Abstract
The protein α-synuclein is central to the pathophysiology of Parkinson’s disease (PD) but its role in the development of neurodegeneration remains unclear. α-synuclein knockout mice develop without gross abnormality and are resistant to MPTP, a mitochondrial inhibitor widely used to model parkinsonism. Here we show that differentiated human dopaminergic neuron-like cells also have increased resistance to MPP+, the active metabolite of MPTP, when α-synuclein is knocked down using RNA interference. In attempting to understand how this occurred we found that lowering α-synuclein levels caused changes to intracellular vesicles, DAT and VMAT2, each of which is known to be important components of the early events leading to MPP+ toxicity. Knockdown of α-synuclein reduced the availability of DAT on the neuronal surface by 50%, decreased the total number of intracellular vesicles by 37% but increased the density of vesicular monoamine transporter (VMAT2) molecules per vesicle by 2.8-fold. However, these changes were not associated with any reduction in MPP+-induced superoxide production suggesting that α-synuclein knockdown may have other downstream effects which are important. We then showed that α-synuclein knockdown prevented MPP+-induced activation of nitric oxide synthase (NOS). Activation of NOS is an essential step in MPTP toxicity and increasing evidence points to nitrosative stress as being important in neurodegeneration. Overall, these results show that as well as having a number of effects on cellular events upstream of mitochondrial dysfunction α-synuclein affects pathways downstream of superoxide production, possibly involving regulation of NOS activity.
Introduction
The protein α-synuclein is central to the pathophysiology of Parkinson’s disease (PD) but its normal function in neurons is unknown. Three missense mutations, A53T (Polymeropoulos et al., 1997), A30P (Krüger et al., 1998) and E46K (Zarranz et al., 2004), in the SNCA gene which encodes α-synuclein cause autosomal dominant PD. Shortly after the discovery of the first of these mutations, the protein was found to be aggregated in Lewy bodies, the pathological hallmark of PD (Spillantini et al., 1997). More recently, duplications (Chartier-Harlin et al., 2004) or triplications (Singleton et al., 2003) of the wild-type SNCA locus with associated increases in α-synuclein expression cause autosomal dominant PD with a severity proportional to the degree of α-synuclein overexpression (Eriksen et al., 2005). This implies α-synuclein has a central role, not just in familial disease, but in sporadic cases as well (Spillantini et al., 1997).
Dopaminergic mouse neurons and human neuroblastoma cells lacking α-synuclein have been shown to be resistant to the mitochondrial inhibitor MPTP or its active metabolite MPP+ (Dauer et al., 2002; Fountaine & Wade-Martins, 2007) although the mechanism underlying this effect is not known. α-synuclein knockout mice are grossly normal and display no neurodegenerative phenotype (Abeliovich et al., 2000) but α-synuclein suppression does decrease the number of synaptic vesicles and leads to subtle changes in dopamine homeostasis (Abeliovich et al., 2000; Murphy et al., 2000; Cabin et al., 2002; Fountaine & Wade-Martins, 2007). In addition, α-synuclein has been implicated in a range of specific cellular activities making it difficult to define its exact role. For example, α-synuclein has been found to regulate synaptic vesicle function (Murphy et al., 2000; Cabin et al., 2002), cytoskeletal proteins (Jensen et al., 1999; Payton et al., 2001), protein kinase C (PKC) (Ostrerova et al., 1999), phospholipases D1 and D2 (PLD1 and 2) (Jenco et al., 1998), phosphoinositol lipid signalling pathways (Narayanan et al., 2005), tyrosine hydroxylase (Perez et al., 2002) and monoamine transporters (Lee et al., 2001; Wersinger et al., 2006a; Wersinger et al., 2006b).
To better understand the role of α-synuclein in the pathway of MPP+ toxicity we have developed RNAi-mediated knockdown of α-synuclein in models of differentiated human dopaminergic neurons. MPP+ enters neurons through the dopamine transporter (DAT) and may be sequestered into vesicular monoamine transporter 2 (VMAT2) positive vesicles which have a protective effect against MPP+ toxicity (Takahashi et al., 1997). MPP+ exerts its toxic function by inhibiting mitochondrial respiration causing a detrimental rise in superoxide levels (Przedborski et al., 1992; Zhang et al., 2000). In addition, MPP+ increases nitric oxide synthase activity, a necessary step for MPP+ toxicity (Halász et al., 2004). Overall in this work we found that α-synuclein knockdown caused a reduction in MPP+ toxicity associated with changes in DAT, VMAT2, intracellular vesicles and NOS activation but with no change in superoxide production.
Experimental procedures
Cell culture
Human SH-SY5Y neuroblastoma cells (ECACC# 94030304) were obtained from the European Collection of Cell Cultures (ECACC) and used within 20 passages of the original vial. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% FBS (Sigma), 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine at 37°C and in 5% CO2. For transfection of siRNAs, SH-SY5Y cells were plated at a density of 3×105 cells per well in a 6 well plate and allowed to adhere over night. For each well to be transfected, 3 μL of Lipofectamine 2000 (LF2000) (Invitrogen) was resuspended in 300 μL of OM and incubated for 5 minutes at room temperature. At the same time, each siRNA was resuspended in 300 μL of OM. After 5 minutes, the siRNA and LF2000 mixtures were combined. Complexes were allowed to form for 20 minutes before the 600 μL mixture was added to the cells. After 6 hours, 900 μL of OM with 30% FBS was added to the wells and media replaced after 24 hours. For cells to be differentiated this method was adapted as follows. Cells were diluted down to 1.33 × 105 cells/ml and 1.5 ml put aside for each well to be transfected. siRNA:LF2000 complexes were made as described above and after they had formed, 300 μl of the complex mixture was added to 1.5 ml of cells and mixed gently. The cells were then allowed to stand for 10 minutes before being plated in prepared cell culture plates. After 6 hours, 900 μl of OM supplemented with 30% FBS was added. After 18 hours, cells were covered in DMEM with 5% FBS, antibiotics, 2 mM L-glutamine and 10 μM retinoic acid. Differentiation of the cells then proceeded using a method adapted from that used by Gimenez-Cassina et al (2006). Briefly, culture dishes were prepared by application of 0.1% poly-Lysine (70-150 kDa) (Sigma) for 24 hours. Plates were then washed thoroughly with water and cells seeded at 2 × 104 per cm2 in normal growth media. The next day the media was replaced with DMEM with only 5% FBS, antibiotics, 2 mM L-glutamine and 10 μM retinoic acid. The medium was changed every second day. After 4 days, the medium was changed to Neurobasal medium (Invitrogen) supplemented with 1 × B-27, 2 mM GlutaMaxI (Invitrogen), 2 mM dibutyryl-cyclic AMP (db-cAMP), 50 ng/ml human recombinant brain derived neurotrophic factor (BDNF) and antibiotics. Cells were then allowed to differentiate for 5 days in this medium.
MESC2.10 cells were maintained as described by Lotharius et al (2002). For differentiation of cells in one well of a 6 well dish, 3.9 μl of LF2000 was diluted in 150 μl of OM and incubated for 5 minutes at room temperature. The siRNA was diluted in 150 μl of OM. Based on our titration experiments, we used siRNAs at a final concentration of 50 nM. After 5 minutes, the siRNA and LF2000 mixtures were combined and complexes allowed to form for 20 minutes. During the incubation MESC2.10 cells were trypsinised, spun down, resuspended in N2 medium with no antibiotics and counted. The cells were diluted to 3.33 × 105 cells/ml and 1.5 ml was put aside for each well. After the siRNA:LF2000 complexes had formed, 300 μl were added to the cells which were then allowed to stand for 10 minutes. The cells were plated in wells prepared with poly-L-lysine and fibronectin as described. After 5 hours the cells had adhered and the transfection medium could be removed. Cells were washed three times with N2 medium and then covered with differentiation medium consisting of DMEM/F12 high glucose with N2 supplement, 2 mM L-glutamine, antibiotics, 1 mM db-cAMP, 2 ng/ml glial cell line-derived neurotrophic factor (GDNF) (Sigma) and 1 μg/ml tetracycline. Cells were differentiated for five days with the medium being changed every second day. Differentiation then proceeded as described.
