Abstract
Background
Based upon alloantibodies produced after sensitizing dogs with transfused blood, more than a dozen blood group systems have been recognized thus far, and some have been classified as dog erythrocyte antigens (DEA).
Hypothesis
A new canine red cell antigen was suspected, based on the development of specific alloantibodies in a Dalmatian previously sensitized by blood transfusions.
Animals
Twenty-six Dalmatians (including 1 Dalmatian in need of blood compatibility studies); 55 canine blood donors.
Methods
Serologic tests, including blood typing, crossmatching, and direct Coombs’ test were performed by standard tube techniques and a novel gel column technology adapted from human blood banking.
Results
By day 40 after transfusion of an anemic Dalmatian, all major crossmatch tests to 55 non-Dalmatian dogs were incompatible. The 2 initial donors, who were compatible before transfusion, were also now incompatible, suggesting the development of an alloantibody to a common red cell antigen. No siblings were available, but 4 of 25 unrelated Dalmatians were crossmatch compatible, suggesting that they were missing the same red cell antigen. The patient was blood typed DEA 1.1, 3, 4, and 5 positive, but DEA 7 negative. Further blood typing and crossmatching results did not support an association to any of these known blood types. The alloantibodies produced were determined to be of the immunoglobulin G class.
Conclusions and Clinical Importance
Based upon the identification of an acquired alloantibody in a Dalmatian, a presumably new common blood type named Dal was identified. Dalmatians lacking the Dal antigen are likely at risk of delayed and acute hemolytic transfusion reactions.
Keywords: Alloantibody, Blood compatibility, Crossmatch, Dog erythrocyte antigen, Transfusion
Because dogs do not have clinically important naturally occurring alloantibodies, the blood group systems in dogs have been recognized largely by experimental investigations of alloantibodies produced after sensitization via blood transfusions.1 More than a dozen blood group systems have been described in dogs2–5; however, it has not been determined if all blood groups reported are serologically distinct, mainly because of the limited availability or loss of typing reagents and the paucity of comparative studies of the different blood group systems and reagents. International standardization has been proposed for 7 different canine blood group systems6,7; however, typing sera are currently only available for 5 of those blood groups, including DEA 1 (1.1, 1.2), 3, 4, 5, and 7. Furthermore, a new canine blood group classification has been proposed in Japan, based upon 4 monoclonal antibodies (Shigeta A, B, D, and E), but its correlation with the DEA system has not been defined, with the exception of SGT A, which likely recognized DEA 3 based on a limited comparative study.8 In addition, little is known about the frequency of blood types in dogs and the biochemistry of the antigen molecules.2,3
More than 2 dozen blood group systems have been recognized in humans besides the well-known human ABO and Rh blood group systems. Approximately 100 additional high-frequency red cell antigens have also been defined as hereditary traits occurring in 92–99% of the general population. They have been identified mainly through the detection of their specific alloantibody in serum after hemolytic transfusion reactions and an incompatible major crossmatch test.9,10 These high-frequency red cell antigens can be of clinical importance, because it is difficult to find units of compatible blood for patients with alloantibodies directed against a high-frequency antigen.
Based upon the identification of an alloantibody in a sensitized Dalmatian and the investigations of other blood donors, including Dalmatians, we describe herein a presumably newly recognized common blood type named Dal. In addition, we applied a gel column crossmatching technique that readily detected these Dal-induced incompatibilities.
Methods
Animals
A female spayed Dalmatian was examined for medical management and hemodialysis for chronic renal failure. After sensitization by blood transfusions, this dog (index dog) required blood compatibility testing when in need of additional transfusions.
A total of 55 privately owned healthy dogs (>25 kg) enrolled in the volunteer blood donor program of the Penn Animal Blood Bank were screened as potential blood donors, including 23 Borzois, 8 mixed-breed dogs, 6 Greyhounds, 6 German Shepherds, 4 Labrador Retrievers, 3 Golden Retrievers, 2 Great Danes, as well as 1 Boxer, 1 Rhodesian Ridgeback, and 1 Doberman Pinscher. (EDTA) anticoagulated blood samples and segments of blood collection tubings (containing donor blood in citrate-phosphate-dextrose-adenine [CPDA]) were used for compatibility testing. In addition, EDTA blood samples were obtained for compatibility testing from 25 Dalmatians not related to the index dog, based upon a 3-generations pedigree and from various geographical areas in the United States (Pennsylvania, New York, Illinois, and Texas). These studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (no. 800839).
