Abstract
Cyclin-Dependent Kinase 1 (CDK1) is the major M-phase kinase known also as the M-phase Promoting Factor or MPF. Studies performed during the last decade have shown many details of how CDK1 is regulated and also how it regulates the cell cycle progression. Xenopus laevis cell-free extracts were widely used to elucidate the details and to obtain a global view of the role of CDK1 in M-phase control. CDK1 inactivation upon M-phase exit is a primordial process leading to the M-phase/interphase transition during the cell cycle. Here we discuss two closely related aspects of CDK1 regulation in Xenopus laevis cell-free extracts: firstly, how CDK1 becomes inactivated and secondly, how other actors, like kinases and phosphatases network and/or specific inhibitors, cooperate with CDK1 inactivation to assure timely exit from the M-phase.
1. Introduction
The cell cycle regulation comprises a network of numerous kinases and phosphatases. CDK1 is a major kinase necessary both for the S-phase and M-phase progression. Identification at the end of XX century of the CDK1 as a major regulator of the cell cycle made the understanding of its own regulation a fascinating topic. The use of Xenopus laevis cell-free extracts has had a large impact on these studies.
CDK1 belongs to the family of Cyclin-Dependent Kinases (CDKs). It has been the first CDK described in yeast (as a product of cdc2 or cdc28 gene depending on species) and human (called p34cdc2). The name of CDKs comes from the association of these kinases with the regulatory subunits called cyclins. Similarly to all other kinases CDK1 has in its amino-terminal domain an xGxPxxxxREx sequence (where x represents any amino acid). This conserved region corresponds to a cyclin-binding domain [1]. There are twenty-one CDK-coding genes in the human genome [2]. However, only a few of them are involved in cell cycle regulation. In Xenopus laevis oocytes and early embryos a handful of CDKs are expressed (CDK1, CDK2, CDK5, CDK9; our unpublished data). Two major CDKs taking part in cell cycle regulation are CDK1 and CDK2. Only CDK1 seems to be involved in M-phase regulation, while CDK2 is a major player in S-phase progression. The similarity in three-dimensional structure of these two kinases helps to understand some aspects of their regulation.
CDK1 activity picks only for a very short period of time upon G2/M transition and falls down rapidly at the M-phase exit. The structure of CDK1 is bilobate, similar to the cyclic AMP-dependent protein kinase, but contains a unique helix-loop segment that interferes with ATP and protein substrate binding. In its monomeric inactive form, CDK1 binds to the ATP in a conformation, which prevents a nucleophilic attack by hydroxyl substrate on the β-γ phosphate bridge of ATP [3].
Cyclins, the regulatory subunits of CDKs, are encoded by at least 15 different genes in human genome [2]. Only some of them are expressed in oocytes and early embryos of Xenopus laevis. B-type cyclins are main regulators of CDK1. In Xenopus laevis early developmental stages five of B-type cyclins (B1–B5) were identified [4]. B1 and B2 cyclins play a major role in M-phase regulation in Xenopus laevis since they are associated with the majority of CDK1s. Upon M-phase exit cyclins are degraded sequentially beginning from cyclin B1 and ending with cyclin B5. This sequential degradation of cyclins reflects inactivation of successive parts of CDK1. Thus just a brief look at the composition and metabolism of CDK1/cyclin B complexes illustrates the complexity of the system, and this is just the tip of the iceberg.
2. M-Phase Control in Xenopus laevis Oocytes via MPF, CSF, and Calcium Signalling
Amphibian oocytes have been excellent model system allowing the discovery of the basic principles of M-phase regulation. Most of the molecules regulating M-phase entry, maintaining, and exit were identified with the help of the amphibian experimental model. The enzymatic complex of CDK1 and cyclin B is the universal regulator of the M-phase. It was first discovered as an activity called Maturation Promoting Factor (MPF) by Masui and Markert in 1971 [5]. MPF drives both meiotic and mitotic cell cycle via M-phase entry induction. Masui and Markert discovered the MPF activity in experiments involving a cytoplasmic transfer between mature and immature oocytes of Rana pipiens and Xenopus laevis. Such a transfer invariably induced resumption of meiotic maturation, that is, M-phase entry in G2-arrested immature oocytes. Initially, the MPF activity was called a Maturation Promoting Factor because of its ability to induce maturation upon injection into immature oocytes. However further studies have shown that the very same factor induces also the mitotic M-phase. Thus the name of Maturation Promoting Factor was changed to M-phase Promoting Factor. Soon after, the MPF was shown to have an M-phase-inducing activity regardless of the species. This suggested the key role of this molecule in the induction of the M-phase of cell cycle in all eukaryotic cells. After numerous efforts, MPF was identified as a complex of Cyclin-Dependent Kinase 1 (CDK1) and its regulatory subunit cyclin B [6–9].
