Abstract
The phosphoinositide 3-kinase (PI3K) signaling pathway controls a wide variety of cellular processes including cell death and survival, cell migration, protein synthesis and metabolism. Aberrant PI3K-dependent signaling, mediated by Akt kinase, has been implicated in many human diseases including cancer, inflammation, cardiovascular disease and metabolic diseases, making this pathway a principle target for drug development. In this article we will summarize the PI3K signaling network and discuss current strategies for pathway inhibition. We will also explore the importance and emerging relevance of Akt-independent PI3K signaling pathways and discuss attempts being made to harness these pathways by inhibiting the binding of a product of PI3K, phosphatidylinositol-(3,4,5)-trisphosphate, to effector pleckstrin homology domains.
Overview of phosphinositide-3-kinase signaling
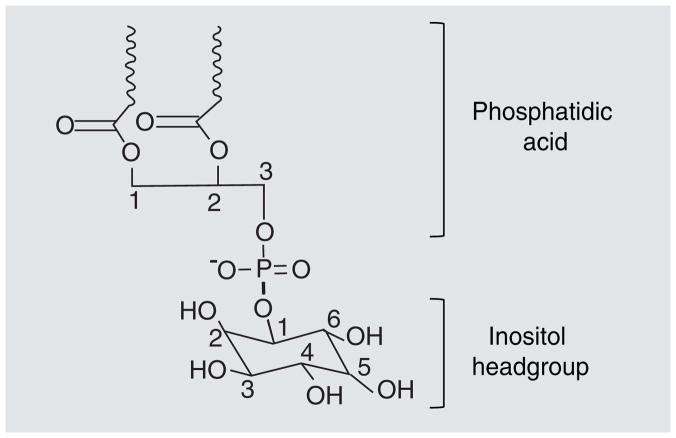
Inositol phospholipids play an important role in cellular signal transduction. Signaling down-stream from inositol phospholipids triggers a wide variety of cellular responses including growth, differentiation, death, vesicle trafficking and motility [1,2]. Association of proteins with inositol phospholipids can induce protein relocalization or conformational changes that modify protein function. In addition to alterations in the protein, the phospholipid itself may be phosphorylated or dephosphorylated as a result of the protein–phospholipid interaction [3]. Inositol phospholipids are composed of a phosphatidic acid connected to an inositol headgroup via its 1′ hydroxyl group, creating phosphatidylinositol (PtdIns) (Figure 1). PtdIns can be phosphorylated in vivo, at its 3′, 4′ and 5′ hydroxyl sites. The phosphorylation pattern around the inositol headgroup controls the cellular function of each phospholipid. Phosphorylation of these hydroxyl groups is carried out by phosphoinositide kinases [4]. These kinases transfer phosphate groups to the inositide ring position after which they are named: for example phosphoinositide 3-kinases (PI3K) phosphorylate the 3′ hydroxyl group. This article will focus on the function and downstream consequences of PI3K activation.
Figure 1. Phosphatidylinositol.
An inositol headgroup is bound to phosphatidic acid via its 1′-hydroxyl group. Phosphatidic acid is composed of a glycerol backbone with fatty acids bound to carbons-1 and -2 (represented by waves in this graphic) and a phosphate group bound to carbon-3. The 4′- and 5′-hydroxyl groups are phosphorylated in PIP2. PI3K generates PIP3 by phosphorylating the 3′-hydroxyl group.
Multiple PI3Ks are expressed in mammalian cells and this family of proteins is divided into three subclasses [1,4,5]. We will focus on class I kinases, which phosphorylate PtdIns, PtdIns-4-P and PtdIns-4-5-P2 (PIP2) in vitro, but may be specific for PIP2 in vivo. Class II kinases preferentially phosphorylate PtdIns and PtdIns-4-P, while Class III PI3Ks only phosphorylate PtdIns. Class I PI3Ks are hetero-dimers comprised of a catalytic and a regulatory subunit. Class I is further divided into Class IA and Class IB. Class IA and Class IB PI3Ks are activated, respectively, by either receptor and nonreceptor tyrosine kinases or by G-protein-coupled receptors. Three mammalian catalytic subunits (p110α, p110β and p110δ) make up Class IA. These catalytic subunits contain a N-terminal adapter binding domain, RAS-binding domain, C2 domain, helical domain and C-terminal catalytic domain. There are five regulatory/adapter subunits that can associate with class IA PI3Ks. These include p85α, p85β, p85γ and two smaller splice variants of p85α: p50α and p55α. Class IB consists of a p110γ catalytic subunit and a p101 regulatory subunit. The regulatory subunits do not possess an intrinsic enzymatic activity, but are responsible for the activation and subcellular localization of the catalytic subunits. These molecules contain rho-GTPase-activating proteins (GAPs) homology domains, src homology (SH) 2 domains, SH3 domains and proline-rich motifs.