Blood Typing
All dogs used in the study were blood typed for DEA 1.1 by using commercially available typing cardsa according to the manufacturer’s instructions and as described.8,11 Furthermore, the novel gel column technology,b recently introduced in human blood banking and adapted for canine blood compatibility testing, was applied. The gel column technique uses a monoclonal anti-DEA 1.1 antibody for DEA 1.1 blood typing,c requires a special centrifuge,d and has been described in detail in a recent comparative study, which concluded that the gel system appeared to be a reliable and rapid laboratory canine DEA 1.1 typing method.8
Blood from the index dog, all crossmatched-compatible Dalmatian blood donors, and a limited number of crossmatched-incompatible donors was also typed for DEA 1.1, 1.2, 3, 4, 5, and 7 by using commercially available polyclonal antisera according to the manufacturer’s protocol.e The addition of canine polyclonal anti-immunoglobulin (Ig) G reagent (canine Coombs’ reagent) was required to facilitate the agglutination reactions with the Michigan State University DEA 1.X and 1.1 reagents.8,f
Crossmatching
Major (donor red blood cells [RBC] and recipient plasma) and minor (donor plasma and recipient RBCs) blood crossmatches were performed according to the recently standardized tube method.g Briefly, plasma was separated from EDTA or CPDA blood of a potential donor and recipient, and a 3–5% washed erythrocyte suspension was prepared. Fifty μL of plasma and 25 μL of the erythrocyte suspensions were mixed and incubated at 37°C for 15 minutes. After standard centrifugation (1000 ×g for 15 seconds),h the tubes were examined for signs of hemolysis and for macroscopic and microscopic agglutination. The degree of agglutination was scored from 1+ to 4+.8 Recipient autocontrols, ie, recipient plasma incubated with recipient RBCs, were also performed with each crossmatch test.
In addition, all major crossmatch and recipient autocontrol test results were assessed by using the novel gel column technology. The procedure was performed according to the manufacturer’s instructions and uses standard “saline gel test cards” from human medicine,i which include 6 microtubes that contain a neutral dextran-acrylamide gel (preservative, <0.1% NaN3). Briefly, anticoagulated blood samples from the recipient and potential donors were centrifuged to separate plasma from RBCs; the plasma from each sample was pipetted into a labeled tube. A 0.8% RBC suspension was obtained by adding 10 μL of packed RBCs to 1 mL of modified low ionic strength saline solution.j For the major crossmatch and recipient autocontrol tests, 25 μL of the patient plasma was pipetted in each labeled gel column. Fifty μL of the patient RBC suspension was added to the autocontrol column. Likewise, 50 μL of each potential blood donor RBC suspension were added to the appropriately labeled gel column. This procedure was repeated with an additional gel column card, but 25 μL of modified bromelin solutionk was added to every microcolumn; the modified bromelin solution was used to potentially enhance access to the antigens on the red cell surface. Both gel cards (with and without modified bromelin) were incubated at 37°C for 15 minutes in the manufacturer’s automated incubator.l The gel cards were then centrifuged for 10 minutes in a special centrifuged and the gel card could then be interpreted: if the RBCs passed through the gel, forming a pellet at the bottom of the column, then the reaction was considered negative. With positive agglutination, the RBCs were either trapped on top or within the gel column. Similar to the grading for blood typing, such positive agglutination reactions could be graded from 1+ to 4+ according to the manufacturer’s instructions.8,12
Characterization of the Index Dog’s Alloantibodies
The recipient’s serum was further investigated to characterize the strength and the class of the transfusion-induced alloantibody. The agglutinin titer of the alloantibody, defined as the highest dilution of serum or plasma in which agglutination could still be detected, was determined by creating serial 2-fold dilutions of the recipient’s serum in phosphate-buffered saline solution (PBS) and then proceeding with the standard tube crossmatch test by using these serodilutions.3,13 The various suspensions were incubated at 4°C and 37°C for 15 minutes. The process was repeated by using red cell suspensions from 5 dogs.
