Abstract
In the past decade, a “default mode network” (DMN) has been highlighted in neuroimaging studies as a set of brain regions showing increased activity in task-free state compared to cognitively demanding task, and synchronized activity at rest. Changes within this network have been described in healthy aging as well as in Alzheimer's disease (AD) and populations at risk for AD, that is, amnestic Mild Cognitive Impairment (aMCI) patients and APOE-ε4 carriers. This is of particular interest in the context of early diagnosis and more generally for our understanding of the physiopathological mechanisms of AD. This paper gives an overview of the anatomical and physiological characteristics of this network as well as its relationships with cognition, before focusing on changes in the DMN over normal aging and Alzheimer's disease. While perturbations of the DMN have been consistently reported, especially within the posterior cingulate, further studies are needed to understand their clinical implication.
1. A Brief Historical and Methodological Introduction on Default Mode Network
Before the emergence of functional magnetic resonance imaging (fMRI), the most classical way to explore brain functional activity associated with different cognitive states consisted in using metabolism or perfusion Positron Emission Tomography (PET). The concept of brain resting-state network arose from observations made when comparing cerebral perfusion during cognitive processing to that measured during passive baseline conditions such as at rest, that is, when subjects lie in the dark and are instructed to think about nothing in particular. Activity decreases in a set of brain areas were then consistently reported during tasks compared to the resting-state, leading to the concept of “deactivations” [1, 2]. In other words, some brain regions appear to be more engaged during rest than during constrained cognitive activity. These observations were then reinforced by works showing greater deactivations with increasing attention-demanding processes [3–7]. In these conditions, deactivations intensity depends on attention load so that a cognitive task requiring low attention levels will induce weak deactivations, while greater deactivations will be associated with tasks requiring high attention levels.
Resting-state brain activity is mainly assessed using 18FDG-, H2O15-PET or fMRI. fMRI is a noninvasive method that utilizes changes in blood oxygen level-dependent (BOLD) signal to identify areas of increased or decreased neuronal activity [8, 9]. In addition, resting-state activity can be investigated either contrasting cognitive and baseline conditions (see above), or assessing the temporal characteristic of brain activity measured at rest with fMRI. Using this technique, resting-state activity was shown to be characterized by low-frequency synchronized oscillations in large-scale functional brain networks. Resting-state activity is also sometimes assessed while subjects are asleep or sedated (see for instance [10–13]). However, findings reported under those specific conditions have to be considered with caution since the resting-brain activity depends on consciousness levels. In case of light sleep or sedation [14] which are characterized by reduced levels of awareness and arousal, at-rest brain activity is modified so that low frequency fluctuations are attenuated compared to the more classical resting-state conditions described above.
There are two main methods that can be used to analyse data obtained from an fMRI resting-state acquisition. In the first category, Independent Component Analysis (ICA) is the most commonly used. It is an exploratory method which allows the detection of independent brain networks (components) from a same dataset without a priori. This is a data-driven method where maps of coactivated brain regions are computed according to temporal correlations in their activity. The second category mainly refers to Region of Interest (seed) based methods, where a brain region has to be selected according to a priori hypotheses. The averaged time course of the BOLD signal in this region is then extracted and correlated with the signal time-course in each voxel of the grey matter. This method is more generally used to explore the cortical functional connectivity at rest or during cognitive tasks, allowing to reveal how components of large-scale distributed neural systems are coupled together in performing specific tasks [15]. Although ICA, functional connectivity and deactivation methods probably give slightly different findings, all are used to study resting-state brain activity without theoretical distinction. Consequently, results will be presented disregarding of the method in what follows. However, results obtained when comparing brain activity at rest versus during a cognitive task will be referred to as “deactivations” while DMN “activity” or “connectivity” will refer to analyses conducted from resting-state scans.
