Abstract
Regulatory T cells (Treg) contribute significantly to the tolerogenic nature of the liver. The mechanisms however underlying liver-associated Treg induction are still elusive. We recently identified the vitamin A (VitA) metabolite, retinoic acid (RA), as a key-controller, which promotes TGF-β–dependent Foxp3+ Treg induction but inhibits TGF-β driven Th17 differentiation. To investigate whether the RA producing hepatic stellate cells (HSC) are part of the liver tolerance mechanism, we investigated the ability of HSC to function as regulatory APC. Different from previous reports, we found that highly purified HSC did not express costimulatory molecules and only upregulated MHC class II after in vitro culture in the presence of exogenous IFN-γ. Consistent with an insufficient APC function, HSC failed to stimulate naïve OT-II TCR transgenic (OT-II) CD4+T cells and only moderately stimulated α-GalCer primed invariant NKT (iNKT) cells. In contrast, HSC functioned as regulatory bystanders and promoted enhanced Foxp3 induction by OT-II T cells primed by spleen dendritic cells (DC) whereas they greatly inhibited the Th17 differentiation. Furthermore, the regulatory bystander capacity of the HCS was completely dependent on their ability to produce RA. Our data thus suggest that HSC can function as regulatory bystanders and therefore by promoting Tregs and suppressing Th17 differentiation, they might represent key-players in the mechanism that drives liver induced tolerance.
Introduction
In spite of continuous exposure to bacterial components and dietary antigens (1) , liver remains immune quiescent and is considered an immunosuppressive and tolerogenic organ (2). This is also demonstrated by the fact that liver grafts cause weak rejection and promote tolerance of co-transplanted tissues (3, 4). In addition, introduction of antigens via the portal vein leads to systemic tolerance (5). On the other hand, its suppressive nature renders liver tissue highly susceptible to chronic viral infections, such as hepatitis virus B and C (6, 7). Forkhead box P3, (Foxp3) expressing Treg, that suppress immune responses (8), are thought to play an important role in liver-mediated tolerance (9). Notably, increased Treg cells are observed both in liver graft transplantations and chronic infections with hepatitis viruses, supporting a role for these cells in the immune suppression (10-13). Nevertheless, although the contribution of Tregs in mediating liver tolerance has been recognized (14-19), little is known about the mechanisms that drive the differentiation and expansion of liver associated Tregs.
Activated CD4 T cells differentiate into various T helper (Th) subtypes, including Th1, Th2 and Th17 effector cells as well as induced Foxp3+Treg (iTreg) depending on the priming conditions and the cytokine milieu (20). Transforming growth factor (TGF)-β is a key cytokine required for the induction of the anti-inflammatory induced iTreg differentiation, whereas it inhibits the differentiation of Th1 and Th2 effectors (21). On the other hand, TGF-β can also function in a pro-inflammatry fashion and together with IL-6, TGF-β drives the differentiation of pro-inflammatory Th17 cells (22-24). The VitA metabolite RA, was recently indentified as a key-regulator of TGF-β-mediated T cell differentiation, able to promote iTreg but inhibit the generation of Th17 (25). Consistent with this, intestinal CD103+ migratory DC biased the generation of iTreg over Th17 effectors through the release of RA during priming (26-28).
HSC are defined as fat-storing cells and about 80% of the body's VitA is stored in HSC lipid droplets (29). HSC reside within the perisinusoidal space of Disse, in close proximity to liver sinusoidal endothelial cells (LSEC) and recent work indicated that HSC have the capacity to function as APC for MHC class II restricted T cells (30). Consequently, it is possible that HSC may have the potential to directly promote iTreg differentiation through the release of RA, which they store. Since the sinusoid has a lot of open pores, HSC can also interact with the lumen of the sinusoid where other APC such as DC and liver macrophages or kupffer cells are present (2, 31). Therefore, HSC might also influence the antigen presenting function of these APC (32), (33) and indirectly provide suppressive effects as RA-secreting regulatory bystanders.
In this study here, we addressed the potential direct- or indirect roles of HSC as tolerogenic regulators that drive the unique differentiation and or expansion of iTreg. Using highly purified sorted HSC, we found that HSC do not present antigen to naïve MHC class II restricted CD4 T cells and they do not induce Foxp3+ Treg cell differentiation or expansion. On the other hand, we show here that HSC function indirectly to mediate RA and TGF-β dependent Treg induction but Th17 inhibition of T cells that were primed by other APC. Our findings therefore designate an unexpected role for HSC to function as regulatory bystanders, capable of enhancing differentiation and accumulation of Treg, which may lie at the basis of the tolerogenic nature of the liver.
Materials and Methods
Mice
C57BL/6, BALB/c, OT-II TCR-transgenic (C57BL/6 background) mice were purchased from the Jackson Laboratories (USA). VitA-deficient mice were generated in our animal facility by feeding VitA-deficient diet starting at d8 to d10 of gestation and continued after birth until 10 weeks of age. All mice were maintained at the La Jolla Institute for Allergy and Immunology vivarium under specific pathogen-free condition. Animal care and experimentation were consistent with the NIH guidelines and were approved by the Institute Animal Care and Use Committee at the La Jolla Institute for Allergy and Immunology.