The PI3K signaling pathway can be activated by growth-factor stimulation of various receptor tyrosine kinases, including EGF receptors, human EGF receptor 2 and IGF-1 receptors among many others [6]. Activation of these receptor tyrosine kinases leads to the formation of secondary adaptor complexes, recruitment of PI3K to the plasma membrane through the binding of the SH2 domain of p85 to the phosphotyrosine residues on adaptor proteins, and the release of the p110 catalytic subunit from inhibition by the regulatory subunit. This results in the synthesis of PtdIns-3–4–5-P3 (PIP3) by PI3K from PIP2, which is abundant in the plasma membrane of cells. PIP3 is a critical phospholipid second messenger involved in the regulation of cell growth, proliferation and survival [7]. This message is relayed through proteins that contain PIP3-binding domains such as pleckstrin homology (PH) domains [8]. PIP3 binding to PH domains affects the localization, conformation and activity of PH domain-containing proteins. The accumulation of the PH domain-containing proteins at sites of PI3K activation at the plasma membrane initiates an array of signaling cascades. Signaling by PI3K is terminated by the action of phosphatase and tensin homologue (PTEN). PTEN negatively regulates PIP3-dependent signaling by dephosphorylating PIP3 back to PIP2. In addition, phosphatases that dephosphorylate downstream effectors of PIP3 also help halt the signal [9,10].
Multiple mechanisms contribute to the regulation of cell function by PI3K effectors, so here we will only focus on some of the best characterized pathways (Figure 2). Two of the most relevant proteins that respond to PIP3 generation are Akt kinase (protein kinase B) and phosphoinositide-dependent kinase 1 (PDK1) [11,12]. Both of these proteins contain PH domains and accumulate at the membrane after PI3K activation [13]. Their localization to the plasma membrane brings these enzymes into close proximity with one another, promoting Akt phosphorylation by PDK1 [14]. Akt is a serine/threonine kinase with three family members (Akt1, Akt2 and Akt3). Akt activation occurs through its phosphorylation at Thr-308 by PDK1 at the plasma membrane [14] and phosphorylation at Ser-473 by mTOR complex (mTORC) 2, resulting in a fully catalytically active kinase [15]. Akt activation is involved in the regulation of many cellular processes including cell death and survival, cell proliferation, protein synthesis and cell metabolism [16,17].
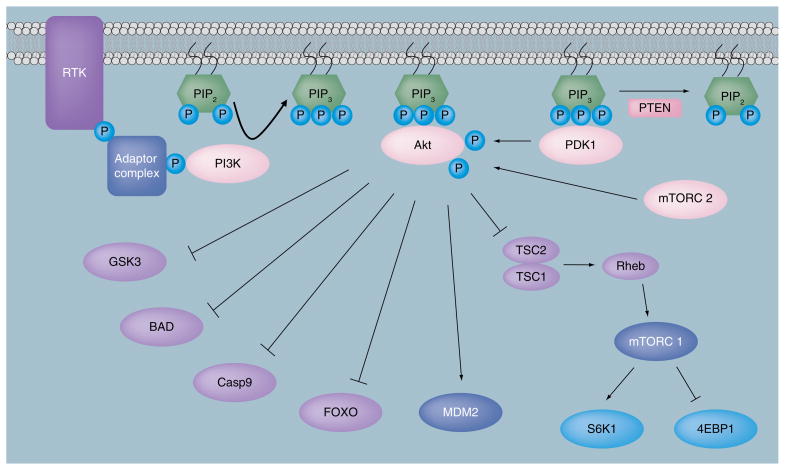
Figure 2. The phosphinositide-3-kinase/Akt signaling pathway.
RTKs, activated in response to growth factor signaling, initiate PI3K signaling. Activated PI3K phosphorylates PIP2 to generate PIP3. Akt and PDK1 then bind to PIP3 via their PH domains and are localized to the plasma membrane. Akt is activated by phosphorylation of Thr-308 by PDK1 and Ser-473 by mTORC2. Activated Akt controls cell death and survival, cell cycle regulation, regulation of protein synthesis, angiogenesis and cell metabolism through activation or inhibition phosphorylations of many downstream substrates. Signaling is terminated when enzymes, such as PTEN, dephosphorylate PIP3.
4EBP1: Eukaryotic initiation factor 4E binding protein 1; Casp9: Caspase 9; FOXO: Forkhead family of transcription factor;
GSK3: Glycogen synthase kinase 3; mTORC: Mammalian target of rapamycin (mTOR) complex; P: Phosphate;
PDK1: Phosphoinositide-dependent kinase 1; PI3K: Phosphinositide-3-kinase; PIP2: Phosphatidylinositol-4–5-P2;
PIP3: Phosphatidylinositol-3–4–5-P3; PTEN: Phosphatase and tensin homolog; Rheb: Ras homolog enriched in brain;
RTK: Receptor tyrosine kinase; S6K1: p70S6 kinase; TSC: Tuberous sclerosis complex.
Akt plays a critical role in the regulation of cell death and survival. Multiple Akt substrates mediate this effect. For example, the B-cell lymphoma (Bcl)-2 homology domain (BH3)-only pro-apoptotic Bcl-2 family member, BAD associates with the anti-apoptotic proteins Bcl-2 and Bcl-XL and causes disruption of outer mitochondrial membrane integrity and cytochrome c release. Phosphorylation of BAD by Akt releases it from the Bcl-2/Bcl-XL complex and increases cell survival [18]. Caspase-9, on the other hand, is an important part of the apoptosis signaling cascade and is inhibited by Akt phosphorylation, which, therefore, also promotes cell survival [17]. In addition, Akt activation can repress the transcription of apoptosis-related genes by phosphorylating members of the forkhead family of transcription factors (FOXOs). Upon phosphorylation, FOXOs are sequestered in the cytosol resulting in inhibition of transcription of target genes that contribute to apoptosis, such as BIM and FasL [19]. Furthermore, Mdm2 phosphorylation by Akt allows Mdm2-dependent ubquitination and degradation of p53 [20]. Finally, direct phosphorylation and inhibition of glycogen synthase kinase (GSK)-3 by Akt has also been found to contribute to increased cell survival [21].