Using ICA and seed-based methods, multiple spatially distributed large-scale functional brain networks have been described and termed as resting-state networks. They mainly include the primary sensory, motor, language, attention and default-mode networks (DMN; see [16] for a recent review about all these networks). Regions included in these networks show a synchronized activity in absence of any specific cognitive activity, that is, at rest, while they are known to be engaged during sensory-, motor-, language- or attention-related tasks, respectively. As for the DMN, it includes brain areas associated with multiple high-order functions described below. This network is now considered as an intrinsic property of the brain, as its activity is widely shared among living beings (for works on monkeys see [17, 18]; for works on rats see [19]) and it seems to emerge in early childhood [20, 21]. As the present paper focuses on the effects of AD onto brain resting-state activity, it will refer to the DMN as it includes the regions known to be the most sensitive to the neurodegenerative processes.
2. Physiology, Anatomy, and Cognitive Role of the DMN
As mentioned above, resting-state networks in general, and the DMN in particular, are defined as sets of anatomically distant brain regions showing temporal correlations in their spontaneous fluctuations, that is, functional connectivity. The DMN mainly includes the posterior cingulate cortex (PCC)/precuneus, dorsal and ventral medial prefrontal, lateral (mainly inferior) parietal cortices, and medial temporal lobes (Figure 1(a)). It is thought to involve multiple subsystems that converge on “hubs” or nodes, such as the PCC, ventral medial prefrontal, and inferior parietal cortices. These hubs are strongly inter-connected and connected to the other regions of the DMN as well [22, 23] (Figure 1(b)). Interestingly, the functional connectivity observed between remote brain regions using resting-state fMRI is consistent with their anatomical connectivity as assessed using Diffusion Tensor Imaging. This suggests that the strength of functional connectivity within the DMN areas at least partly depends on white matter tracts, that is, on the strength of structural connectivity [24–29].
Figure 1.
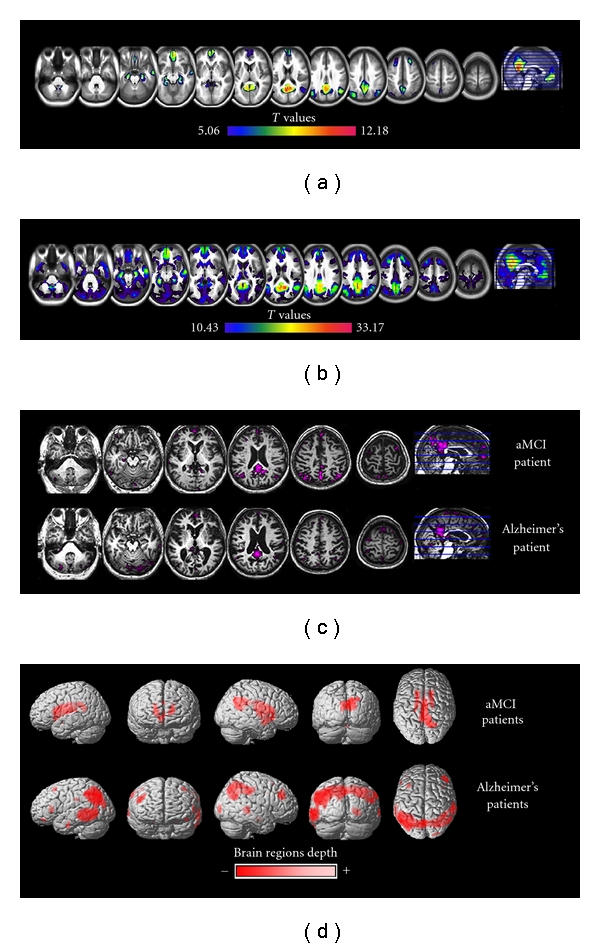
Resting-state fMRI cerebral activity in 71 healthy subjects aged from 19 to 80 years (a) Using an Independent Component Analysis, we identified the Default Mode Network (DMN) encompassing here the posterior cingulate/precuneus, anterior cingulate, orbitofrontal, ventromedial prefrontal, inferior temporal cortices, hippocampi, and angular gyri. (b) Using the posterior cingulate cortex (PCC) as a seed in a functional connectivity analysis, we identified a larger network extending to frontal, occipital, and middle temporal regions, as well as cerebellum, thalami, and motor cortices. Using this same method on (c) amnestic Mild Cognitive Impairment (aMCI) and Alzheimer's disease (AD) patients, a disruption of the connectivity between PCC and anterior then posterior brain areas was observed. (d) 18FDG-PET resting-state measures in two groups of aMCI and AD patients compared to healthy aged controls. While in the former group, hypometabolism was restricted to PCC and subcortical structures, it mainly extended to temporoparietal regions in the latter group.