Antibodies and reagents
The following antibodies were purchased from BD-Biosciences (USA), as purified or conjugated to FITC, PE, PerCP-Cy5.5, APC, PE-Cy7 or biotin: anti-CD4 (L3T4), CD19 (1D3), CD24 (M1/69), CD25 (PC61), CD44 (IM7), CD45.2 (104), CD80 (16-10A1), CD86 (GL1), IA/I-E (M5), TCRβ5 (MR9-4), TCRβ8 (MR5-2), TCRβ chain (H57-597), IL-17 (TC11-18H10), IFN-γ (XMG1.2), desmin (RD301), GFAP (polyclonal) and isotype controls. Anti-mouse Foxp3 (FJK-16s), F4/80 (BM8), biotin conjugated anti-mouse CD8 (53-6.7), CD25 (eBio7D4), CD11b (M1/70), CD11c (N418), CD62L (MEL-14), B220 (RA3-6B2), NK1.1 (PK136), Gr-1 (RB6-8C5) F4/80 (BM8) and TER-119 were purchased from eBioscience (USA). Anti-CD146 (ME-9F1) was purchased from BioLegend (USA). Alexa Fluor 488 conjugated anti-mouse IgG was purchased from Invitrogen (USA). Anti-mouse CD16/32 used for Fc receptor blocking was produced in our laboratory.
Exogenous cytokines, IL-6 (20 ng/ml), TGF-β1 (5 ng/ml) and IFN-γ (100 ng/ml), were from R&D systems (USA). MHC-II restricted OVA peptides (OVA323-339) recognized by OT-II and DO11.10 TCR transgenic T cells were synthesized by Abgent Inc. (USA) and used at 1 μM. All-trans retinoic acid (RA) was purchased from Sigma (USA) and used at 100 nM. RA receptor antagonist LE540 was purchased from WAKO (Japan) and used at 1 μM concentration.
Preparation and purification of HSC and LSEC
Mouse livers were perfused with Ca2+ and Mg2+-free Hank's balanced salt solution and EGTA for 5 min followed by 0.4 mg/ml pronase (Roche; Germany) in HBSS and 0.5 mg/ml collagenase (Roche) in HBSS respectively for 5 min each. Perfused livers were further digested by shaking in a 0.5 mg/ml pronase/collagenase solution with HBSS for 20 min at 37 °C. Digested cells were passed through a 70 μm cell strainer and washed with HBSS. At this stage, cells were determined non-parenchymal cells. For HSC enrichment, non-parenchymal cells were resuspended in 11% Optiprep (Axis-Shield; Scotland) and separated by gradient centrifugation. Next, for HSC and LSEC purification, cells were stained with anti-CD45 and CD146 antibody, and sorted with a FACS-Aria II cell sorter (BD Biosciences) as CD45 negative and autofluorescence positive for HSC and as CD45 negative, CD146 positive and autofluoresent for the LSEC. For some experiments, sorted HSC were cultured for 7 days in RPMI medium with or without IFN-γ.
Naïve T cells, NKT cells and DC isolation
For naïve T cell isolation, spleens were removed, teased into single cell suspensions and filtered through a 70 μm cell strainer. Cells were incubated with a mix of specific biotin-conjugated antibodies including anti-CD8, CD11b, CD11c, CD25, B220, NK1.1 and Ter119 followed by anti-biotin magnetic micro beads (Miltenyi Biotec, Germany) and MACS column purification. Enriched cells were then stained with anti-CD4, CD44, CD25 and TCRvβ5 (for OTII TCR) and CD4+TCR+CD25-CD44low naive T cells were sorted by FACS-Aria II. For NKT cell isolation, cells were similarly stained with anti-CD8, CD11b, CD19, CD24, CD62L, B220, F4/80, Gr-1 and Ter119 antibodies and negatively enriched with EasySpe system (StemCell Technologies, USA) following manufacture's instraction. Enriched cells were then stained with anti-TCRβ and NK1.1 and TCR+NK1.1+ NKT cells were sorted by FACS-Aria II. For DC isolation, spleens were chopped and digested by 1 mg/ml collagenase (Sigma) for 20 min. Digested tissues were mashed through 70μm cell strainer. Subsequently, CD11c positive cells were enriched by positive selection using anti-CD11c micro beads (Miltenyi Biotec). Enriched populations were stained with anti-CD11c and I-A/I-E antibody, and sorted by FACS Aria II as CD11c+MHC II+ cells. In some experiments sorted T cells were labeled with CFSE (Sigma; USA).
Microscopic analysis of HSC
For immunofluorescence staining, sorted HSC was cultured in chamber slide for 2 days. Cells were fixed and permeabilized with a fixation/permeabilization reagent (BD) for 15 min. After washing with permeabilization buffer, cells were stained with anti-desmin or anti-GFAP antibody for 20 min followed by anti-mouse IgG Alexa Fluor 488 for 20 min. For phase contrast microscopic analysis, cells were fixed with 4% formaldehyde for 10 min. Cells were mounted with ProLong Gold antifade reagent with DAPI (Invitorgen) and analyzed with fluorescence microscopy (ZEISS).
In vitro culture
Sorted OVA-specific OT-II TCR transgenic T cells (1 × 105/well) were cultured with DC, HSC or cultured HSC (2 × 104/well) in the presence of OVA peptide in 96-well plates for 4 days. According to the experimental conditions, IL-6, TGF-β1, RA and LE540 were added in the concentration as described above. For the HSC co-culture experiments, HSC or pre-cultured HSC (5 × 103) were added to the culture medium. To enrich HSC, HSC were cultured for 24 hr to remove non-adherent cells, and T cells and DC were cultured with adherent HSC for 4 days in the same condition as described above.