To deduce the immunoglobulin class, serum agglutinin titers were also determined after exposure to 1 of 2 sulfhydryl compounds, which abolish the agglutinating and complement-binding activities of IgM by cleaving their disulfide bonds.3,13 Serum from the index dog was incubated with an equal volume of either PBS, 0.01 M dithiothreitol, or 0.1 M 2-mercaptoethanol at 37°C for 60 minutes, and the agglutinin titer was then determined as described above.
Results
Case Study (Index Dog)
A 24-kg, 4-year-old female spayed Dalmatian with a 2-year history of chronic renal failure was examined because of sudden deterioration of her clinical condition and poor response to standard treatment. The dog’s treatments included 2 to 3 hemodialysis sessions per week because of severe azotemia (blood urea nitrogen [BUN], 206 mg/dL; serum creatinine, 12 mg/dL).14 Because of a nonregenerative anemia (PCV 21%), the dog’s blood type was determined as DEA 1.1 positive), crossmatches were performed, and the dog was transfused with 1.5 units of crossmatch-compatible DEA 1.1 positive packed RBCs from 2 different blood donors (total of 360 mL or 15 mL/kg) over a 3-day period (days 1 to 3). The dog had not previously received a transfusion. The transfusions resulted in an increase in the recipient’s PCV to 27%, considering that 3 hemodialysis treatments were also performed during the same time period. No adverse reactions, such as hemoglobinemia, hemoglobinuria, or both, were observed during or after either transfusion.15 Thereafter, the dog received darbepoetin alfa (1 μg/kg SC every 7 days) and initially responded, with resolution of the anemia by day 28 (PCV, 40%). Although the darbepoetin alfa treatment was continued, the dog’s PCV gradually declined to 16% on day 49. The absolute reticulocyte count was 0 bone marrow/μL, and the cytology of a bone marrow aspirate revealed a myeloid to erythroid ratio of 92: 1, consistent with a pure red cell aplasia, likely attributable to the production of crossreacting anti-erythropoietin antibodies. The dog’s direct Coombs’ test for IgG, IgM, and complement was negative at 4°C and 37°C, and there was no evidence of gastrointestinal blood loss.
The dog was maintained without transfusion support until a compatible donor was found. After extensive crossmatching of 80 potential donors (see below), 1 stored whole-blood unit (450 mL or 19 mL/kg) from a compatible Dalmatian (DEA 1.1 positive) was administered on day 56 without adverse clinical reactions during or after transfusion. The transfusion resulted in the expected rise in the patient’s PCV from 13 to 22%.
Crossmatching Test Results
Blood compatibility studies were performed by using the index dog’s serum and/or plasma collected on days 40 to 55 after the initial transfusion. The major crossmatches with RBCs from 55 non-Dalmatian donors, including 31 DEA 1.1 positive and 24 DEA 1.1 negative dogs, were consistently incompatible (2+ to 3+ agglutination reactions) in the standard tube assay. Furthermore, 3+ to 4+ agglutinin reactions were observed with the gel column technique performed simultaneously, independent of the addition of modified bromelin solution (Fig 1). The index dog’s serum from day 40 was incompatible with the RBCs of the 2 initially used donors (2 DEA 1.1 positive Borzois). All auto-controls of the index dog’s and the donors’ blood were negative when using both crossmatch methods. These results suggested the production of an alloantibody against a common or high-frequency red cell antigen lacking on the index dog’s RBCs. Therefore, siblings of the anemic Dalmatian were sought, but none were available for blood compatibility assessment. Furthermore, blood samples from 25 unrelated Dalmatians were blood typed (19 DEA 1.1 positive and 6 DEA 1.1 negative Dalmatians), and crossmatches were performed; 4 of the 25 crossmatched Dalmatians were found to be compatible when using the tube, as well as the gel column technique. These findings suggested that these 4 Dalmatians were also lacking the same red cell antigen. Finally, 9 of 80 minor crossmatches, testing for the presence of alloantibodies in the plasma of non-Dalmatian and Dalmatian donors, yielded only weak positive (up to 1+ agglutinin) reactions when using the standard tube technique but were all completely negative with the gel test method. None of the blood donors tested had been transfused with blood.
