Abstract
Attachment of telomeres to the nuclear envelope (NE) and their clustering in a chromosomal bouquet during meiotic prophase I is an evolutionary conserved event that promotes chromosome pairing and recombination. In fission yeast, bouquet formation fails when the telomeric protein Rap1 is absent or when the telomeric protein Taz1 fails to recruit Rap1 to telomeres. The mammalian Rap1 orthologue is a component of the shelterin complex and localises to telomeres through an interaction with a Taz1-like telomeric DNA binding factor, TRF2. Here, we investigated the role of mammalian Rap1 in meiotic telomere attachment and clustering by analysing spermatogenesis in Rap1-deficient mice. The results establish that the meiotic three-dimensional nuclear architecture and recombination are not affected by the absence of Rap1. Furthermore, Rap1-deficient meiotic telomeres assemble the SUN1 nuclear membrane protein, attach to the NE, and undergo bouquet formation indistinguishable from the wild-type setting. Thus, the role of Rap1 in meiosis is not conserved between fission yeast and mammals, suggesting that mammals have alternative modes for connecting telomeres to SUN proteins on the meiotic nuclear envelope.
Introduction
Telomeres are key players in meiosis, in particular, in the chromosome pairing and recombinogenic processes that take place during the first meiotic prophase (for reviews see (Scherthan 2006; Zickler and Kleckner 1998). Meiosis is a succession of two specialised cell divisions that reduces the diploid chromosome number to the haploid complement. At the onset of meiotic prophase I, telomeres attach to the nuclear envelope (NE) and undergo NE-bound mobility (Alsheimer 2009; Scherthan 2007). In yeasts and worms, meiotic telomeres connect to the cytoskeleton and perinuclear motor proteins through meiosis-specific telomere protein complexes connected to SUN/KASH-domain nuclear transmembrane protein complexes (Chikashige et al. 2006; Conrad et al. 2007; Penkner et al. 2009; Sato et al. 2009; reviewed in Hiraoka and Dernburg 2009). In mammals, there are two such SUN domain proteins, SUN1 and SUN2. SUN1 and SUN2 localise to telomere attachment sites at the NE (Schmitt et al. 2007), and deletion of SUN1 prevents telomere attachment to the NE and impairs homologue pairing and synapsis (Ding et al. 2007). In mammals, the telomeres attach to the NE during the preleptotene/leptotene stage, after which they move and transiently cluster adjacent to the centrosome. The resulting chromosome “bouquet” is thought to promote homologous chromosome pairing, meiotic recombination and possibly metaphase plate alignment (Scherthan 2006; Tomita and Cooper 2007).
In the synaptic meiosis of budding yeast, the duplex telomere repeats are directly bound by scRap1 (Klein et al. 1992) and telomere attachment and mobility involves actin (Koszul et al. 2008; Trelles-Sticken et al. 2005), the meiotic telomere protein Ndj1 (Conrad et al. 1997, 2007; Rockmill and Roeder 1998; Scherthan et al. 2007; Trelles-Sticken et al. 2000; Wanat et al. 2008), and SUN domain proteins like MSP3 (Conrad et al. 2007, 2008; Wanat et al. 2008).
In the asynaptic meiosis of Schizosaccharomyces pombe, bouquet formation is absolutely required for homologue pairing and for recombination (reviewed by Chikashige et al. 2007) and depends on the presence of the telomeric repeat-binding protein Taz1 (Cooper et al. 1997, 1998; Nimmo et al. 1998). S. pombe telomeres connect via Taz1-spRap1 to the SUN domain proteins Sad1–Kms1 of the NE to reach the microtubule cytoskeleton and cytoplasmic dynein that drives telomere clustering and telomereled horsetail movement of the prophase I nucleus (for review, see Chikashige et al. 2007).
Mammalian telomeres contain the shelterin complex which protects telomeres from the deoxyribonucleic acid (DNA) damage response and regulates telomere maintenance by telomerase (reviewed in Palm and de Lange 2008). Shelterin contains two double-stranded TTAGGG repeat binding proteins, TRF1 and TRF2, that are orthologues of S. pombe Taz1. In addition, shelterin contains a TRF2 binding factor, Rap1, that is distantly related to the Rap1 proteins of budding and fission yeast. Most shelterin components, including the Taz1 orthologues, are essential, thwarting efforts to understand how the mammalian telomeric complex contributes to meiosis. However, we recently found that Rap1 is not required for the essential aspects of telomere protection, and Rap1 null mice are viable and fertile (Martinez et al. 2010; Sfeir et al. 2010). The main phenotype of telomeres lacking Rap1 is their greater tendency to undergo homologous recombination (HR; Sfeir et al. 2010).