In addition to its role in cell death and survival, Akt also regulates protein synthesis and cell growth through activation of mTORC1 and subsequent phosphorylation of ribosomal p70S6 kinase (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4EBP1). mTORC1, similarly to mTORC2, contains the proteins mTOR, mLST8 and deptor. Unlike mTORC2, mTORC1 contains raptor, a scaffolding protein that allows mTOR interaction with its substrates and proline-rich Akt substrate of 40 kDa (PRAS40), a protein that inhibits mTORC1 activity. Akt activates mTORC1 through phosphorylation and inactivation of tuberous sclerosis complex (TSC) 2, which forms a heterodimer with TSC1. TSC2 is a GAP that controls the activation of the small G-protein rheb. When TSC2 is inactivated, rheb promotes mTORC1 activation. Activated mTORC1 in turn phosphorylates and activates S6K1. S6K1 substrates include many proteins involved in translational control, including the S6 ribosomal protein. Activation of mTORC1 also leads to the phosphorylation of 4EBP1 and inhibition of its binding to eukaryotic translation initiation factor (eIF) 4E, thus allowing for formation of the translation initiation complex eIF4F. The eIF4F complex is composed of eIF4E, eIF4G and eIF4A, and initiates cap-dependent translation. In addition to TSC2 phosphorylation, Akt also promotes the activity of mTORC1 by phosphorylating and inactivating PRAS40 [22].
In addition to promoting cell survival and growth, Akt signaling promotes cellular proliferation. Phosphorylation of the cyclin-dependent kinase inhibitors, p27Kip1 and p21Cip1/Waf1, results in their translocation from the nucleus to the cytoplasm where they are unable to inhibit cell cycle progression. In addition, GSK-3β, which phosphorylates and destabilizes several proteins involved in cell cycle progression, such as cyclin D, cyclin E and the transcription factors c-Jun and c-Myc, is inactivated by Akt-mediated phosphorylation, thus increasing the stability and levels of proteins that promote cell-cycle progression [23].
Further highlighting the contribution of Akt to cell proliferation and tumorigenesis, is the important role it plays in promoting angiogenesis. Akt phosphorylates and activates endothelial nitric oxide synthase to produce nitric oxide, which, in turn, stimulates vascular remodeling and angiogenesis. Akt-dependent signaling also leads to increased expression of HIF-1α and VEGF, two molecules that also lead to increased angiogenesis [17].
Along with cell growth and proliferation, Akt signaling regulates cellular metabolism and nutrient uptake in a variety of ways. Akt2, for example, stimulates glucose uptake in cells. Its activation leads to the translocation of glucose transporter (Glut) 4 to the plasma membrane where it can mediate the uptake of glucose. The Rab-GAP AS160, a known Akt substrate, is likely to promote Glut4 vesicle translocation. Glut1, the major glucose transporter in most cells, is also influenced by Akt signaling. Its expression levels are controlled through HIF-1α-induced transcription downstream of mTORC1 activation. Furthermore, Akt stimulates glycogen synthesis by inhibiting GSK-3β and promotes glycolysis by increasing the expression of glycolytic enzymes. Finally, phosphorylation of BAD on Ser-136 by Akt promotes its further phosphorylation on Ser-112 and Ser-155. Tri-phosphorylated BAD nucleates the formation of a mitochondria-associated complex containing glucokinase and other proteins, which increases glucose-driven mitochondrial respiration and insulin secretion by pancreatic β cells [24].
Recent studies have suggested that distinct cellular functions of the three Akt isoforms add significant complexity to the PI3K signaling network (reviewed in [25]). For example, Akt1 has been found to promote tumorigenesis by inhibiting apoptosis, but at the same time to inhibit cancer cell invasion and metastasis through regulation of microRNAs and the Akt1-specific substrate, paladin [26–28]. In a study designed to address the function of each Akt isoform in transgenic polyoma middle T mice models and ErbB2/Neu-driven mammary adenocarcinomas in mice, each isoform was found to have a different effect. Depletion of Akt1 inhibited and depletion of Akt2 promoted tumor induction and growth, while ablation of Akt3 did not have a significant effect [29].
In addition to Akt, other PIP3-binding targets also possess multiple important functions downstream from PI3K activation. The PIP3 effector network is likely to be comprised of at least 40 proteins, although the physiologic role of many of these proteins binding to PIP3 and their specificity towards PIP3 in many cases still remains to be established [30]. PDK1, for example, has been found to posses important Akt-independent functions, including regulation of cancer cell survival [31]. Multiple guanidine exchange factors (GEFs) and GAPs, which regulate members of Rho and Arf GTPase families, contain PH domains and, not surprisingly, PI3K signaling is well established to be an important regulator of actin cytoskeleton remodeling and cell motility both at the level of GTPase activity and at the level of actin polymerization regulated by Arp2/3 complexes [32–34].