FACS analysis of liver cells and cultured T cells
For surface cell analysis of HSC and DC, cells were stained with blocking anti-body followed by anti-CD45 (for HSC) or anti-CD11c and CD40, CD80, CD86 or MHC II anti-body. For intracellular staining with desmin or GFAP, cells were fixed and permeabilized with Fixation/Permeabilization reagent and stained with anti-desmin or GFAP followed by anti-mouse IgG Alexa488. Cultured T cells were stained for Foxp3 analysis or cytokine analysis. For Foxp3 staining, CD4+ T cells were stained intracellular using a Foxp3 staining kit (eBioscience; USA). For cytokine staining, T cells were further cultured with 50 ng/ml PMA (Sigma) and 1 μg/ml ionomycin (Sigma) and 10 μg/ml Brefeldin A (Sigma) for 4 hr at 37o C. Intracellular IL-17 and IFN-γ were then stained with BD Cytofix/Cytoperm Fixation/Permeabilization kit (BD Bioscience) according to the manufacturer's instruction. To detect cells displaying RALDH activity, cells were stained with ALDEFLUOR staining kit (StemCell Technologies) following manufacturer's protocol. Stained cells were analyzed with FACS LSR II or FACS Canto II.
RNA isolation and real-time qPCR
Sorted HSC, LSEC or DC were washed with PBS and re-suspended in TRIZol (Invitrogen, USA). The samples were frozen and kept at -80 °C for later utilization. For RNA isolation, the suspended cells were homogenized and RNA was separated from DNA and proteins by precipitation with chloroform and extraction with isopropanol. cDNA was synthesized from the total RNA using the Superscript II system (Bio-Rad Laboratories, USA) following the manufacturer's instruction. Subsequently, cDNA was subjected to real-time PCR using SYBR green II (Roche) and the following primers: alcohol dehydrogenase (ADH)1 forward; 5’-AGACGGCATGAGCACTGCG-3’, ADH1 reverse; 5’-GATCGTCTGAGCGGCAGACACC-3’, ADH4 forward; 5’-GGCTGATGGCACTACCAGAT-3, ADH4 reverse; 5’-GGGTGACCTTGGCAGTTTTA-3’, ADH7 forward; 5’-GCTGTCCTATGGGGAGTGAA-3’, ADH7 reverse; 5’-CACTCTCCACAACCCCAACT-3’, RALDH1 forward; 5’- ATGGTTTAGCAGCAGGACTCTTC-3’, retinaldehyde dehydrogenase (RALDH)1 reverse; 5’-CCAGACATCTTGAATCCACCGAA-3’, RALDH2 forward; 5’-GACTTGTAGCAGCTGTCTTCACT-3’, RALDH2 reverse; 5’-TCACCCATTTCTCTCCCATTTCC-3’, RALDH3 forward; 5’-GGACAGTCTGGATCAACTGCTAC-3’, RALDH3 reverse; 5’-TCAGGGGTTCTTCTCCTCGAGT-3’, desmin forward; 5’-GAGGTTGTCAGCGAGGCTAC-3’, desmin reverse; 5’-CTTCAGGAGGCAGTGAGGAC-3’, GFAP forward; 5’-GCCACCAGTAACATGCAAGA-3’, GFAP reverse; 5’-CGGCGATAGTCGTTAGCTTC-3’, L32 forward; 5’-GAAACTGGCGGAAACCCA-3’, L32 reverse; 5’-GGATCTGGCCCTTGAACCTT-3’. Gene expression was normalized by L32.
Results
Identification and purification of HSC
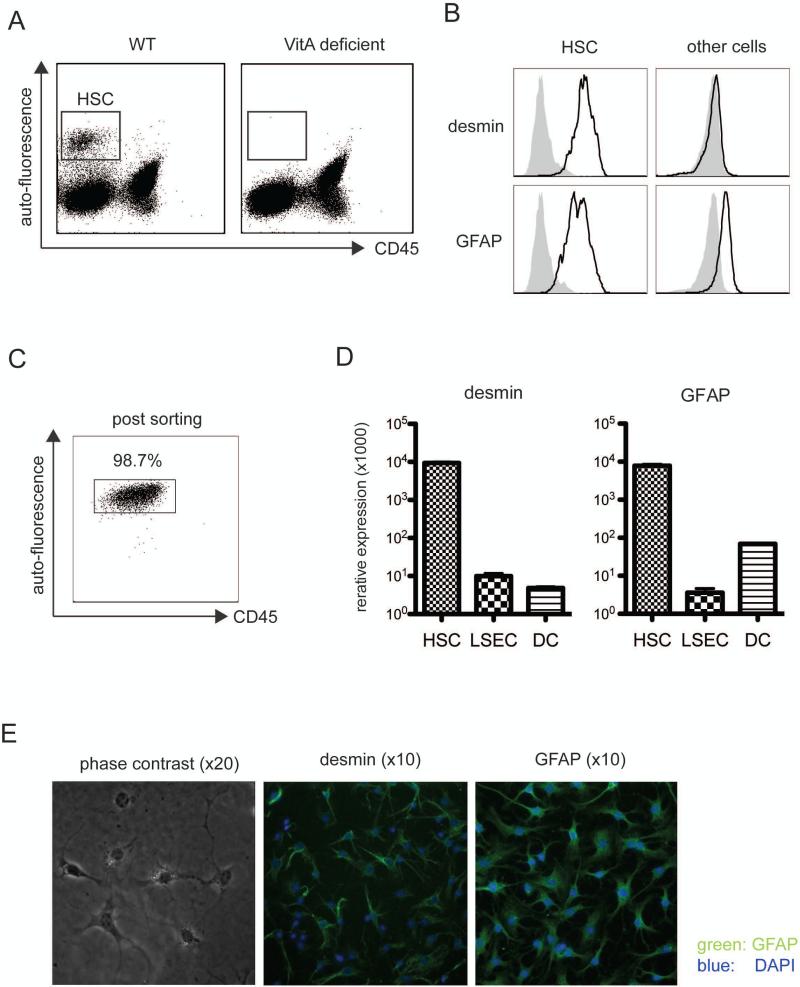
Previous reports showed that liver HSC have the ability to stimulate naïve T cells. However in those cases, the experiments are performed using enriched but not purified cell populations, which might still contain a significant number of cells other than HSC. Therefore, in order to evaluate the specific role of HSC in the differentiation of iTreg, we first established conditions to highly purify the subset of HSC using FACS cell sorting. Although there are no reported HSC specific surface markers, a CD45 negative but VitA-mediated auto-fluorescent positive cell subset (34, 35) was readily detectable among the liver