Meiotic mammalian telomeres also contain shelterin (Scherthan et al. 2000b) and abut the NE in attachment plates or plaques (see, Liebe et al. 2004). Heterozygosity for the shelterin component TRF1 and disruption of the ATM kinase, the SMC1β cohesin, or the ATM kinase target H2AX alter telomere dynamics but not their attachment (reviewed by Scherthan 2007). As in other organisms, the telomere–NE association depends on the SUN domain proteins, SUN1 (Ding et al. 2007; Schmitt et al. 2007) and possibly SUN2, since partially redundant roles for SUN1 and SUN2 have been reported in somatic cells (see Haque et al. 2010). Here, we made use of the Rap1-deficient mouse (Sfeir et al. 2010) to determine the contribution of Rap1 to telomere dynamics and recombination in male prophase I.
Materials and methods
Mice
Rap1 knockout mice were produced by a conditional knockout strategy of exon2 (Rap1Δex2/Δex2; Sfeir et al. 2010). Rap1Δex2/Δex2-targeted mice were maintained in a C57BL/6 J background and are denoted Rap1Δ/Δ in this manuscript. Testes were recovered as described previously (Scherthan et al. 2000a).
Western analysis
Protein extracts were prepared isolated from testes of 4-week-old male mice by homogenisation in extraction buffer [50 mM Tris–HCl at pH 7.4, 1% Triton X-100, 0.1% SDS, 400 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), supplemented with protease inhibitors]. Bradford assay was used to determine the concentration of proteins. Then 10 μg of lysate was separated on an 8% SDS-PAGE gel. After immunoblotting, membranes were probed with antibodies raised against GST-mTRF2 (1254) and GST-mRAP1 (1252).
Testicular preparations, detergent spreading and FISH
Testicular preparations, surface spreading, immunostaining as well as telomere and centromere fluorescence in situ hybridization (FISH) for bouquet stage investigation were carried out as described (Liebe et al. 2006).
Antisera and immunofluorescence
The following affinity-purified antibodies were used in the immunostaining experiments: TRF1, rabbit anti-mouse-TRF1 antisera #1449 (Sfeir et al. 2009); TRF2, rabbit anti-TRF2 #1254 (Celli and de Lange 2005); RAP1, rabbit anti-mouse-Rap1 antiserum (#1252; Celli and de Lange 2005) were used to detect shelterin components. Rabbit polyclonal anti-Mre11 (1:200, Novus Biologicals, Littleton, CO), mouse monoclonal anti-γ-H2AX (1:500, Millipore, Schwalbach), goat anti-Ku70 (sc-1486, 1:25, Santa Cruz, Heidelberg). Guinea pig anti-SUN1 was a kind gift of M. Alsheimer, Univ. of Würzburg, Germany. A monoclonal anti-SYCP3 antibody (Adelfalk et al. 2009) was used to detect axial cores and complete SCs (Lammers et al. 1994). All antisera were diluted in phosphate buffered saline (PBS)/0.1%Tween 20/0.2% BSA/0.1% gelatin (PBTG). All antibodies were tested in individual staining reactions for their specificity and performance. Controls without primary antibodies all were negative (not shown).
Immunostaining was performed as described earlier (Adelfalk et al. 2009; Scherthan et al. 2000b). After incubation with the primary antibodies at 4°C overnight, preparations were rinsed 3×3 min with PBS/Tween 20 and incubated for 30 min at 37°C with a goat anti-rabbit-Cy3 antibody (Dianova, Hamburg, diluted 1/1000). Mouse mmunoglobulins G were detected with anti-mouse-FITC (Dianova, 1/1000). SUN1 was detected using anti-guinea pig Cy3 (Dianova), Ku70 abs were detected with donkey anti-goat Cy2 antibodies (Dianova, 1/500). Triple labelling was achieved using rabbit-anti-mRPA1 and guinea-pig-anti-SUN1 (Schmitt et al. 2007) and mouse anti-SYCP3 antibody as above, with SYCP3 being detected by anti-mouse-Cy5-labelled secondary antibodies (Dianova, 1/500). Finally, preparations were mounted in antifade solution (Vectashield, Vector Labs) containing 0.5 μg/ml DAPI (Sigma) to reveal nuclear DNA. In mouse testicular suspension preparations, the meiotic prophase stage-specific distribution of SYCP3 proteins and/or DAPI-bright heterochromatin clusters (Scherthan et al. 1996) was utilised to identify spermatocytes at various stages of prophase I.