The siRNAs used in this study were (sense sequence only): Scrambled: 5′-GCGACGUUCCUGAAACCACdtdt-3′; SNCA1: 5′-GGAAAGACAAAAGAGGUGdtdt-3′; SNCA2: 5′-GGUGUUCUCUAUGUAGGCdtdt-3′; Scrambled SNCA1: GAGAATAGGGAGGAGAACAtt. All were made by Ambion (Austin, TX, USA).
Immunofluorescence
Cells were differentiated in 24 well dishes containing glass cover slips. After differentiation, cells were fixed in 4% paraformaldehyde for 15 minutes and permeabilised with IF block solution (1% fish gelatine, 0.1% Triton X-100, 10% normal goat serum in TBS) for 30 minutes at room temperature. The following primary antibodies and dilutions were used: mouse monoclonal anti-α-synuclein (1:500) (Abcam, Cambridge, UK) (Fountaine & Wade-Martins, 2007), mouse monoclonal antibody SMI-31 (1:500) (Sternberger Molecular Inc, Baltimore, MD) (Gimenez-Cassina et al., 2006; Howard et al., 2006). The primary antibody was applied in an overnight incubation at 4°C. Cells were washed four times in IF wash solution (0.1% Triton X-100, 0.02% sodium azide in TBS) before application of the appropriate AlexaFluor 488 IgG secondary antibody (Invitrogen). Cells were mounted using Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) and imaged using confocal laser scanning microscopy (Carl Zeiss).
Western blotting
Cells were differentiated in 6 well dishes as described. After differentiation cells were washed once with phosphate buffered saline (PBS) and then scraped into PBS. Cells were pelleted by centrifugation at 1,700 xg for 2 minutes and lysed in WB lysis buffer (50 mM tris pH 8.0, 150 mM NaCl, 1% triton X100, 1x protein inhibitor cocktail (Sigma) by trituration. After incubation for 30 minutes on ice, the lysate was separated by centrifugation for 10 minutes at 1,200 xg at 4°C into soluble and insoluble fractions. Protein content was quantified using the BCA assay (Sigma) in accordance with the manufacturer’s instructions. 10 μg of protein was boiled in 1x Laemelli buffer for 5 minutes, separated by SDS-polyacrylamide gel electrophoresis, and transferred by electrophoresis to 0.45 μm PVDF membrane (Millipore). Blots were blocked in WB blocking buffer (tris buffered saline (TBS), 1% Tween-20, 5% milk) and probed using the primary antibodies suspended in WB hybridising buffer (TBS, 1% Tween-20, 1% milk). The following antibodies were used: mouse monoclonal anti-α-synuclein (Abcam, Cambridge, UK); mouse monoclonal anti-actin (Sigma) (Fountaine & Wade-Martins, 2007). Membranes were washed three times with TBS, 1% Tween-20 and appropriate horse radish peroxidise-conjugated secondary antibodies (Biorad) were applied, suspended in hybridising buffer. After washing, chemiluminescence was produced using an ECL+ kit (Amersham). Images were photographed using a charge couple device (CCD) camera (UVP) and analysed using LabWorks software 4.6 (UVP). Each western blot reproduced here are typical of at least three separate experiments.
Measuring cell viability
Cells were grown in 24 well dishes and, once differentiation was complete, they were washed with Dulbecco’s PBS (D-PBS) supplemented with calcium and magnesium. Cell viability was assessed using vital stains. D-PBS with 4 μM Calcein AM and 4 μM Ethidium homodimer-1 (Invitrogen) was added to the cells and incubated at 37°C for 30 minutes. Cells were then immediately imaged using a 20x objective on an inverted fluorescent microscope (Nikon) and pictures taken with a CCD camera (Hamamatsu Photonics, Shizuoka, Japan). Images from six randomly selected fields of view were captured in each well and live cells were counted blind using Volocity software (Improvision). Results were expressed as the mean number of cells per field of view from three separate experiments.
Dopamine and MPP+ uptake
3H Dopamine (3HDA) and 3HMPP+ uptake measurements were performed on cells in 6 well dishes as described previously (Bönisch, 1998; Reith et al., 1998). The cells were washed three times in Dulbecco’s Phosphate Buffered Saline with calcium and magnesium (D-PBS +Ca +Mg) (Invitrogen). 3HDA and 3HMPP+ were diluted in D-PBS +Ca +Mg to 20 nM with or without 10 μM Mazindol. Uptake was measured over two minutes to measure uptake velocity, or at 15 minutes to measure cell content at saturation. For each experimental well, 1 ml of 3HDA or 3HMPP+ mixture was added for the required time before being aspirated and cells washed twice with ice cold PBS. At the end of the experiment the cells were dissolved in 1 ml 1N NaOH solution. 500 μl of cell lysis solution was added to 5 ml of OptiPhase Supermix (PerkinElmer) and scintillation quantified using a scintillation counter (Beckman Coulter). Results were normalised to protein content measured in a sample of the cell lysate using the BCA method (Sigma).
Electron microscopy
Cells were cultured in 6 cm dishes. The cells were rinsed twice with D-PBS with calcium and magnesium. Cells were then fixed by incubating in 2 ml of 3% PFA/0.05% glutaraldehyde in PBS for 30 minutes at room temperature. The fixative was washed off twice with PBS and cells covered in 3 ml of 0.3% PFA/0.005% glutaraldehyde. The cells were scraped into this solution and stored at 4°C overnight. Cells were transferred to a solution of 2.3 m sucrose in PBS for 24 hours. The cryoprotected cells were slam-frozen (Reichert MM80E; Leica), freeze-substituted at −80°C in methanol for 48 hours, and embedded at −20°C in LR Gold acrylic resin (London Resin Company Ltd) in a Reichert freeze-substitution system (Reichert). Ultrathin sections were prepared by use of a Reichert Ultracut S ultratome, mounted onto formvar-coated nickel grids, incubated for 2 hours with rabbit anti-human DAT polyclonal antibody (dilution, 1:200; Alpha Diagnostics, San Antonio TX, USA) (Fountaine & Wade-Martins, 2007; Yokoyama et al., 2008) or anti-VMAT2 polyclonal antibody (dilution, 1:200; Abcam, Cambridge, UK) (specificity confirmed by western blot on human brain tissue extract (data not shown)) and for 1 hours with Protein A-15 nm gold complex, then lightly counterstained with uranyl acetate and lead citrate. All antibodies were diluted in 0.1 m phosphate buffer containing 0.1% w/v egg albumin. For control sections, the primary antibodies were omitted and replaced with 0.1 M phosphate buffer containing 0.1% w/v egg albumin; immunogold labelling was absent in these conditions. Sections were examined with a JEOL 1010 transmission electron microscope (JEOL USA, Inc.). The number of vesicles and15 nm gold particles were counted in 30 cells and calculated as particles/μm2 by dividing by the cell area. For measurement of cell area micrographs of each cell were taken at a magnification of × 4,000. The cell areas were analysed from scanned micrographs using Axiovision software, version 3.4 (Zeiss). In all cases the analyst was blind to the sample code.
Measuring superoxide levels
Measurement of superoxide levels was performed by measuring the conversion of dihydroethidium (DHE) to 2-hydroxyethidium (2OH-Ethidium) using high performance liquid chromatography (HPLC) as described previously (Zhao et al., 2005). The cells were washed three times in Krebs-HEPES buffer (KHB) (NaCl 99 mM, KCl 4.7 mM, MgSO4 1.3 mM, KH2PO4 1.0 mM, CaCl2 1.9 mM, NaHCO3 2.5 mM, Glucose 11.1 mM, HEPES 20 mM, pH 7.4) and then covered in 500 μl of 25 μM DHE in KHB. Incubation was for 30 minutes at 37°C. The cells were washed three times in KHB, the final wash being at 4°C and were scraped into 500 μl cold KHB. The cells were centrifuged at 3,000 xg for 5 minutes at 4°C. The supernatant was discarded and cells were resuspended in 200 μl ice cold methanol. The lysate was centrifuged at 14,000 xg for 10 minutes at 4°C and the supernatant was transferred to a black 1.5 ml microfuge tube and stored at −80°C until the HPLC assay could be performed.