non-parenchymal cells of wild type (WT) but not VitA deficient (VAD) animals (Fig. 1a). These CD45 negative, autofluorescence positive cells were then stained with anti-desmin and -GFAP antibody, both established intracellular markers for HSC (Fig. 1b). Consistent with this, CD45- but autofluorescent+-sorted cells expressed more than 1000 fold higher desmin and 100 fold higher GFAP mRNA compared to non-fluorescent counterparts (Fig. 1c) and therefore the sorted subset contained more than 98% pure HSC (Fig. 1d). Purified HSC were cultured for 2 days and analyzed for morphology using phase-contrast- and fluorescence-microscopy. HSC displayed a characteristic star like shape with oil droplets and they stained positive for desmin and GFAP (Fig. 1e). These results indicate that a combination of the presence of VitA mediated autofluorescence together with the absence of the cell surface marker CD45, are specific determinants to mark and FACS purify the specific HSC subset derived from liver nonparenchymal cells.
Figure 1. Detection and purification of HSC.
a. Liver non-parenchymal cells form WT or VitA deficient (VAD) mice were enriched by gradient separation, stained with anti-CD45, CD146 and analyzed by FACS. VitA-derived autofluorescence was detected with the pacific blue channel. b. In addition to above staining, cells were further stained for intracellular desmin and GFAP and analyzed by FACS. Cells were gated on auto-fluorescence positive, CD45 negative or auto-fluorescence negative populations. c. Auto-fluorescence positive, CD45 negative cells were sorted and re-analyzed by FACS. d. mRNA was extracted from sorted LSEC, spleen DC or cells as in 1c. GFAP and desmin mRNA expression was measured by qPCR. e. Sorted auto-fluorescence positive, CD45 negative cells (from BALB/c) were fixed and intracellularly stained for anti-GFAP or anti-desmin antibody, mounted with DAPI and analyzed by phase contrast or fluorescence microscopy. All experiments were repeated at least twice.
HSC do not express APC signature surface molecules
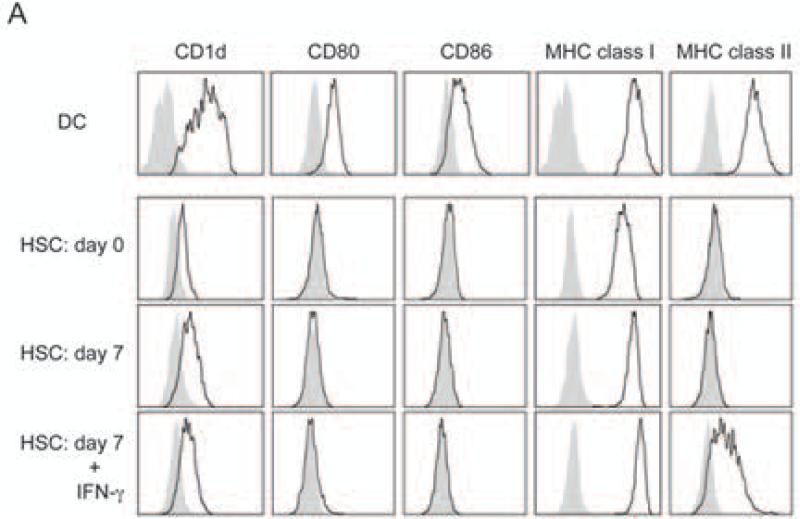
To characterize if highly purified HSC express a phenotype characteristic of APC, we analyzed for the expression of MHC class I and class II molecules as well as CD1d and typical co-stimulatory molecules, including CD80 and CD86 (Fig. 2). In contrast to DC, freshly isolated purified HSC did neither express MHC class II nor CD80 or CD86 co-stimulatory molecules. Previous reports showed that upon in vitro culture in the presence of IFN-γ, HSC did induce these molecules (36, 37) and although we could confirm that culturing in the presence of IFN-γ induced some MHC class II expression, we were unable to detect any CD80 or CD86 expression on cultured highly purified HSC (Fig. 2). These data therefore indicate that highly purified HSC do not express a typical phenotype, characteristic of professional APC.
Figure 2. Phenotypic analyses of HSC.
Spleen DC and HSC were stained with CD1d, CD80, CD86, MHC class I and MHC class II anti-bodies. Cells were analyzed by FACS. For the analysis of cultured HSC, HSC was sorted by FACS and cultured for 7 days with or without IFN-γ before staining. Black line shows stained cells and gray solid shows isotype control staining. All data are representative of at least two experiments.