Microscopic evaluation
A Zeiss Axioplan 2 epifluorescence microscope (Carl Zeiss, Oberkochen) equipped with single-band pass filters for excitation of green, red and blue fluorescence (Chroma Technologies, Bellows Falls) and 10×, 40×, 63× and 100× plan-neofluoar lenses was used for epifluorescence microscopy. Three-dimensional evaluation of immunostained nuclei was performed in some experiments by carefully focusing through the nuclei using a 100× plan-neofluoar lens and the motorized Z drive of the axioplan microscope. Using the ISIS fluorescence image analysis system (Meta-Systems), digital black-and-white images were recorded with a CCD camera and merged to RGB images.
Results and discussion
Presence of shelterin on Rap1Δ/Δ meiotic telomeres
We studied the impact of Rap1 deficiency on telomere dynamics in prophase of meiosis I in the Rap1 (Terf2ip) knockout mouse (Rap1Δex2/Δex2; hereafter denoted Rap1Δ/Δ; Sfeir et al. 2010). This mouse lacks the second exon of the Rap1 gene and consequently lacks functional Rap1 protein in somatic cells. Rap1 was not detectable in immunoblots on testicular extracts of Rap1Δ/Δ mice (Fig. 1), and indirect immunofluorescence on detergent-spread spermatocytes showed loss of Rap1 from meiotic telomeres (Fig. 2a).
Fig. 1.
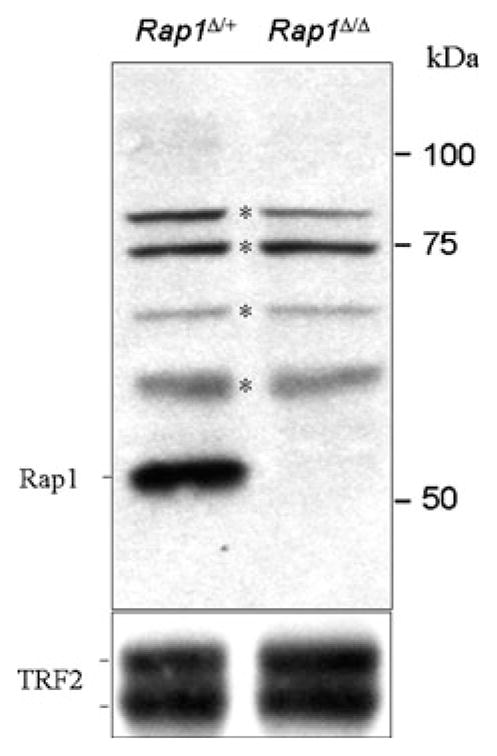
Western blot analysis for Rap1 (1252) and TRF2 (1254) in testis extracts of Rap1Δ/+ and Rap1Δ/Δ mice. Absence of RAP1 protein is noted in testes of Rap1Δ/Δ mice. TRF2 expression is not affected and serves as a loading control. Bands with asterisks are unspecific background of the antibody
Fig. 2.
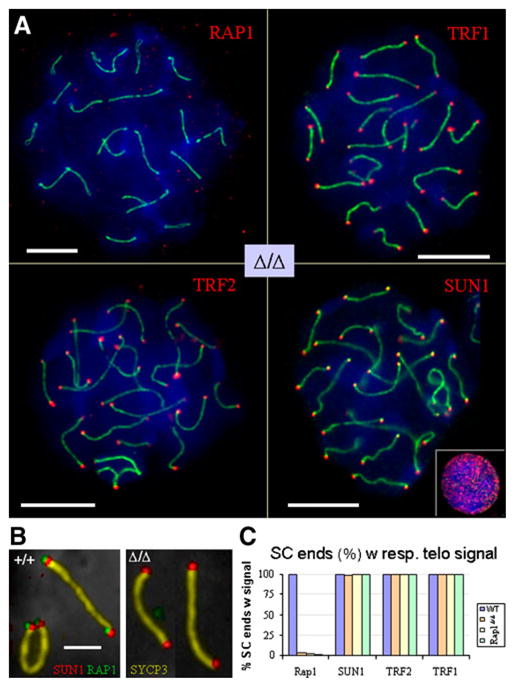
a Immunofluorescence of Rap1Δ/Δ pachytene spermatocytes using anti-Rap1, anti-TRF1, anti-TRF2 and anti-SUN1 antibodies (Cy3, red). Synaptonemal complex was labelled with anti-SYCP3 protein (FITC, green). TRF1, TRF2 and SUN1 colocalise with meiotic telomeres at the ends of all pachytene SCs in Rap1Δ/Δ spreads and at wild-type pachytene telomeres (data now shown and b). The small inset displays the punctate NUP-like distribution pattern of SUN1 (red) of a somatic nucleus. Bars represent 10 μm. b Multi-colour labelling of Rap1+/+ Rap1Δ/Δ pachytene SCs with RAP1 (green, FITC), SUN1 (red, Cy3), SYCP3 (golden, false-colored Cy5) and DNA (gray, inverted DAPI) reveals colocalization of Rap1 with SUN1 at the wild-type but not Rap1Δ/Δ telomere. Bar: 2.5 μm. c Frequency of colocalization of telomere protein signals with SCs ends in one Rap1+/+ and three Rap1Δ/Δ pachytene spermatocytes. 400 SC ends were evaluated for each mouse. RAP1 localises to telomeres (ends) of Rap1+/+ SCs but is absent from Rap1Δ/Δ meiotic telomeres. Shelterin components TRF1, TRF2 as well as the SUN1 nuclear envelope transmembrane protein all localise to Rap1Δ/Δ telomeres. Data were derived from pachytene spermatocyte spreads as shown in (a, b)