DHE, ethidium and 2OH-Eth were separated using a C18 reverse-phase column (Partisil ODS-3 250 × 4.5 mm) (Highcrom), a DG-1580-53 degasser, two PU-2080 Plus pumps, an HG-1580-32 dynamic mixer, a AS-2057 Plus cooled autosampler and a FP-2020 plus Intelligent Fluorescence detector (all from Jasco Ltd). Azure software version 4.02 was used for analysis (Datalys). To prepare samples for HPLC 80 μl of sample was mixed with 80 μl of ice cold 0.1N HCL in fresh black microfuge tubes and vortexed. 120 μl of the mixture was transferred to amber glass HPLC vials for analysis. 100 μl of the sample was loaded for HPLC analysis. The mobile phases were: Buffer A, 0.1% trifluoroacetic acid (TFA) in water; Buffer B, 0.085% TFA in fluorescence grade acetonitrile. Running conditions were: flow rate 1.0 ml/minute with a linear gradient from 70% Buffer A/30% Buffer B to 50% Buffer A/50% Buffer B over 23 minutes. The column was re-equilibriated after every sample for 12 minutes with 70% Buffer A/30% Buffer B.
Measuring NOS activity
NOS enzymatic activity was measured by HPLC quantification of radiolabelled arginine to citrulline conversion (de Bono et al., 2007). Cells were prepared in 24 well dishes. To perform the assay, cells were washed three times with KHB. 100 μl KHB with 5 μM of the arginase inhibitor N(omega)-Hydroxy-nor-L-Arginine (nor-NOHA) (Calbiochem) with or without 1 mM of the competitive NOS inhibitor N-Nitro-L-Arginine Methyl Ester (L-NAME) was added to the cells and incubated at 37°C for 30 minutes. The buffer was then changed to 100 μl KHB with 5 μM nor-NOHA containing 1 mM L-NAME, 1 μM Calcimycin calcium ionophore A23187 or no additive. Ubiquitously labelled 14C-L-arginine (3 μl of 1.85 MBq/ml, Amersham Biosciences) was added to each well and the cultures incubated at 37°C for 2 hours. The supernatants were transferred to 1.5 ml microfuge tubes and the cells lysed by the addition of 200 μl water and freeze-thawing. Cells were scraped from the dish and all 200 μl of solution transferred to the corresponding 1.5 ml microfuge tubes containing the supernatant. Samples were deproteinated by the addition of 300 μl 10% trichloroacetic acid followed by centrifugation. 200 μl of water was added to each tube and the conversion of radioactively labelled arginine to citrulline was measured using HPLC.
14C-arginine, 14C-ornithine, 14C-citrulline and 14C-urea were separated using a 250 × 4.6 mm Supelcosil LC-SCX five cation exchange column (Sigma), a DG-980-50 degasser, two PU-2080 Plus pumps, a MX-2080-32 dynamic mixer, a AS-2057 Plus cooled autosampler (all from Jasco Ltd) and a Lablogic β-RMA model 3 continuous flow liquid scintillation detector (Lablogic Systems Ltd). Azure software version 4.02 was used for analysis. To prepare samples for HPLC 700 μl of sample was transferred to plastic HPLC vials and 600 μl of the sample was loaded for HPLC analysis. The mobile phases were: Buffer A, MQ H2O with 0.1% sodium azide; Buffer B, 200 mM citric acid pH 3.0. Running conditions were: flow rate 1 ml/minute with 100% Buffer A for 5 minutes followed by a linear gradient form 100% Buffer A to 100% Buffer B over 10 minutes then 100% buffer B for 15 minutes. The column was re-equilibrated after each sample for 18 minutes with 100% Buffer A. Scintillant fluid was mixed in line at a ratio of 0.5/1 after elution from the column before passage through the detector. The area under each peak was calculated and adjusted for background levels in 14C-arginine standards and expressed as a proportion of total 14C counts for each sample.
Statistical analysis
All data were presented as means ± SEM. GraphPad Prism software (San Diego CA, USA) was used to analyse the data for statistical significance. Statistical analyses were carried out using one or two-way ANOVA as appropriate. Post hoc comparisons were performed using the Bonferroni adjustment with p < 0.05 as *, p < 0.01 as ** and p < 0.001 as ***.
Results
Developing knockdown in human neuronal cells
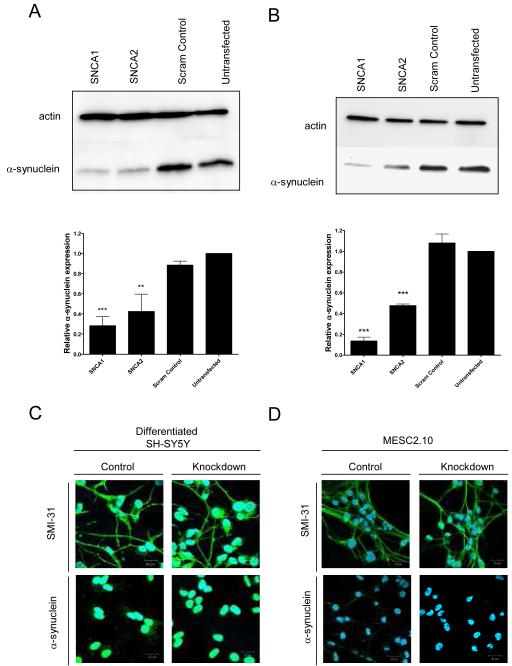
We have previously validated two siRNAs, SNCA1 and SNCA2, capable of knocking down α-synuclein 80-90% in undifferentiated SH-SY5Y cells (Fountaine & Wade-Martins, 2007). For the present study, we used these molecules to knockdown α-synuclein in differentiated SH-SY5Y (Gimenez-Cassina et al., 2006) and MESC2.10 cells (Lotharius et al., 2002). When differentiated according to well described protocols, both cell types are known to produce post-mitotic neuron-like cells (Lotharius et al., 2002; Gimenez-Cassina et al., 2006). To achieve RNAi-mediated knockdown of α-synuclein, we transfected proliferating cells in suspension with siRNAs and then grew the cells under conditions that promote differentiation. Using this method we achieved substantial silencing of α-synuclein in the fully differentiated cells as demonstrated by western blotting (Figure 1a and b). Expression levels of α-synuclein were normalised to the respective actin loading control. In SH-SY5Y cells, SNCA1 produced 80% knockdown compared to untransfected cells. SNCA2 was less effective with 60% knockdown. As a control we used a scrambled version of the SNCA1 siRNA, which did not affect α-synuclein expression. We also found that a single base pair change in SNCA1 was sufficient to abolish silencing of α-synuclein demonstrating the high level of target specificity of SNCA1. In MESC2.10 cells SNCA1 produced 90% knockdown and SNCA2 caused a 50% knockdown. Again, the scrambled control siRNA had no effect. In summary, we established a procedure for reliable and reproducible suppression of α-synuclein expression in differentiated human neuron-like cells.
Figure 1.
Models of α-synuclein knockdown in differentiated human neurons. (A) SH-SY5Y cells were transfected with the siRNAs SNCA1, SNCA2 and a scrambled version of SNCA1 as a control. The cells were then differentiated. At the end of differentiation, protein was analysed by western blot. Expression levels of α-synuclein were quantified SNCA1 produced 80% knockdown compared to untransfected cells. SNCA2 was less effective with 60% knockdown while the scrambled control made no difference to α-synuclein expression. (B) MESC2.10 cells were also transfected with the same siRNAs and cells differentiated. Protein was analysed by western blot. Expression of α-synuclein in MESC2.10 cells was quantified. SNCA1 produced 90% knockdown and SNCA2 50% knockdown. Again, the scrambled control had no effect. Immunofluorescence studies were performed in (C) differentiated SH-SY5Y and (D) MESC2.10 cells using the axonal marker antibody SMI-31 and an anti-α-synuclein antibody. Nuclei are counter stained with DAPI.