Despite the growing amount of knowledge regarding the DMN physiology and anatomy, the cognitive function of this network is still poorly understood. Interestingly, the different brain regions of the DMN are known to be involved in different high-level cognitive functions. Thus, PCC activity is reported during tasks that imply autobiographical episodic memory and self-referential processes [30–35], the medial prefrontal cortex is associated with social cognitive processes [36], the medial temporal lobe is mainly engaged in episodic memory [37, 38], and the inferior parietal cortex, more particularly the angular gyrus, is implicated in semantic processing and attention [39, 40]. Two main hypotheses have been proposed regarding the cognitive role of the DMN. First, it may subtend an introspection activity, implying numerous abilities such as (i) time-travelling in the past, that is, recollection of autobiographical events [13, 34, 41] and in the future, that is, the “prospective brain” [42, 43] and the self-projection based on mental simulations [13, 34, 41], (ii) theory of mind and social cognition, for which human beings may have a genetic predisposition (see [44, 45] for meta-analyses), and (iii) mind wandering and task-unrelated thoughts [46]. First indication of an association between DMN and introspection came from studies using postscan interview to determine the nature of subjects' thoughts during the resting-state scanning [41, 47–49]. Findings all converge to the presence of inner experiences, from autobiographical memories recalling to inner speech or mental images. Reviews and meta-analyses then reinforced this “introspection hypothesis” by underlining the obvious overlap between neural networks of autobiographical memory, theory of mind, future envisioning, and the DMN [13, 45]. According to a second hypothesis, termed as the “sentinel” hypothesis, the DMN is thought to support a broad low-level focus of attention allowing to monitor the external environment for unexpected events [1, 13, 50]. Several experimental works exploring diffuse attention processes support this hypothesis. For instance, DMN activity is linked to high levels of performance on target-detection tasks where targets appear randomly at multiple possible locations. Conversely, performance is not associated with DMN activity when attention is focussed to a specific location [51]. To date, none of both hypotheses has been fully validated, leading to an open field for future investigations.
3. The DMN in Healthy Aging
Several studies have assessed the effects of normal aging on DMN activity, and they consistently reported a significant effect of age. More specifically, they showed significant reduction with age in the activity measured at rest, or weaker deactivation, within superior and middle frontal [52], PCC/precuneus [7, 52–59], middle temporal, superior parietal [7, 52, 60] and medial areas such as medial prefrontal [7, 54, 55, 58, 60, 61], anterior cingulate [53, 54, 61], and hippocampal regions [7, 60]. These disturbances may reflect a reduction in the ability to suspend DMN activity when high-order cognitive processes are required, that is, a difficulty in switching from a “default mode” to a task-related mode of brain function [54, 55]. Older subjects were also found to show greater activity at rest, or greater deactivations, mainly in anterior brain areas, for example, anterior cingulate [16, 54, 56, 58, 62], medial prefrontal, and superior frontal cortices [53]. This increased activity at rest in frontal DMN regions of elderly adults has been interpreted as a reflect of compensatory processes, that is, an attempt to compensate for the decrease of resting-state activity in posterior DMN areas ([56] see below). Thus, the Posterior Anterior Shift in Aging (PASA) model has been proposed to account for the fact that, while deactivations in occipitotemporal areas of the DMN are reduced, bilateral frontal areas deactivations increase in healthy elderly compared to young subjects [56]. However, this model does not fit with all findings, especially those reporting weak deactivations or activations (instead of deactivations) in older subjects' anterior DMN areas [7, 52–55, 58, 60, 61]. In these studies, older subjects failed in generating reinforced frontal deactivations to compensate for posterior DMN disturbances, which is not in agreement with the PASA model. To conclude, further investigations are needed to better understand healthy aging effects on DMN activity and especially to explore possible functional compensation processes in frontal areas.