HSC do not function as APC
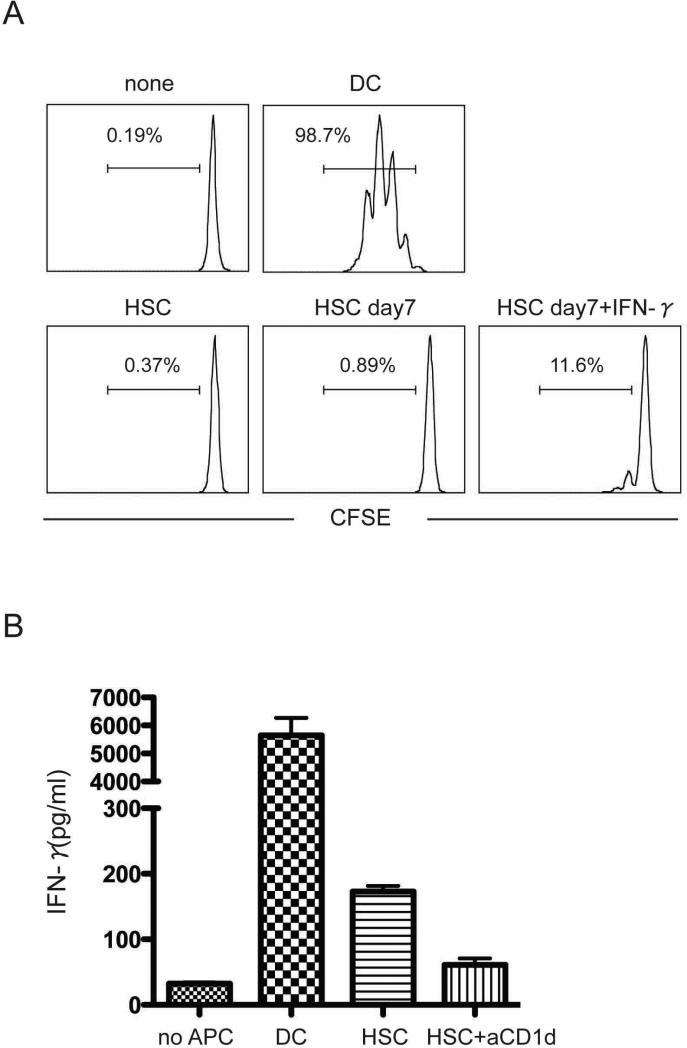
To determine whether HSC can function as professional APC, we cultured purified HSC or spleen DC with CFSE labeled naïve OT-II T cells and measured T cell proliferation in response to antigen-stimulation. Consistent with the absence of MHC class II and co-stimulatory molecules, no OT-II T cell proliferation was observed in response to OVA peptide and freshly isolated highly purified HSC or 7 day cultured HSC (Fig. 3a). IFN-γ activated HSC induced some T cell proliferation, but it was much weaker than that induced by spleen DC. These data confirm that HSC do not function as professional APC in vitro. Because freshly isolated and purified HSC do express significant amounts of CD1d and since the liver is the organ where NKT cells mainly reside, we examined whether HSC could present α-GalCer to CD1-restricted NKT cells in vitro. Purified NKT cells were cultured with freshly isolated HSC and α-GalCer for 1 day and IFN-γ production in supernatant was measured with ELISA. In contrast to the MHC class II restricted OT-II CD4 T cells, NKT cells stimulated by HSC did produce IFN-γ, which was inhibited by anti-CD1d blocking antibody (Fig. 3b). However the amount of IFN-γ produced by HSC stimulated NK T cells was still significantly less as compared to DC stimulated NK T cells, indicating that HSC can present antigen to CD1-restricted NKT cells but they function far less efficient as compared to professional APC.
Figure 3. HSC are not professional APC.
a. Naïve OT-II CD4 T cells were sorted and labeled with CFSE. T cells (1 × 105/well) were cultured with 2 × 104 purified DC, HSC or activated HSC (cultured for 7 days with or without IFN-γ) from WT mice for 4 days in the presence of OVA peptide. T cell division was analyzed with FACS. Cells with more than one division were gated on. b. NKT cells were sorted from spleen as TCR and NK1.1 positive cells. Sorted NKT cells (1 × 104) were cultured with HSC or DC (1 × 104) in round bottom plates for 16-20 hours in the presence of 50 ng/ml α-GalCer. To block CD1d engagement, anti-CD1d antibody (2 μg/ml) was added to the culture. After culture, IFN-γ concentration of cell culture supernatant was measured with ELISA. Data show mean and SD of triplicate cultures. All data are representative of three independent experiments yielding similar results.
HSC function as bystanders to enhance iTreg- but inhibit Th17-differentiation.