To test the effect of α-synuclein knockdown, we compared differentiation in cells transfected with SNCA1, the more potent of our siRNAs with control cells treated with the scrambled SNCA1 siRNA. We evaluated the morphological and gene expression characteristics of the cells stained with antibodies for α-synuclein and the neuronal marker antibody SMI-31 (Figure 1c and d). The SMI-31 antibody recognises axon-specific phosphoepitopes of cytoskeletal proteins, including MAP1B and high-molecular weight neurofilament protein (HMW-NFP) (Ulloa et al., 1993). Consistent with the expression analysis from our western blots, SNCA1 treated SH-SY5Y and MESC2.10 cells showed minimal α-synuclein immunoreactivity compared to scrambled control treated cells. By contrast, the level of immunoreactivity to the SMI-31 was similar in both groups. Cells treated with SNCA1 or scrambled control siRNA both developed an extensive network identical to that seen in untransfected cells indicating that differentiation had occurred normally in cells lacking α-synuclein.
We also examined if α-synuclein reduction adversely affects viability of differentiated neurons (data not shown). In this experiment, we found no difference in the number of cells between control and α-synuclein knockdown groups for differentiation SH-SY5Y cells (unpaired Student’s t-test, p = 0.60) or MESC2.10 cells (unpaired Student’s t-test, p = 0.67). Therefore we found that α-synuclein reduction does not significantly affect neuronal differentiation or cellular survival in normal conditions in either of our two types of human neurons.
Knockdown of α-synuclein protects human neurons from MPP+ toxicity
In our previous experiments we found a significant protective effect with treatment by either SNCA1 or SNCA2 on MPP+ toxicity in undifferentiated SH-SY5Y neuroblastoma cells (Fountaine & Wade-Martins, 2007). To see if this also applied to differentiated human neurons we therefore measured the effect of α-synuclein suppression on neuronal viability in the presence of MPP+ in differentiated SH-SY5Y and MESC2.10 neuronal cells (Figure 2). Cells were transfected with SNCA1 or a scrambled control and, at the end of the differentiation process, were exposed to different concentrations of MPP+. α-synuclein knockdown protected both differentiated SH-SY5Y and MESC2.10 cells from MPP+ toxicity (two-way ANOVA, siRNA effect, p < 0.001 for both cell types). In differentiated SH-SY5Y, 1 mM MPP+ caused a 28% cell loss in the control group but this was completely prevented by α-synuclein knockdown (Bonferroni post hoc comparison, p < 0.05) (Figure 2c). At 5 mM concentration, MPP+ caused a 76% reduction in control cell viability compared to only a 37% reduction in the α-synuclein knockdown group (Bonferroni post hoc comparison, p < 0.01). In MESC2.10 cells, at 1 mM concentration there was a 42% loss in control cell viability which was prevented by α-synuclein knockdown (Bonferroni post hoc comparison, p < 0.001) (Figure 2d).
Figure 2.
α-synuclein knockdown protects differentiated human neurons from MPP+ toxicity. Differentiated SH-SY5Y and MESC2.10 cells were transfected with SNCA1 (knockdown) or a scrambled version of SNCA1 (scram control) and differentiated as normal. At the end of differentiation, cells were exposed to different concentrations of MPP+ for 16 hours and then live cells were stained green using calcein AM and dead cells red using ethidium homodimer-1. Representative photographs are shown of differentiated (A) SH-SY5Y and (B) MESC2.10 cells exposed to normal media or 1mM MPP+ with or without α-synuclein knockdown. 20x objective. (C) Viability was determined by counting live cells in six random 20x fields of view for each condition. Results are from 3 separate experiments performed in triplicate (± SEM). Survival of differentiated SH-SY5Y cells exposed to MPP+ was significantly improved by α-synuclein knockdown (two-way ANOVA, siRNA effect, p < 0.001). (D) MESC2.10 cells were also significantly protected by α-synuclein knockdown from MPP+ exposure (two-way ANOVA, siRNA effect, p < 0.001).
α-synuclein knockdown changes dopamine uptake and DAT localisation
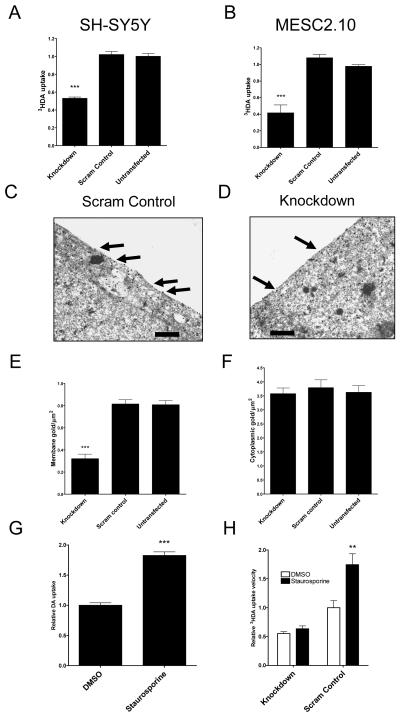
Having established that α-synuclein knockdown protects human neurons from MPP+ toxicity, we were interested to explore why this occurred. MPP+ is taken up through the dopamine transporter DAT, and we therefore investigated whether α-synuclein knockdown caused changes in DAT activity. Dopamine uptake velocity was measured in SH-SY5Y and MESC2.10 cells that had been treated with SNCA1, a scrambled version of SNCA1 or differentiated without siRNA transfection (Figure 3 a and b). There was a significant effect of siRNA treatment on dopamine uptake velocity in both cell types (one-way ANOVA, p < 0.001 for each) with knockdown producing a 48% decrease in dopamine uptake in SH-SY5Y cells and a 58% decrease in dopamine uptake in MESC2.10 cells (Post hoc Bonferroni comparison, p < 0.001 compared to untransfected cells in both models). There was no effect of the scrambled control on dopamine uptake (Post hoc Bonferroni comparison, p > 0.2 compared to untransfected control). These results show that α-synuclein knockdown reduced dopamine uptake velocity in both cell types to a similar degree suggesting a change in DAT function.
Figure 3.
α-synuclein knockdown decreases dopamine transport by reducing DAT surface localisation. (A) SH-SY5Y cells and (B) MESC2.10 were transfected with SNCA1 siRNA (knockdown) or a scrambled version of SNCA1 (scram control). 3HDA uptake velocity was then measured at 20 nM dopamine. Data are normalised to an untransfected control. There was a significant effect of siRNA treatment on 3HDA uptake velocity (One-way ANOVA, p < 0.001 for both SH-SY5Y and MESC2.10 cells) with knockdown being significantly different to controls (Post hoc Bonferroni comparison, p < 0.001 for both cell types). Data are each from three separate experiments performed in triplicate (± SEM). Electron microscopy using DAT immunogold labelling in (C) scrambled control and (D) knockdown cells. The area marked by an asterisk is shown in greater detail in the inset image. Scale bar on all images = 10 μm. (E) Quantification of membrane bound DAT showed a significant effect of siRNA treatment (One-way ANOVA, p < 0.001) with knockdown causing a significant decrease in DAT surface localisation (Post hoc Bonferroni comparison, p <0.001). Scrambled control and untransfected cells were the same. (F) There was no effect of siRNA treatment on total DAT immunoreactivity (One-way ANOVA, p > 0.2). DAT labelling was counted in 30 different cells for each siRNA treatment. (G) SH-SY5Y cells were treated with the PKC inhibitor staurosporine or DMSO carrier only. After 30 minutes, dopamine uptake velocity was determined. Staurosporine caused a significant increase in uptake velocity (unpaired Student’s t-test, p < 0.001). (H) SNCA1 or scrambled control siRNA treated cells were incubated with staurosporine or carrier only and dopamine uptake velocity determined. There was a significant effect of both siRNA treatment and staurosporine on dopamine uptake velocity (two-way ANOVA, p < 0.01 for staurosporine effect, p < 0.001 for siRNA effect). As shown previously, knockdown of α-synuclein significantly reduced dopamine uptake in carrier only treated cells (Post hoc Bonferroni comparison, p < 0.01). While staurosporine significantly increased dopamine uptake velocity in scrambled siRNA treated cells (Post hoc Bonferroni comparison, p < 0.01), it did not alter uptake in α-synuclein knockdown cells.