Masui and Markert [5] demonstrated the presence of another factor that stabilized MPF in MII-arrested oocytes. They called it the CSF for CytoStatic Factor. Identification of the molecular identity of CSF took more time and energy than identification of MPF. The key player in the CSF pathway was discovered recently as a protein called Early mitotic inhibitor 2 (Emi2) [10–13]. Emi2 arrests the Ubiquitin/Proteasome System (UPS) by an inhibitory association with APC/CCdc20 ubiquitin ligase necessary for cyclin B ubiquitination and targeting the CDK1/cyclin B to proteasomes where cyclin B becomes degraded. Thus, CSF holds APC/C in an inactive state assuring MII arrest in oocytes, and Emi2 is the most downstream effector.
Amphibian oocytes, similarly to the majority of other vertebrate oocytes, are ovulated in MII-arrested state. Mos/…/ERK2 MAP kinase pathway stabilizes Emi2 during this period. The stabilization of Emi2 is achieved via phosphorylation on Thr 336, Ser 342, and Ser 344 by the most downstream enzyme of ERK2 MAP kinase pathway, the Rsk90 kinase. These phosphorylations promote Emi2-PP2A interaction and thus antagonize Emi2 phosphorylation by CDK1/cyclin B [14]. Other residues, namely T545 and T551, are phosphorylated by CDK1/cyclin B [15]. These phosphorylations are removed by protein phosphatase 2A (PP2A) assuring the turnover of Emi2 phosphorylation during the oocyte MII arrest (Figure 1).
Figure 1.
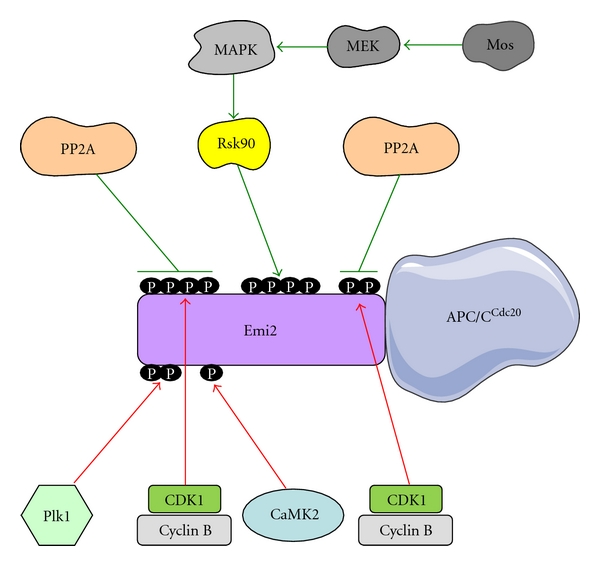
Regulation of Emi2 association with APC/CCdc20. Phosphorylation sites in the upper part of Emi2 are inhibitory for the association and are protected during MII arrest (green arrows and symbols of inhibition), while the sites in the bottom part of Emi2 are activatory for the association and the CSF-arrest exit (red arrows).
Upon fertilization, the spermatozoon entering the oocyte induces a burst of free calcium in the ooplasm. The calcium signaling plays a major role in triggering the developmental program of the embryo. The rapid increase in calcium concentration inactivates CSF. The first step is activation of calcium-dependent kinase 2 (CaMK2), which phosphorylates Emi2. This turns Emi2 into a substrate for Plk1, the kinase which is already active in MII oocytes before fertilization, but unable to phosphorylate Emi2 before it becomes modified by CaMK2 (Figure 1). The double CaMK2- and Plk1-mediated modification of Emi2 following fertilization makes this protein recognizable by SCFβ-TRCP ubiquitin ligase. SCFβ-TRCP triggers Emi2 polyubiquitination followed by its proteasome-dependent degradation and disappearance from the ooplasm. This complex process is necessary to remove the CSF activity and to release the MII arrest of oocytes upon fertilization via APC/C activation and triggering cyclin B polyubiquitination [10–12].