Fig 1.
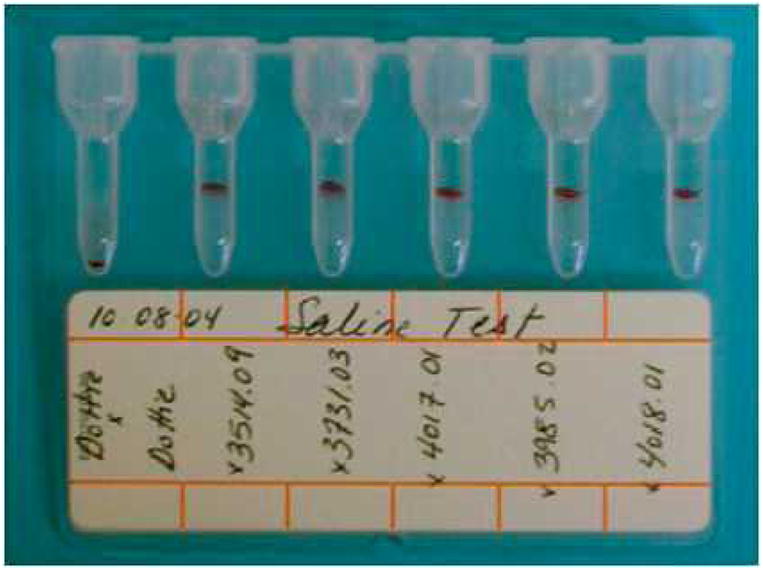
Auto-control and major crossmatch results using the DiaMed gel column technique. Saline crossmatch test (without addition of bromelin) performed on 10-08-2004. Dottie × Dottie: negative index dog’s auto-control; 3514-09, 3731.03, 4017.01, 3985.02, 4018.01: incompatible major crossmatch results (graded 4+ incompatible) of 5 non-Dalmatian donors.
Blood Typing
The Dalmatian index dog was originally typed as DEA 1.1 positive by the typing card method. When using polyclonal typing reagents in tube assays, the dog was DEA 1.1, 3, 4, and 5 positive but DEA 7 negative. Furthermore, similar extended typing procedures of 11 incompatible dogs of various breeds, including 1 incompatible Dalmatian, did not reveal any association with any of these known blood types (Table 1). Both compatible blood donors from before the initial transfusion who were incompatible 40 days after transfusion, were typed as DEA 1.1 and 4 positive but DEA 3, 5, and 7 negative. Two of the 4 compatible Dalmatians were also DEA 1.1 and 4 positive (DEA 3, 5, and 7 negative). The additional 2 compatible Dalmatians were DEA 1.1, 3, 4, and 5 positive (DEA 7 negative). Among the 11 incompatible dogs, all were DEA 4 positive and DEA 7 negative, 5 were DEA 1.1 positive, 1 was DEA 1.2 positive, and 3 were DEA 3 and 5 positive. Based upon these results, all DEAs for which typing reagents were available could be excluded as the cause of the observed blood incompatibility.
Table 1.
Extended blood typing and major crossmatch results obtained by using patient’s serum/plasma before and after transfusion (days 40 to 55).
| Animals | n | DEA Blood Type
|
Major Crossmatch
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1.2 | 3 | 4 | 5 | 7 | Before (day 0) T | After (day 40–55) T | ||
| Index dog | 1 | + | − | + | + | + | − | − | − |
| Initial donors | 2 | + | − | − | + | − | − | − | + |
| Compatible Dalmatians | 2 | + | − | − | + | − | − | ND | − |
| 2 | + | − | + | + | + | − | ND | − | |
| Incompatible Dalmatian | 1 | + | − | − | + | − | − | ND | + |
| Other breeds | 2 | + | − | − | + | − | − | ND | + |
| 2 | + | − | + | + | + | − | ND | + | |
| 2 | − | − | − | + | − | − | ND | + | |
| 1 | − | + | − | + | − | − | ND | + | |
| 1 | − | − | + | + | + | − | ND | + | |
+, Positive agglutination reactions, indicating the presence of a given DEA or an incompatible crossmatch; −, negative agglutination reactions, indicating the absence of a given DEA or a compatible crossmatch; ND, not done; T, transfusion.