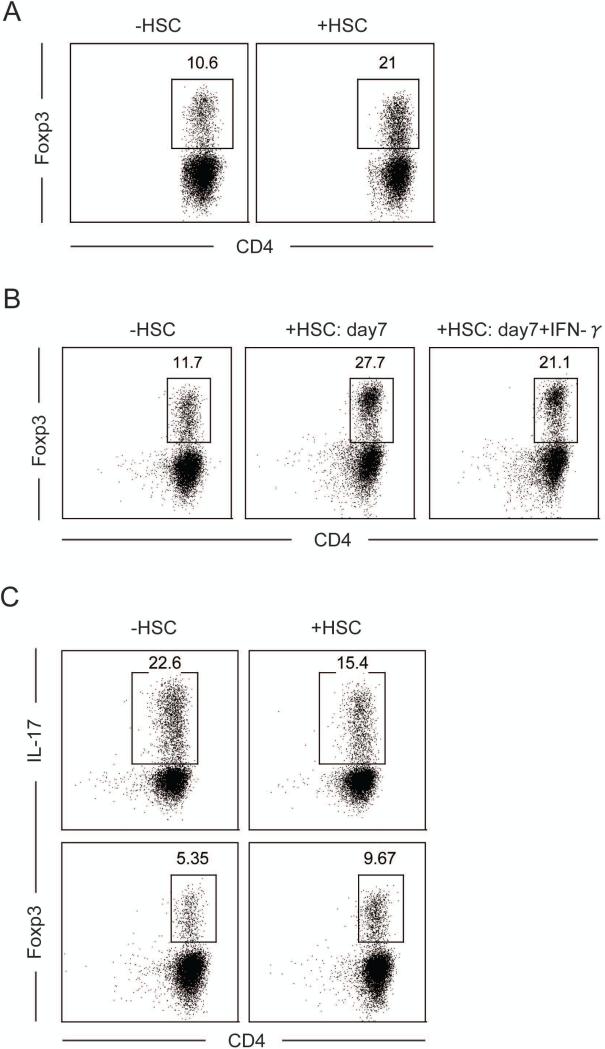
The absence of MHC class II expression and the inability of HSC to activate naïve CD4 T cells render it very unlikely that HSC directly control the priming of CD4 T cells or the generation of iTreg. However, because HSC reside in the space of Disse, in close proximity of liver resident APC, it is possible that HSC can indirectly regulate T cell responses as bystander cells. To determine whether HSC can cooperatively modulate the activation of T cells primed by professional APC, we co-cultured naïve OT-II T cells together with OVA peptide, DC and fleshly isolated and sort-purified HSC. Interestingly, freshly isolated HSC in the presence of DC and exogenous TGF-β, greatly enhanced the generation of Foxp3+T cells from 10.6% to 21% (Fig. 4a). Similarly, cultured HSC with or without IFN-γ also enhanced iTreg differentiation of DC-primed CD4 T cells (Fig. 4b). In contrast, HSC in the presence of DC and TGF-β + IL-6, reduced Th17 differentiation from 22.6% to 15.4%, but still enhanced iTreg differentiation from 5.35% to 9.67% (Fig. 4c). These results suggest that, although HSC do not efficiently present antigens, they do exhibit regulatory bystander functions for both, the generation of TGF-β-dependent iTreg as well as the inhibition of the inflammatory Th17 cells differentiation.
Figure 4. HSC co-operatively enhance Foxp3 and inhibit Th17 differentiation.
a. Purified naïve OT-II T cells and sorted DC were cultured for 4 days with or without freshly purified HSC (5 × 103) and stimulated with TGF-β. After the culture, cells were stained with Foxp3 and analyzed by FACS. b. Same as in 4a except purified HSC were also cultured for 7 days with or without IFN-γ. c. Same as in 4a but cells were stimulated with IL-6 and TGF-β. After culture, cells were stained with Foxp3 or re-stimulated with PMA/ionomycin and brefeldin for 4 hr. Re-stimulated cells were stained for intracellular IL-17 and IFN-γ and analyzed with FACS. Data are representative of more then three independent experiments yielding similar results.
HSC promote iTreg induction and inhibit Th17 differentiation in an RA-dependent fashion
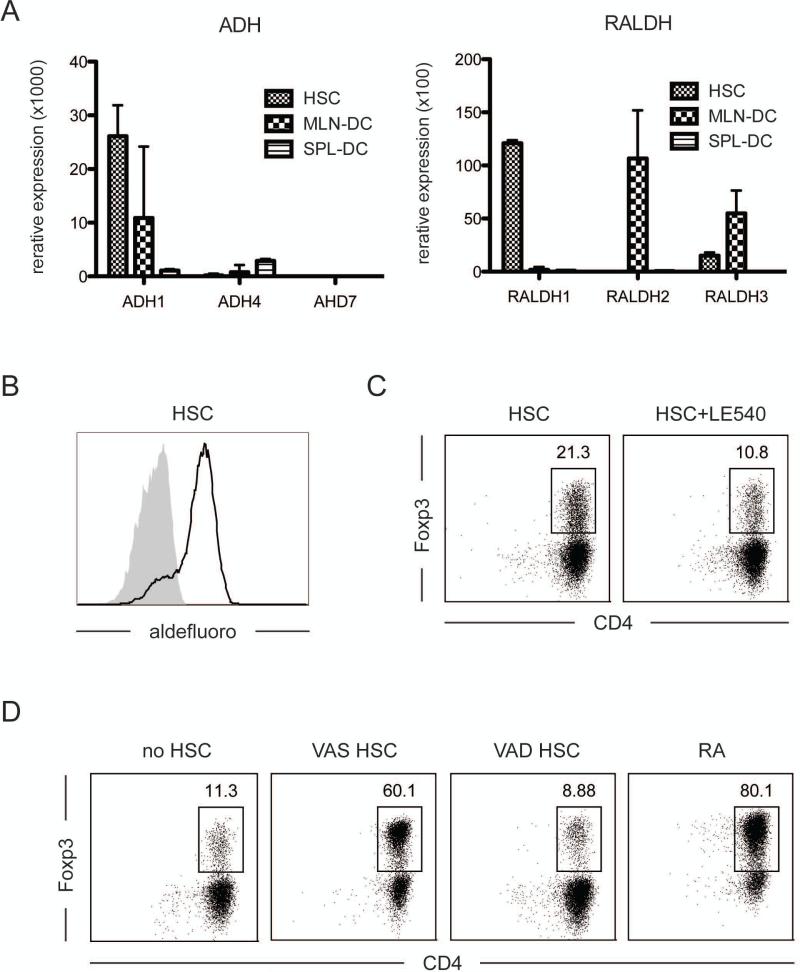
The reciprocal effects on TGF-β-dependent differentiation of activated T cells are identical to those described for RA (25). To investigate whether purified HSC serve their bystander role in an RA-mediated fashion, we measured mRNA expression of ADH1, 4 and 7, which are enzymes involved in the metabolism of VitA to retinol and RALDH1, 2 and 3, which metabolize retinol to RA (38). HSC expressed great amounts of ADH1 and RALDH1 mRNA (Fig. 5a). However, in contrast to MLN DC, which are also known to release RA and drive the iTreg differentiation and which express high levels of RALDH2 (38), HSC expressed RALDH1. The activity of the enzyme to metabolize RA was further confirmed with the ALDEFLUORO assay, which forms a substrate and exhibits fluorescence in response to enzyme activity (Fig. 5b).