Next, we queried the Rap1Δ/Δ spermatocytes for the presence of TRF1, TRF2 and the axial core protein SYCP3 (SCP3; Lammers et al. 1994). Compared to the Rap1-proficient control, the TRF1 and TRF2 signals at the ends of meiotic chromosome cores of leptotene and zygotene spermatocytes (not shown) and SCs (Fig. 2b, c) were unaltered in the Rap1 knockout testis. Pachytene/diplotene nuclei displayed particularly strong TRF1 and TRF2 signals at SCs ends (Fig. 2), which agrees with previous observations (Scherthan et al. 2000b). Enumeration of >400 SC ends in at least 10 well-spread pachytene nuclei of wild-type and three knockout mice revealed a >99% association of chromosome ends with the respective telomere protein (Fig. 2c), indicating that the absence of Rap1 does not disturb the telomere location of shelterin in meiocytes.
Rap1-deficient telomeres attach to the NE
In the mouse, telomere attachment to the NE occurs during preleptotene and precedes homology search and pairing (Scherthan et al. 1996). To investigate telomere dynamics and their re-localisation to the nuclear periphery during the course of meiotic prophase in the absence of Rap1, we used FISH probes to detect telomeres and pericentric major satellite DNA in structurally preserved nuclei of testicular suspensions. TTAGGG telomere FISH signal patterns of testicular suspension nuclei showed a wild-type pattern of distribution, reminiscent of previous investigations (see Liebe et al. 2006). Three dimensionally preserved prophase I spermatocyte nuclei (>1,000/mouse) were inspected for disruption of peripheral telomere location, which revealed a wild-type-like peripheral telomere location (Fig. 3) in controls and three knockout mice, indicating that Rap1 is not required for telomere/NE attachment.
Fig. 3.

Three-dimensional telomere distribution in Rap1+/+ and Rap1Δ/Δ spermatocytes using telomere FISH signals (green, TTAGGG7-FITC) and pericentromeric major satellite DNA (red, Cy3). Localization at the nuclear periphery is noted in leptotene–pachytene spermatocyte nuclei of both genotypes. Bottom, top and equatorial focal planes are indicated in this pachytene nucleus. Most signals are seen in the bottom focal plane, as the nucleus at the glass surface is slightly flattened and displays a larger area in one plane. Nuclear DNA was stained with DAPI (blue). The bar equals 10 μm
At the onset of prophase I, telomeres undergo a dynamic redistribution that involves their fleeting clustering during early zygotene (bouquet topology; Scherthan et al. 1996). Bouquet spermatocytes, which can be assayed through centromere/telomere FISH in testicular suspensions, are rarely encountered (~0.3%) in mouse meiosis (Liebe et al. 2006). When we determined the frequency of bouquet spermatocytes in testicular suspensions of Rap1Δ/Δ and control mice, we noted similar frequencies in Rap1Δ/Δ(0.29%, n=1,056) and wild-type (0.34%, n = 1,164) spermatocytes, which agree with previous reports (Liebe et al. 2006). The difference between the Rap1-proficient and Rap-1-deficient values was not statistically significant (p>0.05; Fisher’s test).
These results establish that Rap1 is dispensable for telomere attachment, dynamic redistribution and clustering in mouse meiosis and contrast with the situation in S. pombe meiosis, where Rap1 is required for bouquet formation (Chikashige and Hiraoka 2001; Kanoh and Ishikawa 2001).