Despite the major role of the Mos/…/ERK2 MAP kinase pathway resulting in Emi2 stability other alternative CSF pathways seem also involved in MII arrest induction and maintenance. For example, CDK2/cyclin E pathway was shown to induce a CSF-like arrest in Xenopus laevis cell-free extracts [16]. A checkpoint kinase Mps1 (Monopolar spindle 1) is necessary for this action of CDK2/cyclin E [16]. Further details of the role of this alternative CSF pathway and especially its elimination upon oocyte activation remain unknown.
The CSF inactivation is not the only effect of the rise in calcium concentration upon fertilization. The calcium burst also triggers a transient activation of a phosphatase called calcineurin [17, 18], which is absolutely necessary for oocyte activation. However, it is unknown whether calcineurin acts on CDK1 or on some of its substrates. Nevertheless, these results pinpoint the network of kinases and phosphatases involved in phosphorylation and dephosphorylation events of numerous proteins as major mean to regulate the cell cycle transition necessary to initiate the embryo development.
3. CDK1 Inactivation without Cyclin B Degradation
Proteolytic degradation of cyclin B via UPS plays a major role in cell cycle regulation. Its perturbation disorganizes the cell cycle progression [19–21]. Inhibition of the proteolytic activity of proteasome with inhibitors like MG115, MG132, or ALLN arrests cells in M-phase with high cyclin B content and equally high CDK1 activity. The same inhibitors block also cyclin B degradation in Xenopus laevis cell-free extracts; however, they neither arrest CDK1 inactivation nor provoke the M-phase arrest [22–24]. This important difference between the reaction of intact cells and cell-free extracts strongly suggests that CDK1 inactivation proceeds without cyclin B degradation at least in Xenopus laevis oocytes and embryo extracts. The reason for such a different reaction of intact cells and cell-free extract to proteasome inhibition remains unclear. It seems reasonable to speculate that in the case of somatic cells some upstream substrate of proteasome pathway must be degraded before cyclin B could be targeted for degradation. In this case the proteosome inhibition would inhibit cyclin B degradation indirectly, via action of remaining upstream substrate of UPS. However, the identity of a potential UPS substrate conditioning cyclin B degradation in intact cells remains unknown.
The first step in cyclin B degradation is its polyubiquitination by APC/C ubiquitin ligase (Figure 2). This process takes place when cyclin B is still associated with CDK1. Thus, APC/C-mediated polyubiquitination targets the proteasome not only cyclin B but the whole complex, which is still active when cyclin B is in the polyubiquitinated state. The proteasome induces or catalyses the dissociation of cyclin B from its CDK1 partner. Nishiyama and colleagues [22] have shown that the activity of the 26S proteasome involved in cyclin B dissociation from CDK1 is associated with its 19S regulatory subunit. It seems that the lid of the proteasome could be involved in this process. The 19S subunits of proteasome may also deubiquitinate and concomitantly denature cyclin B before it becomes loaded into the 20S proteasome catalytic chamber. This hypothetical modification of cyclin B may, as a side effect, trigger its dissociation from CDK1. Takeo Kishimoto's group named the dissociating activity of the proteasome a “nonproteolytic activity” to distinguish it from the classical proteolytic activity [22].
Figure 2.
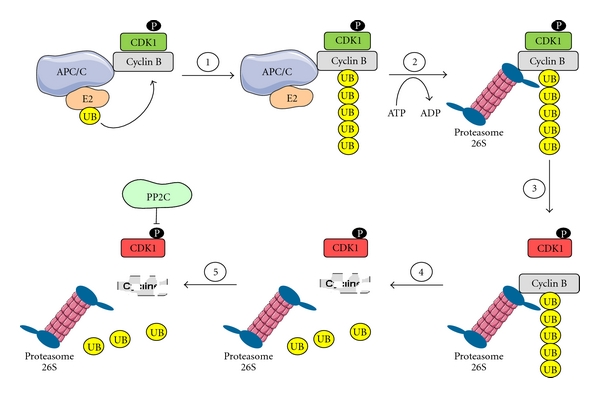
Canonical pathway of CDK1/cyclin B inhibition.