Inhibition of the PI3K pathway
Aberrant PI3K signaling causes a wide variety of diseases. These include cancer, chronic inflammation and allergies, cardiovascular disease and metabolic diseases among others [35–37]. In particular, there is abundant information implicating the PI3K pathway in cancer. Activating point mutations in PI3K, loss of PTEN activity and activating mutations as well as amplifications of Akt have all been observed in cancer [38]. Activating mutations in the p110α gene, PIK3CA, including those that inhibit p110α interaction with the p85 regulatory subunit and those that allow increased access to the plasma membrane, have been identified [38,39]. Mutations that result in the inactivation of PTEN lead to an increase in cellular PIP3 levels and are very frequently observed in some forms of tumors, such as glioblastomas. In addition, a specific AKT1 mutation (E17K) that allows Akt to be constitutively localized to the plasma membrane and activated has also been described [38,40].
In light of the deregulation of the PI3K pathway in various cancers and other diseases, inhibitors of this pathway have been the subjects of intense research. Several classes of inhibitors have been developed and are currently in clinical trials. These include PI3K inhibitors, Akt inhibitors, mTOR inhibitors and dual PI3K-mTOR inhibitors. It is important to note that PI3K signaling is relevant to many human diseases, including inflammation, neurodegenerative conditions and cancer, as discussed in detail, for example, by Marone et al. [41]. However, anticancer agents have been the main focus of drug discovery targeting PI3K signaling to date. This, therefore, will be the main subject of our discussion.
PI3K inhibitors
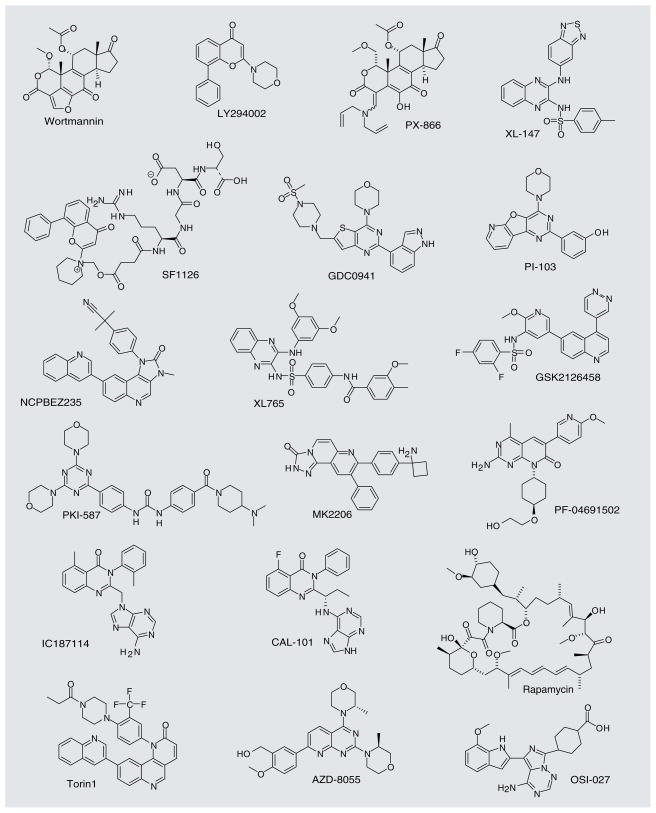
Wortmannin was the first PI3K inhibitor developed (structures of wortmannin and selected other molecules discussed in this section are shown in Figure 3). In 1987, this compound was found to have an inhibitory effect on respiratory burst in neutrophils, and it was eventually shown that its mechanism of action was through irreversible inhibition of PI3K. Wortmannin is a nonspecific inhibitor that cross-reacts with other PI3K-related proteins such as mTOR, DNA-PKCs, ATM, ATR and PI4K. It is also unstable and toxic in animals [42,43]. The first synthetic inhibitor of PI3K, LY294002, was made by Eli Lilly through the analysis of several chromone-containing compounds that resembled a nonspecific PI3K inhibitor, the flavonoid quercetin [44]. LY294002 is less potent than wortmannin and is a reversible inhibitor, but it is more chemically stable. LY294002 has proven to be a very useful research tool to delineate PI3K signaling in the cells, but its poor pharmacological properties, such as limited stability, have precluded clinical development of this molecule [42,45].
Figure 3.
Representative PI3K pathway inhibitors.
More recent PI3K inhibitors were designed with the hope of improving the pharmacologic properties of wortmannin and LY294002. A structural analog of wortmannin, PX-866 (Oncothyreon), also an irreversible inhibitor of PI3K, shows decreased toxicity and is orally bioavailable. It showed broad activity in tumor xenograft models and a good safety profile in Phase I clinical trials [42,43]. One LY294002-like compound that has been developed is SF1126 (Semaphore Pharmaceuticals), which was derived by conjugation of LY294002 to an arginine–glycine–aspartic acid integrin-targeting peptide in order to achieve better pharmacological properties and improve tumor targeting [45]. Similarly to LY294002, this molecule is a pan-PI3K inhibitor that also targets a subset of other PI3K-related kinases, including mTOR and DNA-PK. This molecule demonstrates greater aqueous solubility relative to LY294002, and has been reported to display favorable properties in the ongoing Phase I clinical trials [42].