To better understand the effect of α-synuclein knockdown on intracellular DAT localisation SH-SY5Y cells were imaged by electron microscopy with DAT immunogold labelling (Figure 3c - f). Consistent with the results of the 3HDA uptake, knockdown cells had a 59% reduction in membrane DAT compared to untransfected controls (One-way ANOVA, p < 0.001 with post hoc Bonferroni comparison for knockdown, p < 0.001, compared to untransfected or scrambled control) with no difference in the total DAT immunoreactivity in each group (One-way ANOVA, p > 0.2). In untransfected control cells, 22% (0.8 out of 3.5 particles per μm2) of DAT was localised in the surface membrane compared to only 9% in knockdown cells. In both knockdown and control cells, the remaining DAT was located in the cytoplasm mostly just beneath the plasma membrane. These results show that α-synuclein knockdown influenced the intracellular localisation of DAT, reducing the number of transporters on the cell surface.
Protein kinase inhibition using staurosporine is known to increase DAT cycling to the cell surface, increasing DAT function (Pristupa et al., 1998; Page et al., 2004). The effect of staurosporine was evaluated in SH-SY5Y cells (Figure 3g). Staurosporine significantly increased DAT activity by 1.9-fold compared to the vehicle only control (unpaired Student’s t-test, p < 0.001). To test if α-synuclein was required for staurosporine-mediated increases in DAT activity, cells with and without α-synuclein knockdown were incubated with staurosporine before measuring DAT activity (Figure 3h). There was a significant effect of both staurosporine and knockdown on dopamine uptake velocity (two-way ANOVA, p < 0.01 for staurosporine effect, p < 0.001 for knockdown effect). Staurosporine caused a 1.8-fold increase in DAT activity in control cells (Post hoc Bonferroni comparison, p < 0.01) but had no significant effect in α-synuclein knockdown cells (Post hoc Bonferroni comparison, p > 0.2). Therefore, these data suggest that α-synuclein is required for staurosporine-mediated increases in DAT activity.
α-synuclein knockdown alters intracellular vesicle numbers and VMAT2 expression
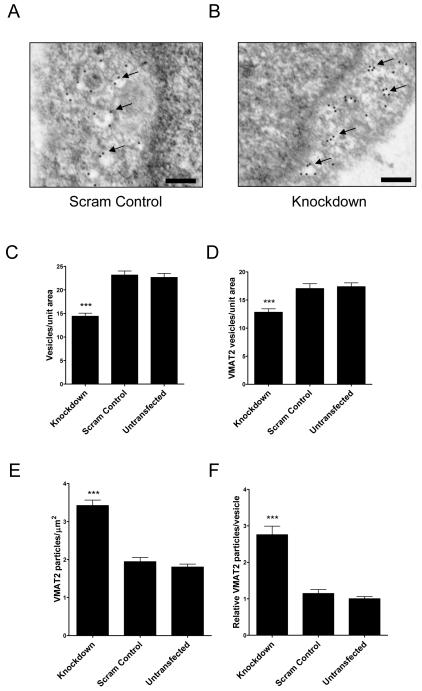
Previous reports have found that α-synuclein knockdown reduces the total number of intracellular vesicles in mouse neurons (Murphy et al., 2000; Cabin et al., 2002). Again, this could affect cellular susceptibility to MPP+ since sequestration of the toxin in dopaminergic vesicles in known to be important in regulating its toxicity (Takahashi et al., 1997). To see what changes occurred in SH-SY5Y cells with α-synuclein knockdown and to further define the types of vesicles involved, electron micrographs from cells treated with SNCA1, scrambled control or untransfected cells were VMAT2 immmunogold labelled (Figure 4a and b). The total number of electron lucent and VMAT2 positive vesicles were counted (Figure 4c and d). There was a significant reduction in total vesicles by 37% in knockdown cells compared to controls (one-way ANOVA, p < 0.001, with post hoc Bonferroni comparison, p < 0.001, compared to untransfected control) consistent with a previous report (Murphy et al., 2000). A decrease was also seen with knockdown in the VMAT2 positive vesicle subpopulation but only by 26% (one-way ANOVA, p < 0.001, with post hoc Bonferroni comparison, p < 0.001, compared to untransfected control) suggesting that the reduction in vesicle number affected non-VMAT2 more than VMAT2 vesicles. There were no differences in vesicle numbers between scrambled control and untransfected cells (Post hoc Bonferroni comparisons, p > 0.2 for both total and VMAT2 positive vesicles). Quantification of total VMAT2 immunogold staining showed a two-fold increase in VMAT2 number in knockdown cells compared to controls (Kruskal-Wallis one-way ANOVA, p < 0.001 with post hoc Dunn’s comparison, p < 0.001, compared to untransfected cells) (Figure 4e). There was a corresponding 2.8-fold increase in VMAT2 particles per vesicle in knockdown cells compared to control (Kruskal-Wallis one-way ANOVA, p < 0.001 with post hoc Dunn’s comparison, p < 0.001, compared to untransfected cells) (Figure 4f). Again, no significant differences were seen in scrambled control versus untransfected cells (post hoc Dunn’s comparisons, p > 0.05). In summary, α-synuclein knockdown decreased the total number of vesicles and increased the density of VMAT2 transporters per vesicle.
Figure 4.
Knockdown of α-synuclein decreases vesicle number but increases VMAT2 expression. SH-SY5Y cells were transfected with SNCA1, a scrambled control or were untransfected. Cells were stained using VMAT2 antibody and immunogold labelling before electron microscopy was performed. Representative electronmicrographs from (A) scrambled control and (B) knockdown cells are shown. The area marked by an asterisk is shown in greater detail in the inset image. Scale bar on all images = 1 μm. (C) Quantification of total number of electron lucent vesicles. There was a significant effect of α-synuclein knockdown (one-way ANOVA, p < 0.001 with Bonferroni post hoc comparison to either control, p < 0.001). (D) Quantification of the number of VMAT2 positive vesicles. There was a significant effect of α-synuclein knockdown (one-way ANOVA, p < 0.001 with Bonferroni post hoc comparison to either control, p < 0.001). (E) Total VMAT2 immunoreactivity was increased by α-synuclein knockdown (Kruskal-Wallis one-way ANOVA, p < 0.001 with Dunn’s post hoc comparison to either control, p < 0.001). (F) The number of VMAT2 molecules per vesicle was also increased (Kruskal-Wallis one-way ANOVA, p < 0.001 with Dunn’s post hoc comparison to either control, p < 0.001).