Characterization of the Index Dog’s Alloantibodies
The index dog’s alloantibody agglutinin titer, measured on 5 occasions with different Dal positive red cells, was 1: 8 to 1: 16, both at 4°C and 37°C. After exposure of the patient’s serum to either dithiothreitol or 2-mercaptoethanol, the agglutinin titer remained unchanged, which implied that the causative immunoglobulins were not destroyed by the sulfhydryl compounds, thereby suggesting anti-Dal alloantibodies of the IgG class. Finally, the serum of the 4 compatible Dalmatians who had never been previously transfused contained no detectable alloantibodies against each other, to other Dalmatians (n = 2), or to dogs of different breeds (n = 2).
Discussion
Based upon the identification of an alloantibody in a sensitized Dalmatian, we describe herein a presumably new common red cell antigen lacking in some Dalmatians. The index dog’s blood transfusion induced alloantibodies, which were determined to be of the IgG class, yielded positive agglutination reactions against all non-Dalmatian blood samples tested. In contrast, the index dog’s serum was compatible with 4 of 25 Dalmatians screened. A correlation between the incompatibility reactions and known DEA for which typing reagents are available was ruled out. In fact, the index dog, which was typed DEA 1.1, 3, 4, and 5 positive (DEA 7 negative), could not have been sensitized to those known blood groups via the first transfusions, because the 2 initial crossmatch-compatible donors were both only DEA 1.1 and 4 positive, but were DEA 3, 5 and 7 negative. Similarly, the extended typing of 11 incompatible dogs (including 1 incompatible Dalmatian) and 4 compatible Dalmatians did not support an association with any of these known DEAs. Thus, the blood typing results indicated the presence of an alloantibody against a novel red cell antigen other than any known DEA for which typing reagents are available. Therefore, we named the red cell antigen recognized by the index dog’s serum Dal, until an official nomenclature is applied.
Based upon this limited survey, the frequency of the Dal antigen was, overall, 93%, and its presence was ubiquitous among the non-Dalmatian group. In human medicine, red cell antigens are classified as high-frequency antigens when they reach an incidence of >92 to 99% in the general population, whereas the frequency may differ in certain ethnic groups.13 Because the amount of the index dog’s serum was limited, it has not been possible to screen large numbers of dogs to determine the precise frequency of the Dal antigen in Dalmatians and other breeds; therefore, we are referring to the antigen as common rather than high frequency.
Antibodies to high-frequency red cell antigens can be very important in human transfusion medicine. Some are capable of causing severe immediate or delayed hemolytic transfusion reactions or hemolytic disease of the fetus and the newborn. Such antibodies can create a critical clinical problem, because compatible blood is often extremely difficult to find.9,16,17 The dog in this study developed the anti-Dal alloantibody after transfusion, whereas 4 other Dal-negative Dalmatians, who had never been transfused, did not have any anti-Dal alloantibodies in their plasma, suggesting that the anti-Dal alloantibody is acquired and only occurs after sensitization by blood transfusion. It has to be considered that, after sensitization via transfusion, the development of anti-Dal alloantibodies may result in ineffective transfusions and even acute life-threatening hemolytic transfusion reactions, if Dal-positive blood products are repeatedly transfused to a Dal-negative dog. Hence, crossmatching of previously transfused Dalmatians and other breed dogs is pivotal to select compatible blood for transfusion.
There are few recognized RBC high-frequency antigens in dogs. Based on several surveys of blood types in dogs, DEA 4 is classified as a high-frequency antigen, with a prevalence of >97%.1,3,18,19 Because of the high incidence of DEA 4, immunization against it takes place in only isolated cases when transfusing dogs. Thus, this blood type was, for a long time, widely considered to have no clinical relevance and was, therefore, not selected against when choosing donors.19–22 However, an acute hemolytic transfusion reaction from anti-DEA 4 alloantibodies in a dog sensitized via transfusion was recently described.22 In a similar fashion, alloantibodies against a common red cell antigen lacking in an anemic Whippet were previously reported when an acute hemolytic transfusion reaction occurred after sensitization by an initial transfusion. In that case, the undefined red cell antigen was only found to be missing in 1 litter mate.23 This antiserum is currently not available for comparison.