Additional classes of inhibitors that are chemically unrelated to wortmannin and LY294002 and more highly selective for Class I PI3K have also been reported. For example, the orally bio-available Class I inhibitor XL-147 is being developed by Exelixis. This molecule, which lacks activity against mTOR, showed robust PI3K inhibition at well-tolerated doses and has been advanced to Phase II clinical trials [46].
Dual PI3K-mTOR inhibitors
Many of the PI3K inhibitors that are under development are actually active against several proteins that are structurally related to PI3K, including mTOR [41]. The development of dual-specific inhibitors that act against multiple p110 isoforms and also inhibit mTOR activity may confer a therapeutic advantage [47]. On one hand, both growth factors and nutrient signaling regulate mTOR; therefore, selective PI3K inhibitors alone do not completely abolish mTOR activity. On the other hand, inhibition of mTOR leads to the augmentation of PI3K signaling through S6K1-dependent negative feedback [48,49]. Thus, concomitant inhibition of both PI3K and mTOR may be able to more completely block PI3K signaling.
PI-103 is a chemically distinct PI3K inhibitor that also inhibits mTOR kinase activity in mTORC1 and mTORC2 [50,51] (structures of PI-103 and selected other molecules, discussed in this section, are shown in Figure 3). Analysis of a panel of PI3K isoform-selective PI3K inhibitors, including the p110α-selective inhibitor PI-103, revealed that even though many of these molecules effectively blocked Akt activation, only PI-103 potently inhibited proliferation of glioblastoma cells [52]. This potent effect of PI-103 was linked to the ability of this molecule not only to inhibit PI3K, but also mTOR. This led to the development of multiple classes of dual PI3K-mTOR inhibitors. PI-103 itself, despite its potent activity, is rapidly metabolized and has a low solubility. Analogs of this compound, such as GDC-0941 (Genentech), display better pharmacokinetic properties. GDC-0941 is more selective towards PI3K than mTOR and is currently in Phase I clinical trials [53,54].
There are several other examples of dual PI3K-mTOR inhibitors in clinical trials. These include the orally bioavailable pan-PI3K-mTOR inhibitor NVP-BEZ235 (Novartis), which showed potent activity in multiple human xenograft models [55–57], GDC-0980 (Genentech), XL-765 (Exelixis), GSK2126458 (GlaxoSmithKline) [58], PKI-587/PF-05212384 (Wyeth) [59] and PF-04691502 (Pfizer) [60]. All of these molecules are currently in Phase I clinical trials.
Isoform-specific PI3K inhibitors
Another strategy for the inhibition of the PI3K pathway is to design compounds that act only on one specific PI3K isoform. This strategy is significant given that the targeting of a specific PI3K isoform may help minimize the toxic side effects of general pathway inhibition. While isoform-specific activities of the various PI3K catalytic subunits are becoming better understood, the complexity of their regulation is also becoming more obvious [5,42]. Each p110 isoform plays a unique role in cellular physiology. On the one hand, some initial efforts focused on specific inhibition of p110α and p110β, which are frequently overactivated in particular forms of cancer. However, p110α and p110β are ubiquitously expressed and homozygous deletion of either subunit is embryonic lethal in mice [61,62]. On the other hand, p110δ and p110γ may play more restricted physiologic roles. These subunits are primarily expressed in the immune system and may play a more significant role in lymphocyte signal transduction, although p110γ may also participate in the regulation of cardiomyocyte function [36]. Furthermore, a comparison of p110γ knockout and p110γ catalytically inactive knock-in mice showed that, although both mice had an impaired immune function, only the knockout mouse displayed a cardiac defect, suggesting a kinase activity independent function for p110γ in cardiac development [47]. A recent study screened and evaluated the isoform selectivity of a panel of PI3K inhibitors [63]. The authors screened a panel of 11 classes of PI3K inhibitors and evaluated their ability to inhibit 15 PI3K family members. The p110δ inhibitors, PIK-23, PIK-39 and IC87114, were among the most selective inhibitors analyzed. Using these isoform-specific inhibitors, the authors found that p110α is vital for insulin signaling. The PI3K isoform most successfully specifically targeted to date is the p110δ subunit [41]. IC87114 (Figure 3), discovered through high-throughput screening of a diverse chemical library by ICOS Corporation [64], was found to be a 0.5-μM inhibitor of PI3Kδ, whereas the IC50 values against PI3Kα, PI3Kβ and PI3Kγ were all equal to or greater than 29 μM [64]. The authors used this molecule to show the role of PI3Kδ in the directional chemotaxis in neutrophils. In addition, this molecule was found to be effective in inhibiting acute myeloid leukemia cell proliferation and survival [65]. IC87114-related compounds, PIK-39, PIK-239 and PIK-294 (ICOS Corporation), are also being explored [41]. CAL-101 (Calistoga Pharmaceuticals; Figure 3) is also a p110δ isoform-specific inhibitor that is showing promising results in Phase I clinical trials [42,66].
In addition to targeting specific PI3K isoforms, molecules downstream of PI3K have also been targeted. It is thought that this approach will reduce toxicity associated with general inhibition of PI3K signaling, while still targeting critical downstream events that are hyperactivated in cancer cells.