There have been an increasing number of studies exploring DMN disturbances in different pathological states including schizophrenia, autism, hyperactivity disorder, epilepsy, multiple sclerosis, and Alzheimer's disease (AD) (see [63] for a review of results in all these pathologies). Studies on AD are the most numerous, which is probably due to the fact that the DMN includes two key areas in AD, that is, the posterior cingulate cortex and hippocampal formation. Indeed, the hippocampus is the region of earliest and most marked atrophy (see [64]; [65] for a meta-analysis on hippocampal atrophy), and the PCC is consistently found to be hypometabolic early in the course of the disease (see [64] for instance; [66] for a recent study; Figure 1(d)). As a consequence, the DMN has been the focus of interest in studies not only on AD patients but also in at-risk populations such as patients with amnestic Mild Cognitive Impairment (aMCI) and asymptomatic APOE-ε4 allele carriers as well.
4. The DMN in APOE-ε4 Carriers
Works aiming at studying subjects with increased risk of developing AD are still rare. However, their results are of high relevance to better understand the pathological processes early in the course of the disease. Except Koch et al. [67] who found no significant differences in the DMN activity between APOE-ε4 carriers and noncarriers, ε4 carriers were found to be characterized by significant changes in brain activity at rest. These disturbances mainly correspond to diffuse decreases in deactivations [68–70] and PCC functional connectivity disruption with the precuneus [68] but also increased functional connectivity between the whole DMN and medial, as well as middle, temporal regions [69]. Furthermore, differences were found in the effect of APOE-ε4 on DMN activity according to the age of the subjects ([71, 72]; see [73] for a review). While young ε4-carriers were characterized by higher DMN activity in retrosplenial, medial temporal, and medial-prefrontal cortices compared to young noncarriers, elderly ε4-carriers showed reduced activity compared to old noncarriers in anterior and posterior cingulate, and cerebellum. As mentioned above regarding age-related effects on DMN activity, increased BOLD signal in young ε4-carriers might also be interpreted as a putative compensatory mechanism to maintain normal cognitive performances. These findings also suggest that the ε4 allele modulates neuronal activity decades before the appearance of the clinical manifestation of the disease [71, 72]. Conversely, attenuated BOLD signal in older ε4-carriers might be attributed to effects of early pathology and especially to interactions between beta-amyloid deposition or clearance and BOLD signal [72]. In addition, disruption of white matter tracts has recently been shown in APOE-ε4 carriers, notably in the cingulum [73] which interconnects DMN areas such as the PCC and the hippocampus. Consequently, white mater disturbances might underlie APOE-ε4-induced DMN activity decreases. Altogether, amyloid plaques and/or white matter disruption could be responsible for DMN functional disturbances characterizing older APOE-ε4 carriers. Finally, according to Trachtenberg et al. [74], future works using fMRI should take into account several considerations which are of importance when investigating the effects of APOE-ε4 on brain activity, such as family history and age, as well as the inclusion of a wider range of APOE genotypes. For instance, there might be a dose-dependent effect so that ε4 homozygotes may be characterized by greater effects on brain functional activity compared to heterozygote subjects. As only two studies with divergent findings have assessed this question to date [68, 75], further investigations are needed to establish clear assertions concerning a dose effect of APOE-ε4.