SUN1 associates with Rap1-deficient telomeres
In S. pombe meiosis, Rap1 mediates telomere attachment and bouquet formation through a Bqt1- and Bqt2-dependent connection with the Sad1–Kms1 protein complex in the NE (reviewed by Chikashige et al. 2007). In mammalian meiosis, telomeres also attach to the NE through an interaction with the trans-membrane SUN domain proteins SUN1 and SUN2, which concentrate at telomeres during prophase I (Ding et al. 2007; Schmitt et al. 2007).
To determine whether the absence of Rap1 alters telomere association with the SUN trans-membrane complex, we performed IF for SUN1 (Schmitt et al. 2007) on Rap1Δ/Δ spermatocytes in combination with detection of SYCP3 and/or Rap1. The results indicated a wild-type SUN1 distribution at all telomeres (SC ends, n ≥ 400 ends/mouse) in spread pachytene nuclei (Fig. 2). As expected, triple staining of SCP3, SUN1 and Rap11 showed this combination of proteins at wild-type telomeres, but not in the Rap1-deficient testis (Fig. 2b).
In budding yeast, telomere maintenance and perinuclear telomere positioning involves Hdf1/Hdf2 (Ku70/Ku80; see Gasser 2000) which are abundant nuclear DSB-binding proteins important for nonhomologous end joining (NHEJ) pathway of DSB repair. During the leptotene/zygotene prophase stages of mammalian meiosis I Ku proteins are down-regulated (Goedecke et al. 1999) and Ku protein levels at mammalian meiotic telomeres are below the detection level of IF (see, Scherthan 2004). Ku70 acts in parallel to shelterin to suppress HR (Celli et al. 2006; Palm and de Lange 2008; Sfeir et al. 2010) and has been detected biochemically at somatic mammalian telomeres (Hsu et al. 1999). In budding yeast, Ku accumulates at meiotic telomeres (Scherthan and Trelles-Sticken 2008). In this study, we observed similar Ku70 staining patterns in wild-type and Rap1Δ/Δ spreads with no enrichment at telomeres in Rap1Δ/Δ spermatocytes (data not shown). The latter would be expected if Ku was involved in telomere/nuclear periphery localisation, as has been observed in vegetative nuclei of budding yeast (Laroche et al. 1998). However, our data in the mouse fail to reveal significant amounts of Ku70 at the meiotic Rap1Δ/Δ telomere, which suggests that the mammalian Ku complex is not compensating for the loss of Rap1 at meiotic telomeres.
Collectively, these data suggest that meiotic telomeres do not require Rap1 for telomere/SUN protein interaction and explain why the telomere/NE attachment is normal in Rap1Δ/Δ meiocytes. It will hence be interesting to determine which proteins are responsible for the interaction with SUN1 at the mammalian meiotic telomere.
Normal DNA repair dynamics in Rap1-deficient meiosis
At somatic telomeres, mammalian Rap1 contributes to the inhibition of homology-directed DNA repair (Sfeir et al. 2010), which is the major pathway for DNA repair during meiosis. The onset of meiotic recombination depends of the induction of numerous DSBs by the Spo11 nuclease (Baudat et al. 2000; Romanienko and Camerini-Otero 2000), which leads to a DNA damage response and global phosphorylation of histone H2AX, now called γ-H2AX. Progress of HR is paralleled by regression of γ-H2AX signal to the XY (sex) body in pachytene/diplotene spermatocytes (Barchi et al. 2005; Mahadevaiah et al. 2001). In prophase I of wild-type and Rap1Δ/Δ mice γ-H2AX formation was normal with strong γ-H2AX labelling in leptotene spermatocytes (Fig. 4a, b) and regression of the γ-H2AX signal to the XY body in pachytene spermatocytes (Fig. 4c, d).
Fig. 4.
a–d Immunofluorescence with anti-γ-H2AX (FITC, green) and anti-SCP3 (Cy3, red) in spermatocytes of control Rap1+/+ and Rap1Δ/Δ mice. a Wild-type leptotene spermatocyte displaying intense γ-H2AX staining over developing SYCP3 axes (red dots). b Strong γ-H2AX chromatin signal in a Rap1Δ/Δ leptotene spermatocyte. c d Intense γ-H2AX labelling of the XY body in spread pachytene spermatocytes of Rap1+/+ and Rap1Δ/Δ mice due to image enhancement that discloses smaller γ-H2AX foci at pachytene SCs, which represent sites of DSB repair by homologous recombination. Small γ-H2AX foci occur at tips of SCs (arrows, telomeres) in wild-type and Rap1Δ/Δ pachytene spermatocytes in similar frequencies (see text).e, f Mre11 signals (Cy3, red) are concentrated on the XY body (arrow) that contains the sex chromosomes. Anti-SCP3 staining was applied to reveal SCs (FITC, green). Nuclear DNA was stained with DAPI (blue). Scale bars, 10 μm
Whilst the XY body chromatin of pachytene spermatocytes is the prominent site of γ-H2AX formation (Barchi et al. 2005; Mahadevaiah et al. 2001), image enhancement can reveal small γ-H2AX foci on pachytene bivalents that represent ongoing repair by homologous recombination (Chicheportiche et al. 2007; Lenzi et al. 2005; Roig et al. 2004).