Figure 5. HSC function in an RA-dependent manner.
a. mRNA was extracted from HSC, spleen DC (for negative control) and MLN-DC (for positive control) and expression of ADH1, 4 ,7 and RALDH1, 2, 3 was measured by qPCR. Gene expression was normalized for the housekeeping gene, L32. b. Liver non-parenchymal cells were stained with ALDEFLUORO followed by CD45 antibody staining. The enzyme activity in auto-fluorescence positive- and CD45-negative cells was detected by FACS. c. Naïve CD4 T cells were cultured with sorted DC and HSC as in figure 4, and in the presence of TGF-β and LE540. Foxp3 expression of cultured T cells was analyzed by FACS. d. HSC from VitA deficient (VAD) or sufficient diet (VAS) fed mice was enriched by density separation. Those cells were further cultured with OT-II T cells, OVA peptide and DC in the presence of TGF-β as described above. RA was used as positive control. Shown are representative data of at least two experiments with similar results.
To determine if the HSC mediated increase of Foxp3 induction was RA dependent, we cultured naïve OT-II T cells with DC and purified HSC in the presence of the retinoic acid receptor antagonist, LE540 (27, 28). T cells co-cultured with DC and HSC in the presence of LE540 did not show the increased iTreg differentiation, indicating that the HSC bystander effect was RA-dependent (Fig. 5c). This was also confirmed using HSC from VitA-deficient (VAD) mice. Since VAD-derived HSC do not display auto-fluorescent, gradient-enriched HSC were used instead. Nevertheless, consistent with the absence of RA, enriched HSC from VAD mice failed to enhance Foxp3 induction on DC primed T cells (Fig. 5d). These data showed that the reciprocal effects of HSC on TGF-β-dependent T cell differentiation is mediated by their ability to release RA. Overall, the results show that the HSC do not function as regulatory APC, but that instead, they harbor the unique ability to function as regulatory bystanders, which are able to control the reciprocal TGF-β-dependent differentiation of primed T cells in an RA-mediated fashion.
Discussion
Although the tolerogenic nature of the liver is well recognized, very little is known about the liver associated Treg induction, which is readily observed in liver allograft transplantations, hepatitis virus infections or portal venous-induced tolerance (9-13). In this study, we aimed to elucidate the underlying mechanisms of liver Treg induction and accumulation. Based on the recently described reciprocal effects of RA that greatly promote TGF-β-driven iTreg generation and the unique ability of HSC to store and metabolize VitA, we reasoned that HSC could be the driving force for the enriched iTreg differentiation in the liver.
The density separation method widely used before to enrich HSC for the study of their phenotype and function has led to inconclusive and at times controversial data. To circumvent this problem, we established a new method to sort-purify HSC. We determined here that liver cells that are positive for VitA specific auto-fluorescence but negative for the cell surface molecule CD45 also display the typical HSC morphology as well as the intracellular expression of desmin and GFAP (Fig. 1 a, b and d). Therefore, these data indicate that the presence of autofluorescence together with the absence of CD45 staining is dependable markers to allow for FACS sort purification of HSC (Fig. 1c). Furthermore, the absence of CD45 expression also indicates, in contrast to published data obtained with enriched HSC (39, 40), that HSC are of non-hematopoietic origin.
Using sort-purified HSC, we show that HSC do not display an APC phenotype or function and when analyzed ex vivo they do not express detectable levels of MHC class II or costimulatory molecules and they fail to prime naive T cells in vitro (Fig. 2 and 3a). The discrepancy of our findings with those of previously published studies, underscores the importance of the degree of purity of the cells that are studied and our study here challenges previously made conclusions and demonstrates, using sort- purified cells, that HSC display neither an APC phenotype nor APC functions.
On the other hand, freshly isolated HSC do express CD1 and IFN-γ activated HSC do induce some MHC class II expression which leads to the in vitro activation of NKT cells and conventional CD4 T cells, respectively, however the observed activation is significantly weaker compared to that of DC (Fig. 3B).
Nevertheless, although HSC function poorly as APC to activate CD4 T cells and therefore they are likely not directly involved in the biased generation of liver iTreg, our results here are the first to define a unique bystander role for HSC that enables them to greatly enhance iTreg differentiation of T cells primed by professional APC (Fig. 4 a-c). These data suggest an indirect role for HSC to drive the immune suppressive and tolerogenic nature of the liver. In this context it was shown indeed that co-transplanted HSC effectively protect islet allografts rejection (41).