α-synuclein knockdown reduces total MPP+ uptake
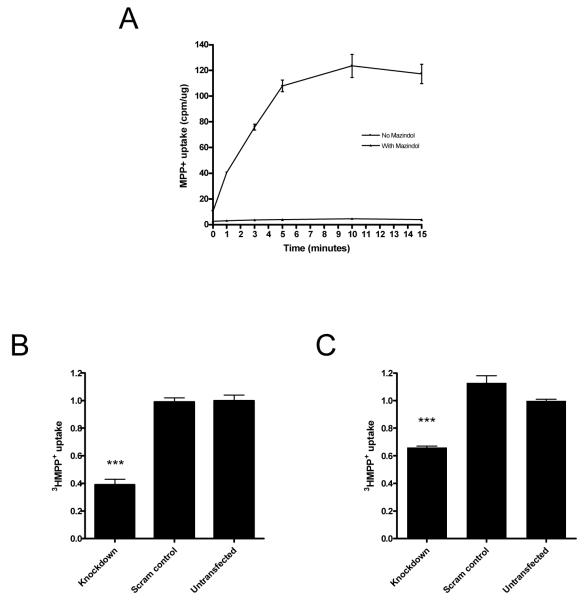
To investigate directly the effect of α-synuclein knockdown on MPP+ uptake we measured both the velocity of uptake and total MPP+ uptake at saturation. SH-SY5Y cells were first exposed to 3HMPP+ for different time periods from 0 to 15 minutes either with or without mazindol (Figure 5a). The uptake was linear for the first 5 minutes, and reached saturation after 10 minutes. Subsequent experiments were conducted with and without α-synuclein knockdown, using a 2-minute incubation time to measure uptake velocity, and after 15 minutes to measure cellular MPP+ content in saturation conditions (Figure 5b and c). Nonspecific uptake was measured in cells incubated with mazindol. In keeping with the results of the DA experiments, we found that α-synuclein knockdown led to a decrease in MPP+ uptake at both incubation times (one-way ANOVA, p < 0.001 for both time points, with post hoc Bonferroni comparison, p < 0.001 and p < 0.01 respectively for 2 and 15 minutes, compared to untransfected cells). There was no effect of the scrambled control on MPP+ uptake (post hoc Bonferroni comparison, p > 0.05 compared to untransfected control). These findings suggest that suppression of α-synuclein reduces not only MPP+ uptake velocity, but also the amount of MPP+ that is taken up by the cells at saturation. Overall, these results show that α-synuclein knockdown reduces total MPP+ uptake into the cell, suggesting that changes in DAT function form part of the mechanism by which cells are protected from MPP+ toxicity.
Figure 5.
α-synuclein knockdown decreases MPP+ uptake velocity and total uptake. (A) SH-SY5Y cells were incubated with 10 nM 3HMPP+ for time periods from 0 to 15 min, with or without 10 μM mazindol. Uptake was normalized to cellular protein in each sample (± SEM). SH-SY5Y cells were transfected with SNCA1 siRNA (knockdown) or a scrambled version of SNCA1 (scram control). 3HMPP+ uptake was then measured at 20 nM for 2 min (B) or 15 min (C). Data are normalised to an untransfected control. There was a significant effect of siRNA treatment on 3HMPP+ uptake velocity (One-way ANOVA, p < 0.001 for both time periods) with knockdown being significantly different to controls (Post hoc Bonferroni comparison, p < 0.001 and p < 0.01, respectively). Data are each from three separate experiments performed in triplicate (± SEM).
Superoxide levels are increased by MPP+ toxicity but are not affected by α-synuclein knockdown
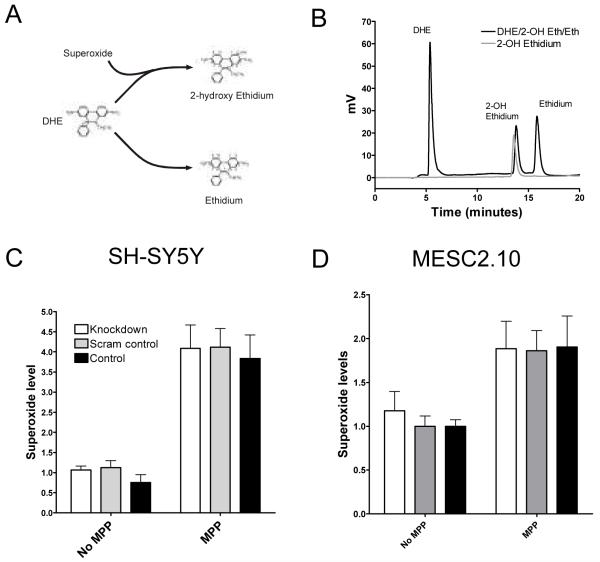
We expected that a reduction in MPP+ uptake would reduce oxidative stress in cells with α-synuclein knockdown. Superoxide is one of the most important oxidative species generated during MPP+ exposure, and we measured levels in our cells using a high performance liquid chromatography (HPLC) based approach for measuring superoxide which allows identification of 2-hydroxyehidium (2OH-Eth), the product formed from the reaction of dihydroethidium (DHE) and superoxide (Zhao et al., 2005) (Figure 6a and b). This technique is now established as the most specific assay available for detecting superoxide (Zhao et al., 2003; Zhao et al., 2005). It has previously been reported that MPP+ leads to a significant increase in superoxide production by inhibiting complex I of the mitochondrial electron transport chain (Przedborski et al., 2000). Superoxide levels were then measured in SH-SY5Y and MESC2.10 cells with or without α-synuclein knockdown (Figure 6c and d). Cells were exposed to normal media or MPP+ for 16 hours. In both cell types, there was a significant increase in superoxide production with MPP+ treatment (two-way ANOVA, MPP+ effect, p < 0.001 for SH-SY5Y cells and p < 0.01 for MESC cells) but α-synuclein knockdown had no effect on superoxide levels (two-way ANOVA, siRNA effect, p > 0.2). MPP+ increased superoxide levels about four-fold in SH-SY5Y cells in all three treatment groups but only two-fold in MESC2.10 cells. Therefore, in this experiment, knockdown of a-synuclein did not affect the rise in superoxide levels caused by MPP+.
Figure 6.
Knockdown of α-synuclein does not affect superoxide levels in MPP+ treated SH-SY5Y or MESC2.10 cells. (A) Dihydroethidium (DHE) is specifically broken down into 2-hydroxy ethidium (2-OH Eth) by superoxide. DHE also forms ethidium, a non-specific product. (B) Standard preparations of 100 nM 2-OH Eth and a mixture of DHE/2-OH Eth and ethidium were separated by HPLC and detected by fluorescence. Elution times: DHE 6 minutes, 2-OH Eth 13 minutes and ethidium 16 minutes. (C) SH-SY5Y and (D) MESC2.10 cells were treated with SNCA1 (knockdown) or scrambled control siRNA and then exposed to 1mM MPP+ or normal media for 16 hours. Superoxide levels were quantified by 2OH-Eth quantification using HPLC. MPP+ had a significant effect on superoxide levels (two-way ANOVA, MPP+ effect, p <0.001 for SH-SY5Y and, p < 0.01 for MESC cells). There was no effect of siRNA treatment on superoxide levels (two-way ANOVA, siRNA effect, p >0.2). Data for each cell type are from three separate experiments each conducted in triplicate (± SEM).
α-synuclein knockdown prevents MPP+ increasing NOS activity
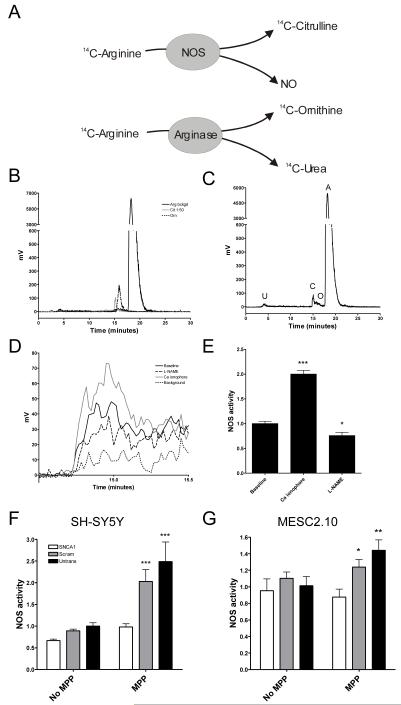
Another critical component in MPP+ toxicity is the production of NO by NOS (Guix et al., 2005). NOS catalyses the production of NO from arginine and in doing so produces citrulline (Figure 7a). To measure NOS activity, therefore, cells were loaded with 14C-arginine and its conversion to 14C-citrulline, 14C-ornithine and 14C-urea was measured by HPLC in a procedure modified from a method recently developed for use in cardiovascular research (de Bono et al., 2007). Cells were treated with the arginase inhibitor N-hydroxy-nor-L-arginine (nor-NOHA) to reduce background arginase activity which can metabolise arginine into ornithine and urea in a side reaction. In preliminary experiments, the separation of 14C-arginine, 14C-citrulline and 14C-ornithine was visualised by eluting individual standard samples of each compound (Figure 7b). The 14C-arginine peak was detected at 20 minutes, 14C-citrulline at 15 minutes and 14C-ornithine at 16.5 minutes. To determine whether this assay of NOS activity could be used in neuronal-type cells, we performed measurements of arginine to citrulline conversion in SH-SY5Y cells (Figure 7c). Significant peaks were identified at 15 and 20 minutes corresponding to 14C-citrulline and 14C-arginine respectively. The 14C-ornithine peak at 16.5 minutes was negligible. An additional small peak was identified at 4 minutes which probably corresponds to 14C-urea.