The APC/C activity is itself positively regulated in large part by CDK1/cyclin B-dependent phosphorylations of its numerous subunits [25, 26]. These phosphorylations trigger cyclin B polyubiquitination, dissociation from CDK1, and degradation resulting in CDK1 inactivation. In addition, the substrate specificity of APC/C changes during mitosis, due to the switch in its regulatory subunit, from Cdc20 (Fizzy) to Cdh1 (Fizzy related). This switch is possible after Cdc20 polyubiquitination by APC/C and its subsequent degradation. Thus, CDK1/cyclin B indirectly regulates itself via controlling APC/C activity at least at two different levels, which in turn determines the stability of cyclin B (reviewed in [27, 28]).
4. CDK1 Inactivation Is Inhibited by Interference with Cyclin B Polyubiquitination
Ubiquitin is a highly evolutionary conserved small (76 amino acids) polypeptide. Its COOH terminus is covalently linked to lysine residues of a substrate (e.g., cyclin B) via an isopeptide bond (for a review see [29]). Polyubiquitination proceeds via multiple rounds of ubiquitination during which the COOH terminus of a new ubiquitin molecule forms an isopeptide bond with the lysine residue of ubiquitin previously attached to the substrate. This process is mediated by three sequentially acting enzymes (E1, E2 and E3, the last being an ubiquitin ligase). Lysine 48 of ubiquitin molecule is one of the major residues involved in polyubiquitination, mediating subsequent targeting of substrates to the proteasome. However, all seven lysine residues present within the molecule of ubiquitin are able to form isopeptide bonds [29–31]. A mutation of lysine 48 (K48) to arginine (R) severely affects the process of polyubiquitination [32, 33]. Such ubiquitin mutant (UbiK48R) was used to perturb ubiquitination of proteins during the M-phase in the cell-free mitotic extracts upstream from the inhibition of the proteolytic activity of the proteasome. The interference with the polyubiquitination pathway via UbiK48R arrests cyclin B dissociation from CDK1 and its degradation [34]. Therefore, the polyubiquitination pathway appears to be necessary for the targeting of cyclin B complexed with CDK1 to the proteasome. This in turn results in the maintenance of high activity of CDK1 and keeping the extract in the M-phase. Thus UPS inhibition at the level of polyubiquitination and not at the level of the proteasome proteolytic activity inhibits effectively CDK1 inactivation. This points to the importance of cyclin B dissociation from CDK1 and not the degradation of cyclin B.
5. Alternative Pathways to Inactivate CDK1
5.1. Dephosphorylation of CDK1 Threonine 161
The phosphorylation of threonine 161 residue of CDK1 is necessary for activation of CDK1. Thus, the dephosphorylation of this site may inactivate CDK1 independently of cyclin B dissociation and proteolysis [35, 36]. The dephosphorylation is catalyzed by the okadaic-acid-(OA-)sensitive type 2C protein phosphatases (PP2Cs) [36]. The comparison of the dynamics of CDK1 inactivation with the dynamics of CDK1 Thr161 dephosphorylation upon M-phase exit has shown that the latter follows CDK1 inactivation [24]. The detailed analysis of the interaction between CDK1 and cyclin B2 (one of five cyclins B potentially present in Xenopus laevis early embryos) upon M-phase exit revealed that the dissociation of cyclin B2 from CDK1 perfectly correlates with the dynamics of CDK1 inactivation and not with CDK1 threonine 161 dephosphorylation [24]. Thus, CDK1 threonine 161 residue dephosphorylation is a relatively late step in CDK1 inactivation and perhaps plays a role in the ultimate switching off of the CDK1, thus protecting the cell against unscheduled and premature reactivation of the kinase after the M-phase exit.
