the number of crossings between compartments or in number of rearings in the illuminated compartment, suggesting an equal pattern of exploratory activity and locomotion during the testing. The lack of differences in anxiety were also confirmed by the fact that the groups showed no differences in defecations (all t<0.493, n.s.).
Figure 4. Light-dark box.
The mean±SE of latency to enter into the dark compartment (a) latency to enter into the illuminated compartment after the first entry into the dark (b) total time spent in the illuminated compartment (c), number of crossings (d), rearings in the illuminated compartment (e) and total defecations (f) for alpha-synuclein knock out (KO, open circles), and wild type (WT, filled circles) mice.
4. Discussion
The results from the present investigation do not support the idea of an involvement of the alpha-synuclein gene in anxiety. With regard to locomotor activity, there were no differences between wild type and alpha-synuclein knock out mice. In the locomotor boxes, animals showed similar activity levels and the same pattern of habituation across time, which is consistent with previous reports showing that targeted deletion of alpha-synuclein does not cause deficits in motor performance (Abeliovich et al., 2000; Senior et al., 2008). Also data from the exposure to the open field and to the light-dark box indicate an absence of differences in locomotion between both groups. Nevertheless, in the elevated plus maze, knock out mice displayed higher activity levels (higher number of total arm entries) in comparison with wild type animals.
With regard to anxiety, the picture appears quite consistent and suggests a lack of involvement of alpha-synuclein in unconditioned anxiety in mice. No statistical differences between groups appeared in the analysis of the behaviour in any of the emotionality tests performed. Animals showed similar levels of anxiety consistently in the elevated plus maze and in the light-dark box, not differing in the main variables as the percentage of entries and time spent in the open arms of the plus maze or in the time spent in the illuminated compartment in the light-dark box. No statistical differences appeared in the open field although a tendency towards a significant difference in entries and time spent in the unprotected area between groups seems to suggest that the knock out mice were more anxious than the wild type in this particular situation, because they were showing thigmotaxis, although statistical significance was not achieved.
A recent paper by George et al (2008) reported that alpha-synuclein transgenic mice showed reduced anxiety-like behaviour. In this study, transgenic mice over-expressing human alpha-synuclein with the A53T mutation either with endogenous mouse alpha-synuclein or without the mouse alpha-synuclein expression, spent more time and displayed a higher percentage of open arm entries than alpha-synuclein knock out mice or wild type controls. Nevertheless, in this investigation knock out and wild type alpha-synuclein mice did not differ in these two variables, presenting a similar anxiogenic profile. Moreover, this experiment failed to find significant statistical differences between knock out and wild type mice in the open field, in agreement with the results from the present paper (George et al., 2008).
In the study by Siegmund et al (2005) innate fear was compared across three different substrains of C57BL/6 mice: the C57BL/6NCrl (B6N) and the C57BL/6JCrl (B6J), that express alpha-synuclein, in comparison with the C57BL/6JOlaHsd (B6Ola) which does not express alpha-synuclein because of a spontaneous ablation of the gene (Specht and Schoepfer, 2004). Siegmund et al (2005) found that in a 30 min exposure to a light-dark box there were no differences between groups in the relative time spent in the dark compartment, the strains displaying similar levels of emotionality in this test. Nevertheless, taking into account only the first 5 min exposure to the test, the B6JOla showed similar avoidance of the illuminated compartment as the B6N, but a higher avoidance levels than the B6J strain, which supports the idea that alpha-synuclein is not responsible of the differences in anxiety showed by these strains in the light-dark box. Furthermore, unpublished results from our laboratory show that B6Ola mice displayed similar levels of emotionality to B6J mice from two different sources (Jackson laboratories and Charles River laboratories), evaluated in the elevated plus maze and in the light-dark box, supporting the lack of involvement of alpha-synuclein in anxiety.