5. The DMN in Alzheimer's Disease Patients
As for AD, DMN activity changes are in line with those found using FDG-PET measure of resting-state brain metabolism, highlighting the major involvement of the PCC/precuneus region (see [64, 76–78] for PET studies; see [6, 53, 79–82] for fMRI studies; Figures 1(c) and 1(d)). For instance, the functional connectivity between the PCC and the hippocampus seems to be impaired in AD (Figure 1(c)), probably as a consequence of early hippocampal structural alterations. This so-called disconnection hypothesis has received strong support from previous works combining structural MRI and PET. Thus, hippocampal atrophy seems to induce PCC functional perturbation, as well as episodic memory impairment, through disruption of the cingulum bundle [64, 77, 78]. Resting-state fMRI studies showing alterations of the temporal synchrony of PCC and hippocampus activity in patients with aMCI compared to healthy controls [83] are consistent with this hypothesis. Decreases in functional connectivity or deactivation disturbances [6] have also been reported within the PCC of aMCI patients and interpreted as the effect of local atrophy [84]. A recent study rather suggests that disconnection precedes gray matter atrophy in the PCC [66]. According to these authors, PCC atrophy would reflect a long-term effect of brain disconnection and lead to the conversion from MCI to AD (see below). Some studies also reported perturbation of resting-state activity within the hippocampus in AD compared to controls [79, 82, 85], but also in patients with aMCI suggesting that it is an early process [86]. According to Xu et al. [87], this region is characterized by a perturbation of low frequency fluctuations synchronisation. The magnitude of these asynchronies depends on pathological stages so that the mean index of hippocampal asynchrony is higher in aMCI than in controls and still higher in patients with probable AD compared to aMCI (see also [88] for similar results). Alteration of DMN activity in AD is not restricted to the PCC and hippocampal region as connectivity disruption between these structures and other brain areas have also been reported [66, 85, 89–92]. According to Gili et al. [66] and Zhang et al. [91], these disruptions seem to spread within the cortex as the disease progresses, that is, respectively, from aMCI to AD and from mild to severe AD. Consistently, Rombouts et al. [6] showed that aMCI deactivations were less marked in the precuneus and medial frontal regions, while in AD patients deactivations were restricted to medial frontal areas. Interestingly, this study [6] also indicated that the precuneus BOLD signal in both groups of patients was delayed during an episodic memory task compared to healthy aged controls. As proposed in normal aging (see above), these findings are thought to reflect a difficulty to switch from a resting-state to a task-related mode of brain function, which would mainly be due to a failure of DMN brain regions to show rapid and efficient synchronisation in their activity.
Increases in DMN activity or connectivity have also been reported in patients with aMCI or AD as compared to healthy aged controls. Thus, aMCI patients were found to be characterized by (i) increases in DMN activity located within the PCC/precuneus [93], (pre)frontal [86, 93], lateral parietal, and middle temporal cortices [84] and (ii) increases in functional connectivity between right parietal cortex and left insula [94]. Increases in AD patients were found to concern (i) DMN activity within the PCC/precuneus [93], frontal [89, 91], occipital [95], parietal, and (pre)frontal [91] cortices and (ii) DMN connectivity between left hippocampus and prefrontal dorsolateral cortex [89] or between PCC and left frontoparietal cortices [92]. Altogether, these results point to the existence of potential compensatory processes emerging in the early stages of the disease and located in several DMN areas. It is worth mentioning that cognitive reserve was found to modulate the effect of the pathology on brain function in general and on DMN activity/connectivity in particular. Cerebral or cognitive reserve relates to the capacity of the brain to cope with neuropathology so as to minimize clinical manifestations [96]. Cognitive reserve for instance was found to differentially affect deactivations in healthy elders versus in aMCI and AD patients [93]. Thus, higher cognitive reserve in healthy elderly was found to be associated with lower deactivations within the DMN and lower task-related activity, both thought to reflect increased neural efficiency. By contrast, aMCI and AD patients with high cognitive reserve showed higher activity in task-related brain areas and increased deactivations within the DMN (PCC/precuneus, anterior cingulate) compared to those with low cognitive reserve. This greater reallocation of processing resources from the DMN to brain areas directly engaged in the experimental task could reflect increased reorganization of functional compensatory resources in patients with high cognitive reserve. To sum up, higher cognitive reserve abilities allow a more-with-less mode of brain functioning in normal aging, while it compensates for pathological processes as they appear. As illustrated here for the DMN, the crucial role of cognitive reserve in age- and pathology-related brain reorganization has been widely demonstrated.
One of the main goals of studies assessing the effects of AD on the DMN is to unravel biomarkers that may be useful for the early diagnosis of the disease. The disruption of hippocampus or PCC connectivity could be a good candidate as it intensifies as the disease progresses [90, 92]. Lower deactivations within the whole DMN and especially within (medial) parietal areas [80, 97] were also found to be associated with conversion from aMCI to AD (see [98] for a review). In addition, Koch et al. [67] suggested that the use of multivariate analyses combining measures of the activity of specific DMN areas to measures of the interconnectivity between these regions improved the diagnosis accuracy. Interestingly, using this approach, the disease pattern observed in patients with AD could be identified in a high proportion of aMCI patients, suggesting that such a combination of resting-state measures may be relevant to identify AD at a predementia stage.