In humans, the lack of a high-frequency antigen may imply homozygosity for a rare allele, reflecting the inheritance of the same rare blood group allele from each parent. In these cases, siblings are considered the most promising source of compatible blood, because offspring of the same parents are far more likely to have the same 2 rare alleles than an individual in the random donor population.13 No sibling of the Dalmatian index dog could be found for compatibility testing, but 2 of the Dal-negative Dalmatians were themselves siblings. Thus, if a sibling is not available, limiting the search for a compatible donor to a population of the same breed as the patient may increase the likelihood of a successful match. To our knowledge, this is the first report of a direct association between a canine blood type and a specific breed.
Because typing sera for DEA 6 and DEA 8, as well as other previously described canine blood groups that no longer exist19 or are not commercially available,8 it remained impossible to determine their relation to the Dal antigen. The availability of canine monoclonal typing reagents has increased, but polyclonal reagents remain the only option for several blood groups and are of limited availability and are often associated with inconsistent hemagglutination reactions. The problem remains that, as of today, no gold standard method for extended blood typing of dogs has been accepted.
The American Association of Blood Banks Standards defines a clinically significant antibody as one that causes decreased RBC survival.13 The immunogenicity, ie, the ability of an antigen to stimulate an immune response, of the Dal antigen was clearly documented by the patient’s production of anti-Dal alloantibodies active at 37°C. The negative Coombs’ test and the lack of agglutination of the patient’s RBCs with its own plasma or serum indicated that an alloantibody (ie, an antibody formed against a specific blood group antigen that is absent in the patient) rather than an autoantibody was responsible for the incompatibility reactions. For ethical reasons, no incompatible blood units were transfused to the patient; thus, the clinical significance of the anti-Dal alloantibodies could not be assessed in vivo.
Throughout this study, a novel gel agglutination assay was used in conjunction with standard serologic methods. This blood compatibility technology, now widely used in human blood banking, was developed in 1985 by Lapierre of Lyon, France, in an attempt to achieve more stable agglutination reaction end points to produce more reproducible results in comparison with traditional tube methodology.24 The procedures used in humans are standardized and provide clear and stable reactions that improve result interpretation. The gel test cards can be saved for up to 24 hours,25 and photocopies are readily achievable. This technology was recently applied for canine DEA 1.1 typing, by using a monoclonal antibody, and initial comparative studies concluded that it is a reliable and rapid laboratory method for canine DEA 1.1 blood typing.8 Similarly, the use of the DEA 1.1 gel test in the present study provided clear typing results, with high sensitivity and specificity.
This gel agglutination assay has been approved in humans by the Food and Drug Administration as an alternative to the tube assay for the detection of red cell antibodies.26 In our study, the results of all major crossmatch tests and autocontrols by using the gel test technology were in accordance with the results of the standard tube assay. The gel test reactions were easily interpretable, because the subjectivity of the process was almost eliminated (Fig 1). In humans, the gel test has proven to be equivalent to standard tube technologies for the detection of unexpected antibodies. In 1 study, the sensitivity and the specificity of gel for potentially significant antibodies were 92% and 96%, respectively. This compares favorably with the 98% (sensitivity) and 90% (specificity) of the routine tube procedure.27 Currently anti-IgG–containing gel tests are being used in humans to improve the sensitivity and the specificity of the crossmatch test, and similar attempts are in progress for canine compatibility testing.
Based upon the identification of an alloantibody in a Dalmatian, we presumably identified a new blood type, named Dal. The Dal red cell antigen seems to be lacking only in some Dalmatians. After sensitization via transfusion, the development of anti-Dal alloantibodies may result in ineffective transfusions or in hemolytic transfusion reactions if Dal positive blood products are subsequently used. Further studies are needed to determine the frequency of the Dal-negative blood type in Dalmatians, as well as other breeds, and to characterize the Dal red cell antigen and its mode of inheritance. In addition, the clinical importance of anti-Dal antibodies in canine transfusion medicine must be investigated.