We investigated γ-H2AX foci as markers for recombinogenic DNA repair events at pachytene telomeres at meiotic chromosome ends of Rap1Δ/Δ and wild-type pachytene spermatocytes (Fig. 4c, d) and detected an average of 0.83 (wt, range 0–4) and 0.57 (Rap1Δ/Δ, range 0–3) telomeric γ-H2AX foci/pachytene cell (n=30 spermatocytes or ~1,100 telomeres per genotype) being an insignificant difference (Student’s t test). These data suggests that HR at Rap1Δ/Δ chromosome ends is similar to the wild type.
Furthermore, we investigated the localisation of Mre11 nuclease of the Mre11/Rad50/NBS1 (MRN) complex that is involved in DSB repair. In meiosis, Mre11 is required for processing of the Spo11-induced DSBs, and Mre11 localises to the XY body of pachytene - diplotene spermatocytes (Eijpe et al. 2000; Goedecke et al. 1999). Mre11 localisation to the XY body was observed in both wild-type and Rap1Δ/Δ pachytene nuclei (Fig. 4e, f). Whilst the Mre11 complex accumulates at dysfunctional somatic telomeres (Celli and de Lange 2005; Takai et al. 2003), Mre11 was not detected at meiotic wild-type and Rap1Δ/Δ telomeres (Fig. 4e, f).
Thus, the induction of DSBs and their recombinational repair appears to proceeds normally in Rap1Δ/Δ spermatogenesis.
Conclusions
These data reveal unexpectedly that Rap1 deficiency has no detectable effect on several aspects of mouse meiosis. Specifically, the association of telomeres with the NE, their interaction with SUN1, the transient clustering of telomeres, and the progression of recombination appear normal in absence of Rap1. The lack of requirement for Rap1 in bouquet formation is strikingly different from the situation in S. pombe where the contribution of Rap1 is key (Chikashige and Hiraoka 2001). Since there are no discernible Rap1 paralogs in the mouse genome and because Rap1 fails to meet with the criteria for the mammalian telomere/NE connector, it appears that either this connection is mediated in a redundant way or that another (shelterin) factor is the main mediator of the SUN telomere attachment to the meiotic nuclear envelope.
Acknowledgments
These studies were supported by supported by NIH grants AG016642 and GM049046 to TdL. HS acknowledges grant support from the DFG (SCHE350/10-1, SPP1384) and thanks D. Gassen for technical assistance. AS is supported by a postdoctoral fellowship from Susan G. Komen for the Cure. TdL is an American Cancer Society Professor. We thank M. Alsheimer, Univ. of Würzburg, Germany, for providing SUN1 antiserum and C. Adelfalk, TU Dresden, Germany, for providing SCP3 antibodies.
Footnotes
Conflicts of interest None
Contributor Information
Harry Scherthan, Email: scherth@web.de, Institut für Radiobiologie der Bundeswehr in Verbindung mit der, Universität Ulm, Neuherbergstr. 11, 80937 Munich, Germany. MPI für Molekulare Genetik, Berlin, Germany.
Agnel Sfeir, Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.
Titia de Lange, Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.