All these studies provided accumulating support for a preferential alteration of the DMN hubs in AD, though. However, the reason for the predominant vulnerability of these regions remains unclear. According to Buckner et al. [13, 23], cortical hubs may be preferentially affected in AD because of their continuous high baseline activity and/or associated metabolism which may induce increased vulnerability (notably to beta-amyloid deposition). This hypothesis is supported by studies showing a relationship between amyloid deposition and impaired DMN function in older people without dementia (see [99] for instance). Further multimodal investigations in at-risk subjects and AD patients are needed to better understand this intriguing overlap between the DMN and the distribution of beta-amyloid deposition in the brain.
6. Synthesis and Perspectives for the Future
In conclusion, at-rest brain activity is one of the most important investigation fields of the past decade in neuroimaging. Major advances have been made in the characterisation of the physiological and anatomical properties of resting state networks, and especially those of the DMN. Healthy aging and AD were shown to have significant and distinct effects on deactivations and DMN activity or connectivity. Changes in PCC resting-state activity or functional connectivity within the DMN for instance may be an accurate and early marker of AD. As mentioned in this paper, previous studies have consistently shown the involvement of this region in the course of the disease, even at presymptomatic or predemented stages, that is, in APOE-ε4 carriers and aMCI populations. Given its accessibility and noninvasive nature compared to metabolism PET measures, fMRI resting-state measurement of PCC activity or connectivity is potentially useful from a clinical point of view. However, further explorations are needed to disentangle the complex and heterogeneous findings, part of which being probably due to the multiple and still suboptimal methods used to explore the DMN. Moreover, several fMRI indices, such as BOLD signal amplitude and temporal derivative (see [6] for instance), may prove to provide complementary information over and above measures of synchronicity. The heterogeneity of findings also certainly reflects the complexity of the disease, as illustrated for instance by the presence of activity increases within the DMN in AD. Those increases may reflect compensatory processes, which may themselves depend on individual cognitive and brain reserve capacity. Finally, further studies are needed to assess the relative accuracy of resting-state fMRI-derived PCC measures as compared to metabolism, hippocampal atrophy, and specific episodic memory measures. It is very likely that, if DMN (PCC) activity proves to be clinically useful, it would have to be considered together with other variables such as education level, and MRI characteristics. An accurate early diagnosis will certainly be achieved only considering overall multiple-source information.
Acknowledgments
The authors are indebted to M. Fouquet, R. La Joie, A. Perrotin, and N. Villain for their participation to data acquisition. They would like to thank B. Landeau and F. Mézenge for assistance with data processing. They also thank F. Doidy, M. Laisney, and F. Viader for their critical reading of the paper. This work is supported by the ANR (Agence Nationale de la Recherche Longévité et Vieillissement, 2007), the Inserm, and Région Basse-Normandie.