The reciprocal effect of HSC bystander regulation to enhance TGF-β-dependent iTreg generation but inhibit TGF-β-dependent Th17 differentiation is identical to the recently defined RA-mediated immune regulation (25). This, together with the notion that HSC form the main storage place for VitA and the fact that purified HSC show high RDH1 and RALDH1 expression and are able to metabolize RA (Fig. 5a and b), provide strong support to suggest that the regulatory bystander function of HSC is mediated via the release of RA during priming of T cells by professional APC. We directly confirmed this possibility by using either RAR inhibitor or VAD HSC and showed under such conditions that the regulatory bystander function of HSC was completely abolished (Fig. 5c and d).
Taken together, we show here that in contrast to published data, HSC do not directly stimulate T cells. However, in proximity of professional APC, HSC have the potential to cooperatively modulate T cell responses. Therefore, HCS function as regulatory bystanders and they may contribute to the suppressive and tolerogenic features of the liver.
Acknowledgements
We would like to thank Drs. Ekihiro Seki and Keiko Iwaisako for providing expertise and help with the isolation and separation of liver stellate cells.
This work was funded by the NIH R01-AI050265 (HC and SI).
Abbreviations
- ADH
alcohol dehydrogenase
- α-GalCer
α-galactosylceramide
- DC
dendritic cells
- Foxp3
forkhead box P3
- HSC
hepatic stellate cells
- iTreg
induced Treg
- iNKT
invariant natural killer T
- LSEC
liver sinusoidal endothelial cells
- Treg
regulatory T cells
- RA
retinoic acid
- RALDH
retinaldehyde dehydrogenase
- RA
retinoic acid
- VitA
vitamin A
- VAD
VitA deficient
- VAS
VitA sufficient
Footnotes
This is manuscript 1353 of the La Jolla Institute for Allergy and Immunology.
References
- 1.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 3.Garnier H, Clot JP, Bertrand M, Camplez P, Kunlin A, Gorin JP, Le Goaziou F, Levy R, Cordier G. [Liver transplantation in the pig: surgical approach]. C R Acad Sci Hebd Seances Acad Sci D. 1965;260:5621–5623. [PubMed] [Google Scholar]
- 4.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu S, Nakafusa Y, Flye MW. Portal vein administration of donor cells promotes peripheral allospecific hyporesponsiveness and graft tolerance. Surgery. 1994;116:229–234. discussion 234-225. [PubMed] [Google Scholar]
- 6.Shoukry NH, Cawthon AG, Walker CM. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu Rev Microbiol. 2004;58:391–424. doi: 10.1146/annurev.micro.58.030603.123836. [DOI] [PubMed] [Google Scholar]
- 7.Thimme R, Lohmann V, Weber F. A target on the move: innate and adaptive immune escape strategies of hepatitis C virus. Antiviral Res. 2006;69:129–141. doi: 10.1016/j.antiviral.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 9.He F, Chen Z, Xu S, Cai M, Wu M, Li H, Chen X. Increased CD4+CD25+Foxp3+ regulatory T cells in tolerance induced by portal venous injection. Surgery. 2009;145:663–674. doi: 10.1016/j.surg.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 11.Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, Banham AH, Klenerman P. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316–324. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Carper K, Zheng XX, Kuhr CS, Reyes JD, Liang Y, Perkins DL, Thomson AW, Perkins JD. The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. 2006;38:3205–3206. doi: 10.1016/j.transproceed.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, Perkins JD. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8:1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 14.Onoe T, Ohdan H, Tokita D, Shishida M, Tanaka Y, Hara H, Zhou W, Ishiyama K, Mitsuta H, Ide K, Asahara T. Liver sinusoidal endothelial cells tolerize T cells across MHC barriers in mice. J Immunol. 2005;175:139–146. doi: 10.4049/jimmunol.175.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 16.Limmer A, Ohl J, Wingender G, Berg M, Jungerkes F, Schumak B, Djandji D, Scholz K, Klevenz A, Hegenbarth S, Momburg F, Hammerling GJ, Arnold B, Knolle PA. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 17.Roland CR, Walp L, Stack RM, Flye MW. Outcome of Kupffer cell antigen presentation to a cloned murine Th1 lymphocyte depends on the inducibility of nitric oxide synthase by IFN-gamma. J Immunol. 1994;153:5453–5464. [PubMed] [Google Scholar]
- 18.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 19.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 26.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 30.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limmer A, Knolle PA. Liver sinusoidal endothelial cells: a new type of organ-resident antigen-presenting cell. Arch Immunol Ther Exp (Warsz) 2001;49(Suppl 1):S7–11. [PubMed] [Google Scholar]
- 33.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, Curbishley SM, Aspinall AI, von Andrian UH, Adams DH. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa T, Tateno C, Asahina K, Fujii H, Kawada N, Obara M, Yoshizato K. Identification of vitamin A-free cells in a stellate cell-enriched fraction of normal rat liver as myofibroblasts. Histochem Cell Biol. 2007;127:161–174. doi: 10.1007/s00418-006-0237-7. [DOI] [PubMed] [Google Scholar]
- 35.Kubota H, Yao HL, Reid LM. Identification and characterization of vitamin A-storing cells in fetal liver: implications for functional importance of hepatic stellate cells in liver development and hematopoiesis. Stem Cells. 2007;25:2339–2349. doi: 10.1634/stemcells.2006-0316. [DOI] [PubMed] [Google Scholar]
- 36.Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, Enrich C, Nicolas JM, Ercilla G, Gallart T, Vives J, Arroyo V, Rodes J. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 37.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 38.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Baba S, Fujii H, Hirose T, Yasuchika K, Azuma H, Hoppo T, Naito M, Machimoto T, Ikai I. Commitment of bone marrow cells to hepatic stellate cells in mouse. J Hepatol. 2004;40:255–260. doi: 10.1016/j.jhep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L, Qian S. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]