5.2. Transient Inhibitory Phosphorylation of CDK1 on Threonine 14 and Tyrosine 15
A study in Xenopus laevis cell-free extracts has shown that a transient phosphorylation of CDK1 on tyrosine 15 (and most probably on threonine 14) could also participate in CDK1 inactivation. D'Angiolella and colleagues have shown that in cycling cell-free extracts the cyclin B1-associated histone H1 kinase activity diminishes more rapidly than the level of cyclin B1 protein [37]. This was not the case for cyclin A-associated activity and the diminution of the level of cyclin A protein. Detailed analysis of M-phase exiting extracts both during mitosis and meiosis (CSF extract treated with calcium) has revealed a transient rephosphorylation of cyclin B-associated CDK1 on tyrosine 15 following the initial drop in CDK1 inactivation. This short-lasting increase in tyrosine 15 phosphorylation could be mediated by a transient increase in association of CDK1 with Wee1 kinase catalysing the reaction of phosphorylation at this site. However, another study has shown that CDK1 dephosphorylation at tyrosine 15 precedes the kinase inactivation [38] (Figure 3). Thus, it is not entirely clear to what extend this kind of regulation influences the dynamics of CDK1 inactivation.
Figure 3.
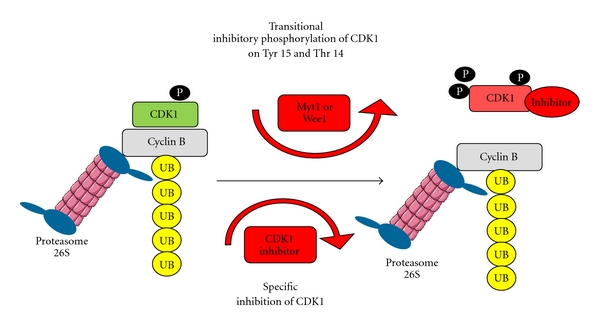
Hypothesis of alternative pathways involvement during CDK1/cyclin B dissociation.
5.3. Specific Inhibitors May Participate in CDK1 Inactivation
Data from yeast and HeLa cells suggest that the specific inhibitors could also be involved in CDK1 inactivation upon mitotic exit. Cdc6 protein, well known as a key S-phase regulator, could play this role [39, 40] (Figure 3). There is no data supporting such a role of Cdc6 in Xenopus laevis cells. However, since Cdc6 was shown to inhibit CDK1 both in yeast and in human cells it seems possible that it also plays the same role in amphibians. CDK1 inhibition specifically during the M-phase exit may lead, together with the transient CDK1 phosphorylation on tyrosine 15, to more rapid and more effective inhibition of CDK1.
5.4. Cooperation of CDK2 and PKA in CDK1 Inactivation
The next alternative pathway of CDK1/cyclin B inactivation implies the participation of CDK2. It was shown that CDK2/cyclin E enables to maintain the high CDK1/cyclin B activity during mitosis in Xenopus laevis cell-free extract [41]. Premature inactivation of CDK2 during the mitotic M-phase induces an increase in protein kinase A (PKA) activity and speeds up CDK1/cyclin B inactivation and cyclin B degradation. Thus, CDK2/cyclin E seems to be coupled with PKA activity in assuring a correct timing of CDK1/cyclin B inactivation and the M-phase exit. However, it remains unknown how CDK2 and PKA act in concert on CDK1 and cyclin B degradation pathway.
Crystallographic studies of another CDK2 enzymatic complex, namely, CDK2/cyclin A that has fundamental role in S-phase regulation, have shown details of the mechanism underlying inactivation of this kinase [2, 42]. CDK1/cyclin B complex has never been studied with such accuracy. As the two complexes are closely related the mechanism proposed for CDK2 may also apply to CDK1. The active site of CDK1 could undergo conformational changes in its PSTAIRE helix and T-loop upon the dissociation of cyclin B, as it happens with CDK2 upon dissociation of cyclin A. Such a conformational change prevents proper interaction of the enzyme with ATP and inactivates the kinase [2, 42]. The cyclin subunit determines substrate specificity of CDKs (reviewed in [43]). Thus, the loss of cyclin subunit should be immediately followed by efficient inactivation of CDK1 to protect the cell against possible phosphorylation of undesired substrates. A part of this job may be performed by the transient inhibitory phosphorylation of tyrosine 15. Another possibility to eliminate unspecific activity of cyclin-free CDK1 would be a direct inhibition via its association with a specific inhibitor, for instance, Cdc6.