Figure 7.
NOS activity is increased by MPP+ but α-synuclein knockdown prevents this. (A) NOS catalyses the conversion of arginine to citrulline and nitric oxide (NO). NOS activity can be measured by quantifying the conversion of radioactively labelled arginine to citrulline. In another common biological reaction arginine can also be converted to ornithine and urea. Therefore, to accurately measure NOS activity it must be possible to accurately distinguish between arginine, citrulline and ornithine. (B) HPLC chromatograms from standard samples of 14C-labelled arginine, citrulline and ornithine. Elution times: citrulline 15 minutes, ornithine 16.5 minutes, arginine 20 minutes. (C) Representative chromatogram showing the products of metabolism of 14C-labelled arginine by SH-SY5Y cells (A, arginine, O, ornithine, C, citrulline, U, urea). (D) Chromatograms showing the citrulline peaks from SH-SY5Y cells treated with calcium ionophore (to stimulate NOS activity) or L-NAME (a NOS inhibitor) compared to untreated cells (baseline). Background citrulline from the 14C-arginine stock is also shown. (E). NOS activity as measured by 14C-citrulline formation was significantly altered by NOS inhibitors and activators (one-way ANOVA, p < 001). Calcium ionophore caused a significant increase in NOS activity (Post hoc Bonferroni comparison, p < 0.001) while L-NAME reduced NOS activity (Post hoc Bonferroni comparison, p <0.05). Results are from three experiments performed in triplicate (± SEM). (F) SH-SY5Y and (G) MESC2.10 cells were untransfected or treated with SNCA1 (knockdown) and scrambled control siRNA. Cells were either exposed to MPP+ or normal media overnight. There was a significant effect of MPP+ on NOS in SH-SY5Y (two-way ANOVA, MPP+ effect, p <0.001) but not MESC2.10 cells (two-way ANOVA, MPP+ effect, P > 0.05). There was, however, a significant effect of siRNA treatment on both cell types (two-way ANOVA, siRNA effect, p < 0.001 for SH-SY5Y and p < 0.05 for MESC). MPP+ increased NOS activity considerably in both scrambled control (Bonferroni post hoc comparison, p < 0.001 for SH-SY5Y cells and p < 0.05 for MESC2.10 cells) and untransfected cells (Bonferroni post hoc comparison, p < 0.001 for SH-SY5Y cells and p < 0.05 for MESC2.10 cells) compared to α-synuclein knockdown cells. Results for each cell type are from three separate experiments each performed in triplicate (± SEM).
To confirm that the 14C-citrulline peak identified at 15 minutes represents NOS activity, cells were incubated with calcium ionophore or the competitive NOS inhibitor, N-Nitro-L-Arginine Methyl Ester (L-NAME) (Figure 7d). As expected, the presence of the calcium ionophore increased citrulline production by 2-fold (one-way ANOVA, p < 0.001 with post hoc Bonferroni comparison p < 0.001 compared to baseline) while L-NAME reduced citrulline production significantly (post hoc Bonferroni comparison, p < 0.05).
The effect of α-synuclein knockdown on this increase in NOS activity was then assayed in SH-SY5Y or MESC2.10 cells transfected with SNCA1, a scrambled control or cells left untransfected (Figure 7f and g). MPP+ caused a significant rise in NOS activity (two-way ANOVA, MPP+ effect, p < 0.001) increasing activity 2.3-fold in scrambled control and 2.5-fold in untransfected cells in SH-SY5Y cells. This was reduced by α-synuclein knockdown (two-way ANOVA, siRNA effect, p < 0.001) with levels only rising 47% compared to baseline (post hoc Bonferroni comparison, p < 0.001 compared to both scrambled and untransfected controls). There was no statistically significant effect of MPP+ on NOS activity on MESC cells (two-way ANOVA, MPP+ effect, p > 0.2) but there was a trend to higher NOS activity with untransfected cells increasing 44% and scrambled cells 12% compared to baseline. α-synuclein knockdown did significantly reduce the increase in NOS activity seen in response to MPP+ exposure (two-way ANOVA, siRNA effect, p < 0.05), with levels being lower than in scrambled or untransfected cells exposed to MPP+ (post hoc Bonferroni comparison, p < 0.05 and p < 0.01 respectively).
Discussion
There have now been several studies showing that α-synuclein knockout mice are resistant to MPTP (Dauer et al., 2002; Schlüter et al., 2003; Drolet et al., 2004; Robertson et al., 2004) and here we have demonstrated that this is also true in human neuronal-like cells. We found that α-synuclein knockdown reduced the amount of DAT at the cell surface decreasing DAT activity by 50%. The amount of VMAT2 per vesicle was increased 2.8-fold although the total number of intracellular vesicles was decreased by 37%. These changes were associated with a 61% decrease in initial MPP+ uptake velocity and a 34% decrease in total cellular MPP+ content at saturation. This decrease in MPP+ exposure did not cause any change in superoxide accumulation. We did find, however, that α-synuclein knockdown prevented MPP+-driven increases in NOS activity.
The overall effect of α-synuclein knockdown on monoamine transport and storage pathways caused a reduction in MPP+ uptake which is likely to have contributed to the protective effect of α-synuclein knockdown. Several previous studies have examined the effects of α-synuclein on monoamine transporter and vesicle function. Consistent with our findings, Murphy et al found a significant reduction in the number of synaptic vesicles in mouse neurons with α-synuclein knockdown (Murphy et al., 2000). In vitro studies of DAT function have found that human α-synuclein overexpression can cause either a decrease (Wersinger & Sidhu, 2003) or an increase (Lee et al., 2001; Gosavi et al., 2002) in activity, depending on the levels of α-synuclein overexpression (Jeannotte & Sidhu, 2007). The relevance of this effect in vivo remains to be determined. α-synuclein and monoamine transporters do interact in mouse neuronal tissue (Wersinger et al., 2003) but direct measurement of DAT and VMAT2 activity in single α-synuclein knockout mice has failed to show any significant difference (Dauer et al., 2002). α/β synuclein double knockout mice have small but statistically significant decreases in DAT function (Chandra et al., 2004).
In the first study of MPTP toxicity in α-synuclein knockout mice, Dauer et al showed that neurons from knockout mice were resistant to MPP+, but not rotenone, a mitochondrial inhibitor that functions independently of monoamine transporters (Dauer et al., 2002). A reasonable hypothesis was that α-synuclein knockdown reduces susceptibility to MPP+ toxicity by changing DAT or VMAT2 function, preventing MPP+ from reaching the mitochondria but they were unable to demonstrate this effect. Indeed, subsequent work has demonstrated that α-synuclein knockout mice are also resistant to other mitochondrial inhibitors that function independently of DAT suggesting α-synuclein may mediate other pathways in mitochondrial inhibitor toxicity (Klivenyi et al., 2006).
Our results suggest that part of any potential role for α-synuclein downstream of the change in MPP+ metabolism may involve alterations related to NO biology. It is unclear whether the changes in NOS activation seen here with α-synuclein knockdown are directly due to decreased MPP+ exposure or other α-synuclein-dependent mechanisms. MPP+ is known to increase intracellular NO levels (Dennis & Bennett, 2003) and our results suggest that this is due to increased NOS activity. NO production is recognised as crucial step in mitochondrial inhibitor toxicity because NO reacts with superoxide to form peroxynitrite, a highly reactive molecule (Halliwell, 2006) and suppression of NOS activity confers a marked protective effect in mice and primates treated with MPTP (Schulz et al., 1995; Hantraye et al., 1996; Przedborski et al., 1996; Matthews et al., 1997a; Matthews et al., 1997b; Liberatore et al., 1999; Dehmer et al., 2000; Klivenyi et al., 2000). NOS-related effects on α-synuclein also appear to be important in PD. For example, α-synuclein nitration increases its aggregation (Paxinou et al., 2001), polymorphisms in iNOS and nNOS are associated with PD (Levecque et al., 2003) and α-synuclein is selectively nitrated in Lewy bodies from PD brains (Giasson et al., 2000).