Finally, our recent studies in feline blood typing and compatibilities indicate that the novel gel column technique is also useful in the laboratory setting for feline AB typing28 and crossmatching.29 Thereby we discovered a common red cell antigen named Mik in domestic shorthair cats which can be associated with naturally-occurring alloantibodies and may caused acute hemolytic transfusion reactions.29 With the rising awareness and improved alloantibody technologies many more red cell antigens are going to be identified.
Acknowledgments
The authors thank the clinicians at the Veterinary Hospital of the University of Pennsylvania, the owners of the Dalmatian patient, and all other Dalmatian and blood donors for their assistance. These studies were, in part, supported by the National Institutes of Health (RR02512) and DEA 1.1 typing and saline gel cards and reagents were generously provided by DiaMed AG, Cressier FR, Switzerland.
Footnotes
RapidVet-H Canine 1.1, DMS Laboratories, Flemington, NJ
DiaMed AG, Cressier FR, Switzerland
DiaMed-Vet ID Card “DEA 1.1,” DiaMed AG, Switzerland
ID-centrifuge 12 S II, DiaMed Microtyping System, Switzerland
Midwest Animal Blood Services, Inc, Stockbridge, MI
Midwest Animal Blood Services, Inc, Stockbridge, MI
Scott, et al. Standard crossmatching protocol, AVHTM meeting, 2003
Clay Adams Sero-Fuge 2002
DiaMed ID card “NaCl, enzyme test and cold agglutinins”, Switzerland
LISS, ID-Diluent “Vet 2,” DiaMed AG, Switzerland
Bromelin, ID-Diluent “Vet 1,” DiaMed AG, Switzerland
ID-incubator 37 SI, DiaMed Microtyping System, Switzerland
References
- 1.Swisher SN, Young LE. The blood group systems of dogs. Physiol Rev. 1961;41:495–520. doi: 10.1152/physrev.1961.41.3.495. [DOI] [PubMed] [Google Scholar]
- 2.Bell K. Blood groups of domestic animals. In: Agar NS, Board DG, editors. Red Blood Cells of Domestic Mammals. Amsterdam: Elsevier Press; 1983. p. 137. [Google Scholar]
- 3.Giger U, Gelens CJ, Callan MB, et al. An acute hemolytic transfusion reaction caused by dog erythrocyte antigen 1. 1 incompatibility in a previously sensitized dog. J Am Vet Med Assoc. 1995;206:1358–1362. [PubMed] [Google Scholar]
- 4.Giger U. Blood typing and cross matching to ensure compatible transfusions. In: Bonagura JD, editor. Kirk’s Current Veterinary Therapy; Philadelphia, PA: WB Saunders; 2000. pp. 396–399. [Google Scholar]
- 5.Hohenhaus AE. Importance of blood groups and blood group antibodies in companion animals. Transfus Med Rev. 2004;18:117–126. doi: 10.1016/j.tmrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Vriesendorf HM, Westbroek DL, D’Amoro JD, et al. Joint Report of 1st International Workshop on Canine Immunogenetics. Tissue Antigens. 1973;3:145–172. [PubMed] [Google Scholar]
- 7.Vriesendorf HM, Albert ED, Templeton JW, et al. Joint Report of the Second International Workshop on Canine Immunogenetics. Transplant Proc. 1976;8:289–311. [PubMed] [Google Scholar]
- 8.Giger U, Stieger K, Palos H. Comparison of various canine blood-typing methods. Am J Vet Res. 2005;66:1386–1392. doi: 10.2460/ajvr.2005.66.1386. [DOI] [PubMed] [Google Scholar]
- 9.Seltsam A, Wagner FF, Salama A, Flegel WA. Antibodies to high-frequency antigens may decrease the quality of transfusion support: An observational study. Transfusion. 2003;43:156–1566. doi: 10.1046/j.1537-2995.2003.00565.x. [DOI] [PubMed] [Google Scholar]
- 10.Arndt PA, Garratty G. A retrospective analysis of the value of monocyte monolayer assay results for predicting the clinical significance of blood group alloantibodies. Transfusion. 2004;44:1273–1281. doi: 10.1111/j.1537-2995.2004.03427.x. [DOI] [PubMed] [Google Scholar]