6. Kinase/Phosphatase Network upon M-Phase Exit
In HeLa cells CDK1 inactivation is not sufficient to assure the successful transition to interphase. The efficient transition to interphase requires a phosphatase activity (or activities) dephosphorylating CDK1 mitotic substrates [44]. The activation of these phosphatases is clearly proteasome-dependent, but independent of cyclin B degradation. PP2A was shown to be the major phosphatase dephosphorylating CDK1 substrates in interphase Xenopus laevis eggs extract [45]. PP2A is a heterotrimer composed of the catalytic C-, scaffolding A-, and regulatory B-type subunits represented by different isoforms. The B-type subunits are responsible for the substrate specificity of the whole complex [46]. Mochida and colleagues [45] have shown that PP2A containing the B55δ subunit is the major phosphatase controlling the exit from the M-phase via dephosphorylation of CDK1 phospho-substrates. It was proposed that the newly discovered Greatwall kinase could play a role of a phosphatase suppressor [47]. Greatwall kinase was discovered in a screen for Drosophila mutants defective in chromosome condensation [48]. Greatwall is a ubiquitous evolutionarily conserved protein kinase, known in humans as MAST-L kinase, belonging to the AGC family of Ser/Thr kinases [49]. Depletion of this kinase from M-phase extracts induced activation of an okadaic-sensitive phosphatase that acts on CDK1 substrates and on the mitotic exit. Addition of this kinase to interphase extracts inhibited dephosphorylation of CDK1 substrates [50, 51]. Moreover, the inability of Greatwall-depleted cell-free extracts to enter M-phase was reverted by removal of PP2A-B55 delta [50]. It was shown recently by two independent laboratories that a small protein called cAMP-regulated phosphoprotein-19 (ARPP-19), a close relative of another small protein alpha-endosulfine (ENSA), was an ideal substrate for Greatwall kinase in Xenopus laevis cell-free extracts [52, 53]. Thus, the network of kinases and phosphatases governing CDK1 substrates dephosphorylation, and thus, the transition to interphase following the M-phase was discovered.
7. Conclusions
Several pathways control CDK1 inhibition upon M-phase exit. The major pathway called here canonical involves dissociation of cyclin B from CDK1 and is followed by cyclin B degradation and disappearance from the cell. Other probably minor pathways, including phosphorylation and dephosphorylation of CDK1 at different sites, and active inhibition of CDK1 kinase activity, may play supplementary role in shortening the process of CDK1 inactivation. This hypothetical role of CDK1 accelerator could be of particular importance for very fast cleaving embryos such as amphibian embryos. CDK2/cyclin E and PKA seem also to exercise an important control over the timing of CDK1/cyclin B inactivation. The alternative pathways in CDK1/cyclin B inactivation may be important in certain unique conditions when the canonical pathway becomes ineffective. For example, in rat one-cell embryos treated with MG132 the M-phase exit probably occurs without cyclin B degradation [54]. Also in mouse oocytes undergoing maturation and fertilized in vitro by numerous spermatozoids CDK1/cyclin B is inactivated rather via threonine 161 dephosphorylation than full cyclin B degradation [55]. Surprisingly, recent studies of the minimal control of CDK network in fission yeast suggest that modulation of CDK1 activity and not its proteolytic or phosphorylation-dependent regulation could play an ancestral role during evolution [56]. This discovery will certainly stimulate further studies on noncanonical pathways regulating M-phase exit, and it is possible that the canonical pathway will become noncanonical and vice versa.
Acknowledgment
The authors are grateful to Dr. Malgorzala Kloc (Houston, Tex) for the discussions and critical reading.