Even though the two papers mentioned above are in agreement with the results reported in the present paper, a recent study reported that the expression of alpha-synuclein was increased in the hippocampus of rats with high levels of innate anxiety in comparison with non-anxious rats (Chiavegatto et al., 2009). This study compared two different inbred strains of rats, the more fearful Lewis (LEW) rats and the Spontaneously Hypertensive (SHR) rats, which also differ in alcohol preference and intake (higher in the LEW strain in comparison with the SHR). It is difficult to compare our results with this investigation, though it is plausible that local expression of alpha-synuclein in certain brain areas, as in the hippocampus, could determine higher levels of anxiety in rodents. Nevertheless, these strains differ in expression of a number of genes, so that it is not possible to attribute differences in anxiety to levels of alpha-synuclein expression.
Also we have to take into account when using knock out animals that compensational mechanisms can occur. The synucleins are a family of three members, alpha-, beta- and gamma-synuclein that share considerable N-terminal amino acid sequence homology (Jakes et al., 1994; Nakajo et al., 1993). When one or more synucleins are deleted, the remaining members of the family are upregulated in some brain regions (Chandra et al., 2004; Robertson et al., 2004) and similar changes in gene regulation occur in response to targeted deletion of alpha-synuclein or gamma-synuclein, suggesting a degree of functional overlap (Kuhn et al., 2007). For example, Senior et al (2008) demonstrated increased locomotor activity in double (alpha/gamma) knock out mice in comparison with wild type animals, whereas no differences in activity were found in single alpha-synuclein knock out mice in a 5 min grey open field test (Senior et al., 2008). These apparently compensatory changes suggest that there is a degree of functional redundancy in the synuclein gene family. We cannot rule out the possibility that this functional redundancy is responsible to the lack of differences between the wild type and knock out mice from the present study. Furthermore, anxiety is not a simple trait but an heterogeneous and complex phenomenon, determined by multiple genes and by their interaction with environmental factors, which make it difficult the isolation of particular genes (Clement et al., 2002). Nevertheless, we can conclude from the present paper that the absence of alpha-synuclein does not affect unconditioned anxiety in mice.
Acknowledgements
This work was carried out within the framework of the IMAGEN consortium. IMAGEN receives research funding from the European Community’s Sixth Framework Programme (LSHM-CT-2007-037286). This paper reflects only the author’s views and the Community is not liable for any use that may be made of the information contained herein. This study was also supported by The Wellcome Trust Programme Grant to VLB.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol Psychiatry. 2004;56:984–6. doi: 10.1016/j.biopsych.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Greifenberg V, Bayerlein K, Biermann T, Reulbach U, Hillemacher T, Kornhuber J, Bleich S. Alpha-synuclein protein levels are increased in alcoholic patients and are linked to craving. Alcohol Clin Exp Res. 2005;29:763–5. doi: 10.1097/01.alc.0000164360.43907.24. [DOI] [PubMed] [Google Scholar]
- Boyer F, Dreyer JL. Alpha-synuclein in the nucleus accumbens induces changes in cocaine behaviour in rats. Eur J Neurosci. 2007;26:2764–76. doi: 10.1111/j.1460-9568.2007.05878.x. [DOI] [PubMed] [Google Scholar]
- Brenz Verca MS, Bahi A, Boyer F, Wagner GC, Dreyer JL. Distribution of alpha- and gamma-synucleins in the adult rat brain and their modification by high-dose cocaine treatment. Eur J Neurosci. 2003;18:1923–38. doi: 10.1046/j.1460-9568.2003.02913.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–71. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–9. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Izidio GS, Mendes-Lana A, Aneas I, Freitas TA, Torrao AS, Conceicao IM, Britto LR, Ramos A. Expression of alpha-synuclein is increased in the hippocampus of rats with high levels of innate anxiety. Mol Psychiatry. 2009;14:894–905. doi: 10.1038/mp.2008.43. [DOI] [PubMed] [Google Scholar]