References
- Adelfalk C, Janschek J, Revenkova E, Blei C, Liebe B, Gob E, Alsheimer M, Benavente R, de Boer E, Novak I, Hoog C, Scherthan H, Jessberger R. Cohesin SMC1beta protects telomeres in meiocytes. J Cell Biol. 2009;187:185–99. doi: 10.1083/jcb.200808016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsheimer M. The dance floor of meiosis: Evolutionary conservation of nuclear envelope attachment and dynamics of meiotic telomeres. Genome Dyn. 2009;5:81–93. doi: 10.1159/000166621. [DOI] [PubMed] [Google Scholar]
- Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, de Rooij DG, Burgoyne PS, Jasin M, Keeney S. Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol. 2005;25:7203–15. doi: 10.1128/MCB.25.16.7203-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–98. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–8. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- Celli GB, Denchi EL, de Lange T. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat Cell Biol. 2006;8:885–90. doi: 10.1038/ncb1444. [DOI] [PubMed] [Google Scholar]
- Chicheportiche A, Bernardino-Sgherri J, de Massy B, Dutrillaux B. Characterization of Spo11-dependent and independent phospho-H2AX foci during meiotic prophase I in the male mouse. J Cell Sci. 2007;120:1733–42. doi: 10.1242/jcs.004945. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Hiraoka Y. Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr Biol. 2001;11:1618–23. doi: 10.1016/s0960-9822(01)00457-2. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Haraguchi T, Hiraoka Y. Another way to move chromosomes. Chromosoma. 2007;116:497–505. doi: 10.1007/s00412-007-0114-8. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Dominguez AM, Dresser ME. Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast. Science. 1997;276:1252–5. doi: 10.1126/science.276.5316.1252. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Lee CY, Wilkerson JL, Dresser ME. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proceeding of the National Academy of Sciences of the United States of America. 2007;104:8863–8. doi: 10.1073/pnas.0606165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad MN, Lee CY, Chao G, Shinohara M, Kosaka H, Shinohara A, Conchello JA, Dresser ME. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell. 2008;133:1175–87. doi: 10.1016/j.cell.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Cooper JP, Nimmo ER, Allshire RC, Cech TR. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature. 1997;385:744–7. doi: 10.1038/385744a0. [DOI] [PubMed] [Google Scholar]
- Cooper JP, Watanabe Y, Nurse P. Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature. 1998;392:828–31. doi: 10.1038/33947. [DOI] [PubMed] [Google Scholar]
- Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–72. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Goedecke W, Heyting C. Localisation of RAD50 and MRE11 in spermatocyte nuclei of mouse and rat. Chromosoma. 2000;109:123–32. doi: 10.1007/s004120050420. [DOI] [PubMed] [Google Scholar]
- Gasser SM. A sense of the end. Science. 2000;288:1377–9. doi: 10.1126/science.288.5470.1377. [DOI] [PubMed] [Google Scholar]
- Goedecke W, Eijpe M, Offenberg HH, van Aalderen M, Heyting C. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet. 1999;23:194–8. doi: 10.1038/13821. [DOI] [PubMed] [Google Scholar]
- Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–98. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Dernburg AF. The SUN rises on meiotic chromosome dynamics. Dev Cell. 2009;17:598–605. doi: 10.1016/j.devcel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Hsu HL, Gilley D, Blackburn EH, Chen DJ. Ku is associated with the telomere in mammals. Proceeding of the National Academy of Sciences of the United States of America. 1999;96:12454–8. doi: 10.1073/pnas.96.22.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F. spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol. 2001;11:1624–30. doi: 10.1016/s0960-9822(01)00503-6. [DOI] [PubMed] [Google Scholar]
- Klein F, Laroche T, Cardenas ME, Hofmann JF, Schweizer D, Gasser SM. Localization of RAP1 and topoisomerase II in nuclei and meiotic chromosomes of yeast. J Cell Biol. 1992;117:935–48. doi: 10.1083/jcb.117.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszul R, Kim KP, Prentiss M, Kleckner N, Kameoka S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell. 2008;133:1188–201. doi: 10.1016/j.cell.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Offenberg HH, van Aalderen M, Vink AC, Dietrich AJ, Heyting C. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol. 1994;14:1137–46. doi: 10.1128/mcb.14.2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche T, Martin SG, Gotta M, Gorham HC, Pryde FE, Louis EJ, Gasser SM. Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr Biol. 1998;8:653–6. doi: 10.1016/s0960-9822(98)70252-0. [DOI] [PubMed] [Google Scholar]