References
- 1.Endicott JA, Nurse P, Johnson LN. Mutational analysis supports a structural model for the cell cycle protein kinase p34. Protein Engineering. 1994;7(2):243–253. doi: 10.1093/protein/7.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Gopinathan L, Ratnacaram CK, Kaldis P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. In: Kubiak JZ, editor. Cell Cycle in Development. 1st edition. Vol. 53. Springer; 2011. pp. 365–389. (Results and Problems in Cell Differentiation). [DOI] [PubMed] [Google Scholar]
- 3.Jeffrey PD, Russo AA, Polyak K, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:294–295. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 4.Hochegger H, Klotzbücher A, Kirk J, et al. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development. 2001;128(19):3795–3807. doi: 10.1242/dev.128.19.3795. [DOI] [PubMed] [Google Scholar]
- 5.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. Journal of Experimental Zoology. 1971;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 6.Lohka MJ, Hayes MK, Maller JL. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(9):3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arion D, Meijer L, Brizuela L, Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- 8.Labbé JC, Capony JP, Caput D, et al. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO Journal. 1989;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from xenopus. Cell. 1990;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- 10.Tung JJ, Hansen DV, Ban KH, et al. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Current Biology. 2005;15(16):1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437(7061):1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Grimison B, Lewellyn AL, Maller JL. The anaphase-promoting complex/cyclosome inhibitor Emi2 is essential for meiotic but not mitotic cell cycles. Journal of Biological Chemistry. 2006;281(46):34736–34741. doi: 10.1074/jbc.M606607200. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T, Ohsumi K, Kishimoto T. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature. 2007;446(7139):1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- 15.Wu JQ, Hansen DV, Guo Y, et al. Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(42):16564–16569. doi: 10.1073/pnas.0707537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimison B, Liu J, Lewellyn AL, Maller JL. Metaphase arrest by cyclin E-Cdk2 requires the spindle-checkpoint kinase Mps1. Current Biology. 2006;16(19):1968–1973. doi: 10.1016/j.cub.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 17.Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449(7160):336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449(7160):341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- 19.Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 20.Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 21.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama A, Tachibana K, Igarashi Y, et al. A nonproteolytic function of the proteasome is required for the dissociation of CDc2 and cyclin B at the end of M phase. Genes and Development. 2000;14(18):2344–2357. doi: 10.1101/gad.823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesnel F, Bazile F, Pascal A, Kubiak JZ. Cyclin B dissociation from CDK1 precedes its degradation upon MPF inactivation in mitotic extracts of Xenopus laevis embryos. Cell Cycle. 2006;5(15):1687–1698. doi: 10.4161/cc.5.15.3123. [DOI] [PubMed] [Google Scholar]
- 24.Chesnel F, Bazile F, Pascal A, Kubiak JZ. Cyclin B2/cyclin-dependent kinase1 dissociation precedes CDK1 Thr-161 dephosphorylation upon M-phase promoting factor inactivation in Xenopus laevis cell-free extract. International Journal of Developmental Biology. 2007;51(4):297–305. doi: 10.1387/ijdb.072292fc. [DOI] [PubMed] [Google Scholar]
- 25.Listovsky T, Zor A, Laronne A, Brandeis M. Cdk1 is essential for mammalian cyclosome/APC regulation. Experimental Cell Research. 2000;255(2):184–191. doi: 10.1006/excr.1999.4788. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nature Reviews Molecular Cell Biology. 2007;8(11):894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- 27.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Reviews Molecular Cell Biology. 2006;7(9):644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 28.Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annual Review of Cell and Developmental Biology. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng J, Schwartz D, Elias JE, et al. A proteomics approach to understanding protein ubiquitination. Nature Biotechnology. 2003;21(8):921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 30.Pickart CM. Back to the future with ubiquitin. Cell. 2004;116(2):181–190. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 31.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nature Reviews Molecular Cell Biology. 2005;6(8):599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 32.Chau V, Tobias JW, Bachmair A, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243(4898):1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 33.Finley D, Sadis S, Monia BP, et al. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Molecular and Cellular Biology. 1994;14(8):5501–5509. doi: 10.1128/mcb.14.8.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazile F, Pascal A, Karaiskou A, Chesnel F, Kubiak JZ. Absence of reciprocal feedback between MPF and ERK2 MAP kinase in mitotic Xenopus laevis embryo cell-free extract. Cell Cycle. 2007;6(4):489–496. doi: 10.4161/cc.6.4.3860. [DOI] [PubMed] [Google Scholar]