Overall, our work shows that α-synuclein knockdown protects human neuron-like cells from MPP+ toxicity, at least in part by reducing exposure of the cells to MPP+ toxicity by altering monoamine uptake and storage pathways. However, our observations suggest that the change in MPP+ uptake and storage seen with α-synuclein knockdown is not sufficient on its own to cause the observed reduction in MPP+ toxicity. In the present work we found that superoxide levels were significantly raised by MPP+ regardless of α-synuclein expression. Since superoxide is known to be toxic, it is likely that changes downstream of its production played some role in protecting cells with α-synuclein knockdown (Cleeter et al., 1992). Activation of NOS is an essential step in MPP+ toxicity and this was prevented by α-synuclein knockdown. While it is unclear how this may occur, understanding α-synuclein’s effects on NOS activation may be a promising avenue of research. The importance of nitrosative stress in neurodegenerative disease is becoming increasingly clear and we suggest α-synuclein may have a role in its regulation.
Acknowledgements
The authors would like to thank Marie Stolbrink for performing the blind counting of vesicle, DAT and VMAT2 on the electron micrographs. T.M.F. was a Commonwealth Scholar supported by the Commonwealth Scholarship Commission in the United Kingdom, L.L.V. is supported by a Fundação para a Ciência e Tecnologia (Portugal) Studentship, and R.W-M. was a Wellcome Trust Research Career Development Fellow.
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- DHE
dihydroethidium
- nor-NOHA
N-hydroxy-nor-L-arginine
- L-NAME
N-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- VMAT
vesicular monoamine transporter
References
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Bönisch H. Transport and drug binding kinetics in membrane vesicle preparation. Methods Enzymol. 1998;296:259–278. doi: 10.1016/s0076-6879(98)96020-7. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Südhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Schapira AH. Irreversible inhibition of mitochondrial complex I by 1-methyl-4-phenylpyridinium: evidence for free radical involvement. J Neurochem. 1992;58:786–789. doi: 10.1111/j.1471-4159.1992.tb09789.x. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JP, Warrick N, Bendall JK, Channon KM, Alp NJ. Radiochemical HPLC detection of arginine metabolism: measurement of nitric oxide synthesis and arginase activity in vascular tissue. Nitric Oxide. 2007;16:1–9. doi: 10.1016/j.niox.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- Dennis J, Bennett JP., Jr. Interactions among nitric oxide and Bcl-family proteins after MPP+ exposure of SH-SY5Y neural cells I: MPP+ increases mitochondrial NO and Bax protein. J Neurosci Res. 2003;72:76–88. doi: 10.1002/jnr.10539. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Przedborski S, Petrucelli L. Gene dosage and pathogenesis of Parkinson’s disease. Trends Mol Med. 2005;11:91–96. doi: 10.1016/j.molmed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fountaine TM, Wade-Martins R. RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res. 2007;85:351–363. doi: 10.1002/jnr.21125. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gimenez-Cassina A, Lim F, Diaz-Nido J. Differentiation of a human neuroblastoma into neuron-like cells increases their susceptibility to transduction by herpesviral vectors. J Neurosci Res. 2006;84:755–767. doi: 10.1002/jnr.20976. [DOI] [PubMed] [Google Scholar]
- Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ. Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J Biol Chem. 2002;277:48984–48992. doi: 10.1074/jbc.M208194200. [DOI] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Halász AS, Pálfi M, Tábi T, Magyar K, Szöko E. Altered nitric oxide production in mouse brain after administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridin or methamphetamine. Neurochem Int. 2004;44:641–646. doi: 10.1016/j.neuint.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, Beal MF. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- Jeannotte AM, Sidhu A. Regulation of the norepinephrine transporter by alpha-synuclein-mediated interactions with microtubules. Eur J Neurosci. 2007;26:1509–1520. doi: 10.1111/j.1460-9568.2007.05757.x. [DOI] [PubMed] [Google Scholar]
- Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R. alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274:25481–25489. doi: 10.1074/jbc.274.36.25481. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Andreassen OA, Ferrante RJ, Lancelot E, Reif D, Beal MF. Inhibition of neuronal nitric oxide synthase protects against MPTP toxicity. Neuroreport. 2000;11:1265–1268. doi: 10.1097/00001756-200004270-00024. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Levecque C, Elbaz A, Clavel J, Richard F, Vidal JS, Amouyel P, Tzourio C, Alpérovitch A, Chartier-Harlin MC. Association between Parkinson’s disease and polymorphisms in the nNOS and iNOS genes in a community-based case-control study. Hum Mol Genet. 2003;12:79–86. doi: 10.1093/hmg/ddg009. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Beal MF, Fallon J, Fedorchak K, Huang PL, Fishman MC, Hyman BT. MPP+ induced substantia nigra degeneration is attenuated in nNOS knockout mice. Neurobiol Dis. 1997a;4:114–121. doi: 10.1006/nbdi.1997.0141. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Beal MF. S-Methylthiocitrulline, a neuronal nitric oxide synthase inhibitor, protects against malonate and MPTP neurotoxicity. Exp Neurol. 1997b;143:282–286. doi: 10.1006/exnr.1996.6406. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan V, Guo Y, Scarlata S. Fluorescence studies suggest a role for alpha-synuclein in the phosphatidylinositol lipid signaling pathway. Biochemistry. 2005;44:462–470. doi: 10.1021/bi0487140. [DOI] [PubMed] [Google Scholar]
- Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page G, Barc-Pain S, Pontcharraud R, Cante A, Piriou A, Barrier L. The up-regulation of the striatal dopamine transporter’s activity by cAMP is PKA-, CaMK II- and phosphatase-dependent. Neurochem Int. 2004;45:627–632. doi: 10.1016/j.neuint.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton JE, Perrin RJ, Clayton DF, George JM. Protein-protein interactions of alpha-synuclein in brain homogenates and transfected cells. Brain Res Mol Brain Res. 2001;95:138–145. doi: 10.1016/s0169-328x(01)00257-1. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, Niznik HB. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G. The parkinsonian toxin MPTP: action and mechanism. Restor Neurol Neurosci. 2000;16:135–142. [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Xu C, Carroll FI, Chen NH. Inhibition of [3H]dopamine translocation and [3H]cocaine analog binding: a potential screening device for cocaine antagonists. Methods Enzymol. 1998;296:248–259. doi: 10.1016/s0076-6879(98)96019-0. [DOI] [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Schlüter OM, Fornai F, Alessandrí MG, Takamori S, Geppert M, Jahn R, Südhof TC. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience. 2003;118:985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Matthews RT, Muqit MM, Browne SE, Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Díaz-Nido J, Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J. 1993;12:1633–1640. doi: 10.1002/j.1460-2075.1993.tb05808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger C, Jeannotte A, Sidhu A. Attenuation of the norepinephrine transporter activity and trafficking via interactions with alpha-synuclein. Eur J Neurosci. 2006a;24:3141–3152. doi: 10.1111/j.1460-9568.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Rusnak M, Sidhu A. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. Eur J Neurosci. 2006b;24:55–64. doi: 10.1111/j.1460-9568.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Takagi S, Watanabe Y, Kato H, Araki T. Role of reactive nitrogen and reactive oxygen species against MPTP neurotoxicity in mice. J Neural Transm. 2008;115:831–842. doi: 10.1007/s00702-008-0019-6. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang J, Graham DG, Montine TJ, Ho YS. Enhanced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J Neuropathol Exp Neurol. 2000;59:53–61. doi: 10.1093/jnen/59.1.53. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]