Akt inhibitors
Multiple studies have pursued the development of Akt kinase inhibitors (reviewed in [38,67–69]). The development of selective ATP competitive inhibitors of Akt has proven particularly challenging due to the high degree of similarity of its ATP binding pocket to that of other AGC kinases. For example, GSK690693 inhibits all three isoforms of Akt and some related AGC family kinases [70]. Several interesting examples of allosteric Akt inhibitors have been reported. A high-throughput screen identified an Akt inhibitor, AKTi-1/2 (commercially known as Akt VIII), which displays selectivity towards Akt1 and Akt2 relative to Akt3 [71]. Differences in the primary sequence of the kinase domains of the Akt isoforms has been proposed to explain the selectivity of this inhibitor. A recently published crystal structure revealed that the binding site for Akt inhibitor VIII is on the interface of the interacting PH and kinase domains, locking Akt1 into the inactive conformation in which both PIP3 binding via the PH domain and ATP binding by the kinase domain are inhibited [72]. MK-2206 (Merck; Figure 3) is another allosteric inhibitor that targets all three Akt isoforms (displaying ~fivefold lower activity towards Akt3). MK-2206 displayed a pronounced synergistic effect with several chemotherapeutic agents in xenograft studies [73] and is currently in Phase I clinical trials [38,73]. Another pan class I PI3K inhibitor, BKM120 (Novartis) [74], was recently reported and is in clinical trials. The detailed properties of this molecule have not yet been published.
mTOR inhibitors
Rapamycin (Wyeth) is an mTORC1 inhibitor that interacts with FKBP12 and binds specifically to mTOR (structures of rapamycin and selected other molecules, discussed in this section, are shown in Figure 3). Rapamycin analogs (rapalogs) include temsirolimus (Wyeth) and everolimus (Novartis). The US FDA has approved these mTORC1 inhibitors for the treatment of renal cell carcinoma [75,76]. In addition to mTORC1, mTOR is also a key component of mTORC2. Rapalogs display efficacy against some forms of cancer; however, their clinical development against many other forms of human tumors has not been successful [75].
While mTORC1 primarily acts to regulate translation through p70S6K and 4EBP1 pathways, rapamycin-resistant mTORC2 regulates survival through phosphorylation of Akt on Ser-473 and other AGC kinases. Since mTORC1 mediates only a subset of mTOR-dependent functions and because even some of the mTORC1-dependent pathways, for example 4EBP1 phosphorylation, are relatively resistant to rapamycin, significant effort has been made to target the kinase activity of mTOR [77,78]. Small molecules that inhibit the kinase activity of mTOR present in both mTORC1 and mTORC2 are expected to be better inhibitors of the PI3K signaling pathway. A low-nanomolar ATP-competitive mTOR kinase inhibitor, Torin1, has recently been described and was shown to efficiently inhibit both mTOR complexes, resulting in promising activity in U87MG glioblastoma xenografts [79]. Several other potent orally bioavailable ATP-competitive selective mTOR inhibitors have been described, including AZD-8055 (AstraZeneca) [80] and OSI-027 (OSI Pharmaceuticals) [81], which are in Phase I–II clinical trials [38].
Akt-independent PI3K signaling & inhibition
As described previously, although PI3K pathway inhibitors are still in relatively early stages of clinical development, they are showing promising initial results as anticancer agents. It is also important to consider, however, that PI3K signaling is not limited to the regulation of the Akt and mTOR. Additional PI3K-dependent signaling branches most likely also contribute to disease, and exploiting these pathways could lead to alternative means of combating cancer as well as to a better understanding of PI3K signaling mechanisms.
Owing to the understanding of the role of PDK1 in PI3K signaling, a significant number of PDK1 inhibitors have been developed (reviewed in detail in [82]). Unfortunately, many of these molecules, for example, UNC-1, displayed very limited selectivity towards the PDK1 ATP binding pocket. However, an allosteric mechanism that contributes to PDK1 activity towards its substrates may present a new direction for the development of truly potent and selective PDK1 inhibitors. PDK1 contains a substrate-docking site (termed PIF) proximal to the ATP binding site, which binds to the phosphorylated hydrophobic motifs of target kinases. PIF/hydrophobic motif binding is required for increased catalytic activity of PDK1 towards multiple substrates. Discovery of this mechanism opens up the possibility for developing new classes of selective allosteric PDK1 inhibitors and several recent studies established principle feasibility of this approach [83,84].
As mentioned above, the roles of PI3K and PDK1 in tumorigenesis are not limited to the activation of Akt. To demonstrate this point Vasudevan et al. have recently shown that not all cancers with constitutively active PIK3CA mutations rely on Akt phosphorylation or depend on Akt for growth [31]. The authors showed that some PIK3CA mutant cancers actually display low levels of Akt phosphorylation. Furthermore, they showed that these cancer cell lines also show a decrease in dependence on Akt for their tumorigenicity while maintaining dependence on PIK3CA. The decreased role of Akt was attributed to a reduction in cellular PIP3 levels, apparently caused by robust PTEN activity. Furthermore, the authors observed significant activity and expression of PDK1, which was critical for the tumorigenicity of the cells. The increased PDK1 activity could be explained by a higher affinity of the PDK1 PH domain towards PIP3 compared with that of Akt PH domain, therefore, allowing PDK1 to be activated by lower PIP3 concentrations. The authors further determined that another AGC kinase, serum/glucocorticoid-regulated kinase (SGK kinase) 3, is likely the target of the increased PDK1 activity in these Akt-independent cells [31].