- Clement Y, Calatayud F, Belzung C. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57:57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–85. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Fornai F, Lenzi P, Ferrucci M, Lazzeri G, di Poggio AB, Natale G, Busceti CL, Biagioni F, Giusiani M, Ruggieri S, Paparelli A. Occurrence of neuronal inclusions combined with increased nigral expression of alpha-synuclein within dopaminergic neurons following treatment with amphetamine derivatives in mice. Brain Res Bull. 2005;65:405–13. doi: 10.1016/j.brainresbull.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Fountaine TM, Wade-Martins R. RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res. 2007;85:351–63. doi: 10.1002/jnr.21125. [DOI] [PubMed] [Google Scholar]
- George S, van den Buuse M, San Mok S, Masters CL, Li QX, Culvenor JG. Alpha-synuclein transgenic mice exhibit reduced anxiety-like behaviour. Exp Neurol. 2008;210:788–92. doi: 10.1016/j.expneurol.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Haebig K, Bonin M, Ninkina N, Buchman VL, Poths S, Riess O. Whole genome expression analyses of single- and double-knock-out mice implicate partially overlapping functions of alpha- and gamma-synuclein. Neurogenetics. 2007;8:71–81. doi: 10.1007/s10048-007-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. Faseb J. 2001;15:916–26. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Liang T, Spence J, Liu L, Strother WN, Chang HW, Ellison JA, Lumeng L, Li TK, Foroud T, Carr LG. alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and -nonpreferring rats. Proc Natl Acad Sci U S A. 2003;100:4690–5. doi: 10.1073/pnas.0737182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Marinus J, Leentjens AF, Visser M, Stiggelbout AM, van Hilten JJ. Evaluation of the hospital anxiety and depression scale in patients with Parkinson’s disease. Clin Neuropharmacol. 2002;25:318–24. doi: 10.1097/00002826-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Pablo J, Basile M, Izenwasser S, Lieberman A, Perrin RJ. Cocaine abusers have an overexpression of alpha-synuclein in dopamine neurons. J Neurosci. 2003;23:2564–71. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: a critical review. Clin Psychol Rev. 2005;25:734–60. doi: 10.1016/j.cpr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–63. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- Nuti A, Ceravolo R, Piccinni A, Dell’Agnello G, Bellini G, Gambaccini G, Rossi C, Logi C, Dell’Osso L, Bonuccelli U. Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol. 2004;11:315–20. doi: 10.1111/j.1468-1331.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–30. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–9. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes Brain Behav. 2002;1:96–110. doi: 10.1034/j.1601-183x.2002.10205.x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ouyang Q, Pablo J, Mash DC. Cocaine abuse elevates alpha-synuclein and dopamine transporter levels in the human striatum. Neuroreport. 2005;16:1489–93. doi: 10.1097/01.wnr.0000175617.39054.ba. [DOI] [PubMed] [Google Scholar]
- Richard IH. Anxiety disorders in Parkinson’s disease. Adv Neurol. 2005;96:42–55. [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–36. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–10. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27:947–57. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord. 2000;15:669–77. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Langnaese K, Wotjak CT. Differences in extinction of conditioned fear in C57BL/6 substrains are unrelated to expression of alpha-synuclein. Behav Brain Res. 2005;157:291–8. doi: 10.1016/j.bbr.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R. Deletion of multimerin-1 in alpha-synuclein-deficient mice. Genomics. 2004;83:1176–8. doi: 10.1016/j.ygeno.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Meldrum BS, Weidmann R, Schneider C, Grutzner M. Does the excitatory amino acid receptor antagonist 2-APH exhibit anxiolytic activity? Psychopharmacology (Berl) 1986;90:166–9. doi: 10.1007/BF00181234. [DOI] [PubMed] [Google Scholar]
- Terra MB, Barros HM, Stein AT, Figueira I, Jorge MR, Palermo LH, Athayde LD, Goncalves MS, Spanemberg L, Possa MA, Filho L. Daruy, Da Silveira DX. Social anxiety disorder in 300 patients hospitalized for alcoholism in Brazil: high prevalence and undertreatment. Compr Psychiatry. 2006;47:463–7. doi: 10.1016/j.comppsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J. 2001;77:89–93. doi: 10.1136/pmj.77.904.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–92. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa E. Gomez, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]