References
- 1.Ghatan PH, Hsieh JC, Wirsen-Meurling A, et al. Brain activation induced by the perceptual maze test: a PET study of cognitive performance. NeuroImage. 1995;2(2):112–124. doi: 10.1006/nimg.1995.1014. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson M, Schiffer W, Joseffer S, et al. Task-specific deactivation patterns in functional magnetic resonance imaging. Magnetic Resonance Imaging. 1999;17(10):1427–1436. doi: 10.1016/s0730-725x(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 3.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 4.Newman SD, Twieg DB, Carpenter PA. Baseline conditions and subtractive logic in neuroimaging. Human Brain Mapping. 2001;14(4):228–235. doi: 10.1002/hbm.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 6.Rombouts SARB, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Human Brain Mapping. 2005;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? Journal of Cognitive Neuroscience. 2007;19(6):1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. Journal of Neuroscience. 2003;23(10):3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raichle ME, Mintun MA. Brain work and brain imaging. Annual Review of Neuroscience. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga M, Horovitz SG, van Gelderen P, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic Resonance Imaging. 2006;24(8):979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Shmueli K, van Gelderen P, de Zwart JA, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38(2):306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boly M, Phillips C, Tshibanda L, et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Annals of the New York Academy of Sciences. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 14.Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. NeuroImage. 2010;49(1):823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magnetic Resonance Imaging. 2007;25(10):1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 18.Rilling JK, Barks SK, Parr LA, et al. A comparison of resting-state brain activity in humans and chimpanzees. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17146–17151. doi: 10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Rane P, Huang W, et al. Mapping resting-state brain networks in conscious animals. Journal of Neuroscience Methods. 2010;189(2):186–196. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damaraju E, Phillips JR, Lowe JR, et al. Resting-state functional connectivity differences in premature children. Frontiers in Systems Neuroscience. 2010;4:p. 23. doi: 10.3389/fnsys.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cerebral Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasi D, Volkow ND. Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. NeuroImage. 2008;43(3):554–561. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teipel SJ, Bokde ALW, Meindl T, et al. White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage. 2010;49(3):2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 28.Van Den Heuvel M, Mandl R, Luigjes J, Pol HH. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. Journal of Neuroscience. 2008;28(43):10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17(2):1080–1086. [PubMed] [Google Scholar]
- 31.Piolino P, Giffard-Quillon G, Desgranges B, Chételat G, Baron JC, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. NeuroImage. 2004;22(3):1371–1383. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 32.D’Argembeau A, Collette F, Van Der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25(2):616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Ries ML, Schmitz TW, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. NeuroImage. 2006;29(2):485–492. doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Schneider F, Bermpohl F, Heinzel A, et al. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157(1):120–131. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 37.Milner B. The medial temporal-lobe amnesic syndrome. Psychiatric Clinics of North America. 2005;28(3):599–611. doi: 10.1016/j.psc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Viard A, Piolino P, Desgranges B, et al. Hippocampal activation for autobiographical memories over the entire lifetime in healthy aged subjects: an fMRI study. Cerebral Cortex. 2007;17(10):2453–2467. doi: 10.1093/cercor/bhl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience. 2004;7(3):217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- 41.Andreasen NC, O’Leary DS, Cizadlo T, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152(11):1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 42.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 43.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 44.Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the "default system" of the brain. Consciousness and Cognition. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 46.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 48.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delamillieure P, Doucet G, Mazoyer B, et al. The resting state questionnaire: an introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Research Bulletin. 2010;81(6):565–573. doi: 10.1016/j.brainresbull.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Shulman GL, Corbetta M, Fiez JA, et al. Searching for activations that generalize over tasks. Human Brain Mapping. 1997;5(4):317–322. doi: 10.1002/(SICI)1097-0193(1997)5:4<317::AID-HBM19>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 51.Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cerebral Cortex. 2007;17(7):1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Damoiseaux JS, Beckmann CF, Arigita EJS, et al. Reduced resting-state brain activity in the "default network" in normal aging. Cerebral Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 53.Lustig C, Snyder AZ, Bhakta M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(2):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- 55.Grady CL, Protzner AB, Kovacevic N, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral Cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Qué PASA? the posterior-anterior shift in aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller SL, Celone K, DePeau K, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambataro F, Murty VP, Callicott JH, et al. Age-related alterations in default mode network: impact on working memory performance. Neurobiology of Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch W, Teipel S, Mueller S, et al. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? NeuroImage. 2010;51(1):280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esposito F, Aragri A, Pesaresi I, et al. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magnetic Resonance Imaging. 2008;26(7):905–913. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 62.Gould RL, Brown RG, Owen AM, Bullmore ET, Howard RJ. Task-induced deactivations during successful paired associates learning: an effect of age but not Alzheimer’s disease. NeuroImage. 2006;31(2):818–831. doi: 10.1016/j.neuroimage.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 63.Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magnetic Resonance Materials in Physics, Biology and Medicine. 2010;23(5-6):409–421. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- 64.Chételat G, Desgranges B, De la Sayette V, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126(9):1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- 65.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 66.Gili T, Cercignani M, Serra L, et al. Regional brain atrophy and functional disconnection across Alzheimer's disease evolution. Journal of Neurology, Neurosurgery and Psychiatry. 2011;82(1):58–66. doi: 10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- 67.Koch W, Teipel S, Mueller S, et al. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. doi: 10.1016/j.neurobiolaging.2010.04.013. Neurobiology of Aging. In press. [DOI] [PubMed] [Google Scholar]
- 68.Persson J, Lind J, Larsson A, et al. Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia. 2008;46(6):1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 69.Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. NeuroImage. 2009;47(4):1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pihlajamäki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals. Behavioural Neurology. 2009;21(1-2):77–91. doi: 10.3233/BEN-2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filippini N, Ebmeier KP, MacIntosh BJ, et al. Differential effects of the APOE genotype on brain function across the lifespan. NeuroImage. 2011;54(1):602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Heise V, Filippini N, Ebmeier KP, Mackay CE. The APOE varepsilon4 allele modulates brain white matter integrity in healthy adults. doi: 10.1038/mp.2010.90. Molecular Psychiatry. In press. [DOI] [PubMed] [Google Scholar]
- 74.Trachtenberg AJ, Filippini N, Mackay CE. The effects of APOE-ε4 on the BOLD response. doi: 10.1016/j.neurobiolaging.2010.03.009. Neurobiology of Aging. In press. [DOI] [PubMed] [Google Scholar]
- 75.Lind J, Persson J, Ingvar M, et al. Reduced functional brain activity response in cognitively intact apolipoprotein E ε4 carriers. Brain. 2006;129(5):1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- 76.Chételat G, Desgranges B, Landeau B, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer’s disease. Brain. 2008;131(1):60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- 77.Villain N, Desgranges B, Viader F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. Journal of Neuroscience. 2008;28(24):6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Villain N, Fouquet M, Baron J-C, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer's disease. Brain. 2010;133(11):3301–3314. doi: 10.1093/brain/awq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. Journal of Neuroscience. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rombouts SARB, Damoiseaux JS, Goekoop R, et al. Model-free group analysis shows altered BOLD FMRI networks in dementia. Human Brain Mapping. 2009;30(1):256–266. doi: 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(5):1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sorg C, Riedl V, Mühlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bai F, Zhang Z, Yu H, et al. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neuroscience Letters. 2008;438(1):111–115. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 85.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Computational Biology. 2008;4(6):11 pages. doi: 10.1371/journal.pcbi.1000100. Article ID e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qi Z, Wu X, Wang Z, et al. Impairment and compensation coexist in amnestic MCI default mode network. NeuroImage. 2010;50(1):48–55. doi: 10.1016/j.neuroimage.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 87.Xu Y, Xu G, Wu G, Antuono P, Rowe DB, Li SJ. The phase shift index for marking functional asynchrony in Alzheimer’s disease patients using fMRI. Magnetic Resonance Imaging. 2008;26(3):379–392. doi: 10.1016/j.mri.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225(1):253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- 89.Wang L, Zang Y, He Y, et al. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. NeuroImage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 90.Allen G, Barnard H, McColl R, et al. Reduced hippocampal functional connectivity in Alzheimer disease. Archives of Neurology. 2007;64(10):1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- 91.Zhang HY, Wang SJ, Xing J, et al. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s disease. Behavioural Brain Research. 2009;197(1):103–108. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H-Y, Wang S-J, Liu B, et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 2010;256(2):598–606. doi: 10.1148/radiol.10091701. [DOI] [PubMed] [Google Scholar]
- 93.Bosch B, Bartrés-Faz D, Rami L, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46(4):451–461. doi: 10.1016/j.cortex.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Bai F, Liao W, Watson DR, et al. Abnormal whole-brain functional connection in amnestic mild cognitive impairment patients. Behavioural Brain Research. 2011;216(2):666–672. doi: 10.1016/j.bbr.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 95.He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. NeuroImage. 2007;35(2):488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 96.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 97.Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS ONE. 2007;2(10) doi: 10.1371/journal.pone.0001104. Article ID e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wermke M, Sorg C, Wohlschläger AM, Drzezga A. A new integrative model of cerebral activation, deactivation and default mode function in Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(1):S12–S24. doi: 10.1007/s00259-007-0698-5. [DOI] [PubMed] [Google Scholar]
- 99.Sperling RA, LaViolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