These data showing differential utilization of PIP3 substrates under specific conditions, also highlight that differences in PIP3/PH domain interactions can have a significant impact on the outcomes of PI3K signaling. Therefore, a more complete understanding of PIP3/PH domain interactions may allow us to take advantage of the differential binding affinities and selectivities of various PH domains towards phosphoinositides [85].
Structures & PIP3 binding properties of PH domains
Pleckstrin homology domains consist of typically 100–120-amino acid domains and were first described in the platelet protein pleck-strin [86,87]. PH domains occur in a variety of species ranging from yeast to mammals, and are present in approximately 250 different human proteins. The structures of many PH domains have been solved by x-ray crystallography or NMR. These domains share low-sequence identities, but they all are composed of a seven-stranded β-sandwich with a C-terminal α-helix. Three loop regions within the structure, the β1/β2, β3/β4 and β6/β7 loop, are highly variable in length and sequence [88]. Although each of the many PH domains contain this common core fold, it is clear that they do not have a single cellular function [89]. One of the first suggestions that PH domains may be involved in membrane targeting came when Harlan et al. found that the N-terminal PH domain of pleckstrin could bind to PIP2 present in phospholipid vesicles in a concentration-dependent manner [90]. They also found that several other PH domains, including the C-terminal PH domain of pleckstrin and the PH domains in T-cell-specific kinase, ras-GAP and the β-adrenergic receptor kinase, were able to bind to the PIP2-containing vesicles [90]. They surmised that the functional relevance of this interaction was to localize PH domain-containing proteins to the cellular membrane where many of these proteins were known to function [90].
As previously mentioned, the PH domain family has high sequence variability and varying ability to bind phosphoinositides. One study found that only one of 33 PH domains present in the Saccharomyces cerevisiae genome was able to bind phosphoinositides with both high affinity and high specificity. The other PH domains bound phospholipids nonspecifically or weakly [91]. Approximately, only 10–20% of PH domain-containing proteins are able to specifically localize to the cell membrane in response to the selective recognition of a phosphoinositide [88]. Only a small fraction of these PH domains exhibit affinity for a specific phospholipid, including the PLCδ PH domain, which selectively binds PIP2 and the Btk and Grp1 PH domains, which selectively bind PIP3.
Much research has been conducted in order to increase our ability to accurately predict which PH domains will interact with phospholipids. One such study used a yeast-based assay to determine a consensus motif that predicts the ability of a PH domain-containing protein to interact with PIP2 and PIP3. This motif (KXn[K/R]XR), in the β1–β2 loop, contains the basic residues arginine and lysine, which interact with the negatively charged phosphates on PIP2 and PIP3 [92]. Another such study has compared the crystal structures of the PH domain of DAPP1, which binds PIP3 and PtdIns(3,4)P2 and Grp1, which only binds PIP3, in complex with Ins(1,3,4,5)P4. The comparison of these structures enabled the authors to begin to understand the structural basis for the different phosphoinositide-binding specificities of Grp1 and DAPP1. They found that, in contrast to DAPP1, the interaction of the PH domain with the 5-phosphate was critical for Grp1 to bind Ins(1,3,4,5)P4 with high affinity. This interaction difference explained the reason DAPP1 binds both PIP3 and PtdIns(3,4)P2, while Grp1 only binds PIP3 [93]. The authors assert that this information about the necessity of interaction with the 5-phosphate will allow predictions about whether a novel PH domain will specifically bind PIP3.
Despite the large number of PH domains present in the mammalian genome, only a small number appear to actually bind to PIP3. Studies to elucidate which PH domain-containing proteins change intracellular localization in a receptor activation-dependent manner have largely used green fluorescent protein (GFP) fusions and epitope tagged proteins as reporters (reviewed in [88]). Park et al. recently undertook a study to develop a model system that could be used to predict which PH domain-containing proteins are regulated by PIP3 [30]. The authors had previously shown that GFP-fused PH domains can be used as biosensors to monitor phospholipid levels in cells [94,95]. They created a library of 130 yellow fluorescent protein-conjugated PH domains and measured translocation to the plasma membrane following PDGF stimulation. The group found approximately 27 PH domains that were localized to the plasma membrane following stimulation. They then used the sequences of these domains to predict which other PH domains might respond to PDGF stimulation. Interestingly, they found that amino acids scattered across the PH domain, not just those specifically located in the PIP3-binding pocket, are important determinants of PIP3 regulation. Ultimately, the authors identified 40 PIP3-regulated PH domains.
The PH domain-containing proteins identified by Park et al. are associated with diverse cellular functions including actin cytoskeleton regulation, vesicular transport, cell size and growth. Notably, many of the PIP3-regulated PH domain-containing proteins are involved in cytoskeleton remodeling. For example, PHLDB2 is a PIP3 binding PH domain-containing protein that localizes to the plasma membrane in a PI3K-dependent fashion. It is required for cytoplasmic linker-associated protein microtubule stabilization at the cell cortex and the recruitment of filamin A, an actin-crosslinking protein, to the plasma membrane after PIP3 formation [96,97]. Myosin-X is an actin-binding myosin motor that contains three PH domains and is involved in filopodia formation and mitotic spindle formation [98]. Pleckstrin-2, PLEK2, is also involved in actin rearrangement in a PI3K-dependent manner [99]. Park et al. also found PH domain-containing proteins that are known to regulate both gene expression and the actin cytoskeleton, such as SH3BP2 an adapter protein known to induce transcription in T cells [100]. Multiple tyrosine kinases (TEC, BTK and ITK and CNKSR2) were regulated by PIP [30]. Finally, a variety of proteins involved in vesicular transport were regulated by PIP3. These included the pleckstrin homology, Sec7 and coiled-coil domain proteins, cytohesin-1, ARF nucleotide-binding site opener and Grp1, which are ARF GEFs that regulate Arf1 and Arf6-mediated vesicle trafficking [101]. All of these data underscore the tremendous diversity of PIP3 signaling functions beyond regulation of PDK1 and Akt kinases.