- Lenzi ML, Smith J, Snowden T, Kim M, Fishel R, Poulos BK, Cohen PE. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis i in human oocytes. Am J Hum Genet. 2005;76:112–27. doi: 10.1086/427268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebe B, Alsheimer M, Hoog C, Benavente R, Scherthan H. Telomere attachment, meiotic chromosome condensation, pairing, and bouquet stage duration are modified in spermatocytes lacking axial elements. Mol Biol Cell. 2004;15:827–37. doi: 10.1091/mbc.E03-07-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebe B, Petukhova G, Barchi M, Bellani M, Braselmann H, Nakano T, Pandita TK, Jasin M, Fornace A, Meistrich ML, Baarends WM, Schimenti J, de Lange T, Keeney S, Camerini-Otero RD, Scherthan H. Mutations that affect meiosis in male mice influence the dynamics of the mid-preleptotene and bouquet stages. Exp Cell Res. 2006;312:3768–81. doi: 10.1016/j.yexcr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–6. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Martinez P, Thanasoula M, Carlos AR, Gomez-Lopez G, Tejera AM, Schoeftner S, Dominguez O, Pisano DG, Tarsounas M, Blasco MA. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat Cell Biol. 2010;12(8):768–80. doi: 10.1038/ncb2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo ER, Pidoux AL, Perry PE, Allshire RC. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature. 1998;392:825–8. doi: 10.1038/33941. [DOI] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Penkner AM, Fridkin A, Gloggnitzer J, Baudrimont A, Machacek T, Woglar A, Csaszar E, Pasierbek P, Ammerer G, Gruenbaum Y, Jantsch V. Meiotic chromosome homology search involves modifications of the nuclear envelope protein Matefin/SUN-1. Cell. 2009;139:920–33. doi: 10.1016/j.cell.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Rockmill B, Roeder GS. Telomere-mediated chromosome pairing during meiosis in budding yeast. Genes Dev. 1998;12:2574–86. doi: 10.1101/gad.12.16.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig I, Liebe B, Egozcue J, Cabero L, Garcia M, Scherthan H. Female-specific features of recombinational double-stranded DNA repair in relation to synapsis and telomere dynamics in human oocytes. Chromosoma. 2004;113:22–33. doi: 10.1007/s00412-004-0290-8. [DOI] [PubMed] [Google Scholar]
- Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–87. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Sato A, Isaac B, Phillips CM, Rillo R, Carlton PM, Wynne DJ, Kasad RA, Dernburg AF. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell. 2009;139:907–19. doi: 10.1016/j.cell.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H. Interphase cytogenetics in understanding chromosome and telomere dynamics during prophase I: Implications for meiotic telomere movements. In: Schmid M, Nanda I, editors. Chromosomes today. Vol. 14. Kluwer, Dordrecht: 2004. pp. 127–147. [Google Scholar]
- Scherthan H. Telomeres. CSH Press; Cold Spring Harbor: 2006. Meiotic telomeres; pp. 225–259. [Google Scholar]
- Scherthan H. Telomere attachment and clustering during meiosis. Cell Mol Life Sci. 2007;64:117–24. doi: 10.1007/s00018-006-6463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Trelles-Sticken E. Absence of yKu/Hdf1 but not myosin-like proteins alters chromosome dynamics during pro-phase I in yeast. Differentiation. 2008;76:91–8. doi: 10.1111/j.1432-0436.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- Scherthan H, Weich S, Schwegler H, Heyting C, Harle M, Cremer T. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol. 1996;134:1109–25. doi: 10.1083/jcb.134.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Jerratsch M, Dhar S, Wang YA, Goff SP, Pandita TK. Meiotic telomere distribution and Sertoli cell nuclear architecture are altered in Atm- and Atm-p53-deficient mice. Mol Cell Biol. 2000a;20:7773–83. doi: 10.1128/mcb.20.20.7773-7783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Jerratsch M, Li B, Smith S, Hulten M, Lock T, de Lange T. Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores. Mol Biol Cell. 2000b;11:4189–203. doi: 10.1091/mbc.11.12.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Wang H, Adelfalk C, White EJ, Cowan C, Cande WZ, Kaback DB. Chromosome mobility during meiotic prophase in Saccharomyces cerevisiae. Proceeding of the National Academy of Sciences of the United States of America. 2007;104:16934–9. doi: 10.1073/pnas.0704860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proceeding of the National Academy of Sciences of the United States of America. 2007;104:7426–31. doi: 10.1073/pnas.0609198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kabir S, van Overbeek M, Celli GB, de Lange T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327:1657–61. doi: 10.1126/science.1185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–56. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Tomita K, Cooper JP. The telomere bouquet controls the meiotic spindle. Cell. 2007;130:113–26. doi: 10.1016/j.cell.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Trelles-Sticken E, Dresser ME, Scherthan H. Meiotic telomere protein Ndj1p is required for meiosis-specific telomere distribution, bouquet formation and efficient homologue pairing. J Cell Biol. 2000;151:95–106. doi: 10.1083/jcb.151.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelles-Sticken E, Adelfalk C, Loidl J, Scherthan H. Meiotic telomere clustering requires actin for its formation and cohesin for its resolution. J Cell Biol. 2005;170:213–23. doi: 10.1083/jcb.200501042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat JJ, Kim KP, Koszul R, Zanders S, Weiner B, Kleckner N, Alani E. Csm4, in collaboration with Ndj1, mediates telomereled chromosome dynamics and recombination during yeast meiosis. PLoS Genet. 2008;4:e1000188. doi: 10.1371/journal.pgen.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. The leptotene-zygotene transition of meiosis. Annu Rev Genet. 1998;32:619–97. doi: 10.1146/annurev.genet.32.1.619. [DOI] [PubMed] [Google Scholar]