- 11.Kohn B, Reitemeyer S, Giger U. Bestimmung der Blutgruppe DEA 1.1 und deren Bedeutung beim. Hund Kleintierpraxis. 1998;43:77–86. [Google Scholar]
- 12.Harmening DM, Walker PS FA Davis Company. Modern Blood Banking and Transfusion Practices. 4. Philadelphia, PA: FA Davis; 1999. Alternative technologies in routine blood bank testing; pp. 545–558. [Google Scholar]
- 13.Friday J. Standards for Blood Banks and Transfusion services. 22. Bethesda, MD: American Association of Blood Banks; 2003. [Google Scholar]
- 14.Fischer JR, Pantaleo V, Fraucey T, et al. Veterinary hemodialysis: Advances in management and technology. Vet Clin North Am Small Anim Pract. 2004;34:935–967. doi: 10.1016/j.cvsm.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Callan MB, Oakley DA, Shofer FS, et al. Canine red blood cell transfusion practice. J Am Anim Hosp Assoc. 1996;32:303–311. doi: 10.5326/15473317-32-4-303. [DOI] [PubMed] [Google Scholar]
- 16.Daniels GL, Delong EN, Hare V, et al. Gil: A red cell antigen of very high frequency. Immunohematology. 1998;14:49–52. [PubMed] [Google Scholar]
- 17.Daniels G, Poole J, de Silva M, et al. The clinical significance of blood group antibodies. Transfus Med. 2002;12:287–295. doi: 10.1046/j.1365-3148.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 18.Swisher SN, Bull R, Bowdler J. Canine erythrocyte antigens. Tissue Antigens. 1973;3:164–165. [Google Scholar]
- 19.Hale AS. Canine blood groups and their importance in veterinary medicine. Vet Clin North Am. 1995;25:1323–1333. doi: 10.1016/s0195-5616(95)50157-3. [DOI] [PubMed] [Google Scholar]
- 20.Andrews GA. Red blood cell antigens and blood groups in the dog and cat. In: Feldman BF, Zinkel JG, Jain NC, editors. Schalm’s Veterinary Hematology. 5. Baltimore, MD: Lippincott Williams & Wilkins; 2000. pp. 767–773. [Google Scholar]
- 21.Abrams-Ogg ACG. Practical blood transfusion. In: Day MJ, Mackin A, Littlewood JD, editors. Manual of Canine and Feline Haematology and Transfusion Medicine. Gloucester: British Small Animal Veterinary Association; 2000. pp. 263–303. [Google Scholar]
- 22.Melzer KJ, Wardrop KJ, Hale AS, Wong VM. A hemolytic transfusion reaction due to DEA 4 alloantibodies in a dog. J Vet Intern Med. 2003;17:931–933. [PubMed] [Google Scholar]
- 23.Callan MB, Jones LT, Giger U. Hemolytic transfusion reactions in a dog with alloantibody to a common antigen. J Vet Intern Med. 1995;9:277–279. doi: 10.1111/j.1939-1676.1995.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 24.Lapierre Y, Rigal D, Adam J, et al. The gel test: A new way to detect red cell antigen-antibody reactions. Transfusion. 1990;30:109–113. doi: 10.1046/j.1537-2995.1990.30290162894.x. [DOI] [PubMed] [Google Scholar]
- 25.Malyska H, Weiland D. The gel test. Lab Med. 1994;25:81–85. [Google Scholar]
- 26.Langston MM, Procter JL, Cipolone KM, Stroncek DF. Evaluation of the gel system for ABO and D typing. Transfusion. 1999;39:300–305. doi: 10.1046/j.1537-2995.1999.39399219288.x. [DOI] [PubMed] [Google Scholar]
- 27.Judd WJ, Steiner EA, Knafl PC. The gel test: Sensitivity and specificity for unexpected antibodies to blood group antigens. Immunohematology. 1997;13:132–135. [PubMed] [Google Scholar]
- 28.Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res. 2005;66:1393–1399. doi: 10.2460/ajvr.2005.66.1393. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein NM, Blais MC, Harris K, Oakley DA, Aronson L, Giger U. A newly recognized blood group in domestic shorthair cats: The Mik red cell antigen. J Vet Intern Med. 2007;21:287–292. doi: 10.1892/0891-6640(2007)21[287:anrbgi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]