- 35.Solomon MJ, Lee T, Kirschner MW. Role of phosphorylation in p34(cdc2) activation: identification of an activating kinase. Molecular Biology of the Cell. 1992;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Smedt V, Poulhe R, Cayla X, et al. Thr-161 phosphorylation of monomeric Cdc2. Regulation by protein phosphatase 2C in xenopus oocytes. Journal of Biological Chemistry. 2002;277(32):28592–28600. doi: 10.1074/jbc.M202742200. [DOI] [PubMed] [Google Scholar]
- 37.D’Angiolella V, Palazzo L, Santarpia C, Costanzo V, Grieco D. Role for non-proteolytic control of M-phase promoting factor activity at M-phase exit. PLoS ONE. 2007;2(2) doi: 10.1371/journal.pone.0000247. Article ID e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chesnel F, Vignaux F, Richard-Parpaillon L, Huguet A, Kubiak JZ. Differences in regulation of the first two M-phases in Xenopus laevis embryo cell-free extracts. Developmental Biology. 2005;285(2):358–375. doi: 10.1016/j.ydbio.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Calzada A, Sacristán M, Sánchez E, Bueno A. Cdc6 cooperates with Sic1 and Hct1 to inactivate mitotic cyclin-dependent kinases. Nature. 2001;412(6844):355–358. doi: 10.1038/35085610. [DOI] [PubMed] [Google Scholar]
- 40.Yim H, Erikson RL. Cell division cycle 6, a mitotic substrate of polo-like kinase 1, regulates chromosomal segregation mediated by cyclin-dependent kinase 1 and separase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(46):19742–19747. doi: 10.1073/pnas.1013557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Angiolella V, Costanzo V, Gottesman ME, Avvedimento EV, Gautier J, Grieco D. Role for cyclin-dependent kinase 2 in mitosis exit. Current Biology. 2001;11(15):1221–1226. doi: 10.1016/s0960-9822(01)00352-9. [DOI] [PubMed] [Google Scholar]
- 42.Cavalli A, Dezi C, Folkers G, Scapozza L, Recanatini M. Three-dimensional model of the cyclin-dependent kinase 1 (CDK1): Ab initio active site parameters for molecular dynamics studies of CDKs. Proteins. 2001;45(4):478–485. doi: 10.1002/prot.10013. [DOI] [PubMed] [Google Scholar]
- 43.Brown NR, Lowe ED, Petri E, Skamnaki V, Antrobus R, Johnson LN. Cyclin B and cyclin A confer different substrate recognition properties on CDK2. Cell Cycle. 2007;6(11):1350–1359. doi: 10.4161/cc.6.11.4278. [DOI] [PubMed] [Google Scholar]
- 44.Skoufias DA, Indorato RL, Lacroix F, Panopoulos A, Margolis RL. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. Journal of Cell Biology. 2007;179(4):671–685. doi: 10.1083/jcb.200704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO Journal. 2009;28(18):2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends in Biochemical Sciences. 2008;33(3):113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Haccard O, Wang R, et al. Roles of greatwall kinase in the regulation of Cdc25 phosphatase. Molecular Biology of the Cell. 2008;19(4):1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J, Fleming SL, Williams B, et al. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. Journal of Cell Biology. 2004;164(4):487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. Journal of the Federation of American Societies for Experimental Biology. 1995;9(8):576–596. [PubMed] [Google Scholar]
- 50.Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase greatwall (Gwl) promotes inactivation of PP2A/B55δ, a phosphatase directed against CDK phosphosites. Molecular Biology of the Cell. 2009;20(22):4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vigneron S, Brioudes E, Burgess A, Labbé JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO Journal. 2009;28(18):2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gharbi-Ayachi A, Labbé JC, Burgess A, et al. The substrate of greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330(6011):1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 53.Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330(6011):1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 54.Josefsberg LBY, Kaufman O, Galiani D, Kovo M, Dekel N. Inactivation of M-phase promoting factor at exit from first embryonic mitosis in the rat is independent of cyclin B1 degradation. Biology of Reproduction. 2001;64(3):871–878. doi: 10.1095/biolreprod64.3.871. [DOI] [PubMed] [Google Scholar]
- 55.Ajduk A, Ciemerych MA, Nixon V, Swann K, Maleszewski M. Fertilization differently affects the levels of cyclin B1 and M-phase promoting factor activity in maturing and metaphase II mouse oocytes. Reproduction. 2008;136(6):741–752. doi: 10.1530/REP-08-0271. [DOI] [PubMed] [Google Scholar]
- 56.Coudreuse D, Nurse P. Driving the cell cycle with a minimal CDK control network. Nature. 2010;468(7327):1074–1079. doi: 10.1038/nature09543. [DOI] [PubMed] [Google Scholar]