PH domain inhibitors
Direct inhibition of PIP3 binding to PH domains represents a valuable PI3K pathway inhibition strategy that has yet to be fully exploited. Given the importance of PI3K signaling and the diversity of PIP3 effector proteins, both an in-depth understanding of the contribution of these effector proteins to PI3K signaling and the ability to inhibit these interactions will provide multiple benefits in both the clinical and research settings. PIP3/PH domain interactions represent appealing drug targets. First, PIP3 binding is a universal signal transduction step required for all downstream PI3K targets. However, unlike direct inhibition of PI3K, antagonizing PIP3, at least in principle, provides an opportunity to achieve selectivity towards particular targets by exploiting the structural differences across individual PH domains. Therefore, this approach could provide a universal method to selectively target any downstream mediator of PI3K. Second, PIP3 binding to a PH domain is an example of a small molecule–protein interaction, which should be amendable to inhibition by heterologous small molecules. Third, PIP3 is a rare and rapidly turned-over second messenger, resulting in a highly transient signal that could be efficiently antagonized by transient addition of small-molecule inhibitors. Finally, typical affinities of PIP3/PH domain interactions are in the nanomolar to low-micromolar range, which represents a feasible target range for small molecule inhibition. Indeed, numerous of recent approaches to inhibiting the interactions between PIP3 and PH domains have been explored. Both lipid-based antagonists and nonlipid small molecule antagonists have been reported, as described below.
Lipid-based antagonists
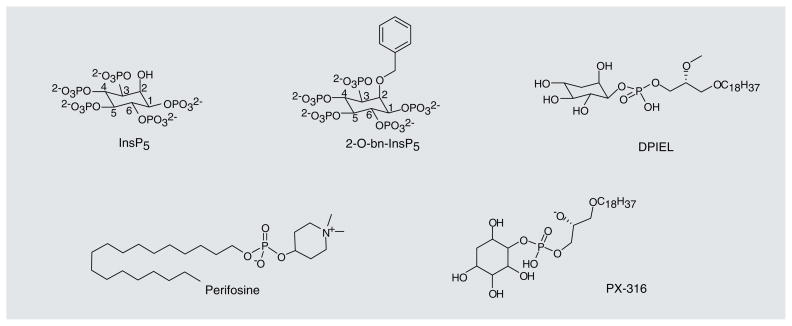
The first proposal to specifically inhibit the PIP3–PH domain interaction came from the Falasca group [102,103]. They used modified water-soluble headgroups of phosphoinositides, inositol polyphosphates (IPPs) (representative structures of the lipid-based PIP3–PH domain antagonists, described in this section, are shown in Figure 4), to compete with PIP3in PH-domain binding pockets and inhibit membrane translocation. The authors showed that, among several IPPs tested, inositol(1,3,4,5,6) pentakisphosphate (inositol[1,3,4,5,6]P5/IP5) and Ins(1,4,5,6)P4 were able to inhibit IGF-1 induced [H3]-thymidine incorporation into MCF-7 breast cancer cells and inhibit the growth of small-cell lung carcinoma cells in semisolid colony formation and liquid growth assays. Two ovarian cancer cell lines that have increased copy number of the PIK3CA gene (SKOV-3 and OVCAR-432) and one human colon carcinoma cell line that grows in a PI3K-independent manner (SW620) were also tested in the liquid growth assay. As expected, Ins(1,4,5,6) P4 was only able to inhibit the growth of the two ovarian cancer cell lines. Ins(1,4,5,6)P4 was also able to inhibit the membrane translocation of the Akt PH domain fused to GFP in response to growth factor stimulation in COS-7 cells [104]. The authors later found that inositol(1,3,4,5,6) P5 was able to induce apoptosis in SKOV-3 cells, inhibit Ser-473 phosphorylation of Akt, and inhibit Akt activity in SKOV-3 and SCLC-H69 cells [105]. In addition, inositol(1,3,4,5,6)P5 demonstrated an anti-angiogenic effect in vitro, since it was able to inhibit growth factor-induced formation of capillary tubes by human umbilical vein endothelial cells. Inositol(1,3,4,5,6)P5 also had an anti-angiogenic effect in an in vivo angiogenesis assay [106]. Finally, inositol(1,3,4,5,6)P5 was able to reduce tumor growth in nude mice after subcutaneous implantation of SKOV-3 human ovarian cancer cells [106]. As an extension of the IPP approach, this group more recently demonstrated that an optimized IP5 derivative, 2-O-bn-InsP5, shows enhanced anti-apoptotic and antitumor activity. 2-O-bn-InsP5 represents a distinct improvement, as it is able to promote apoptosis in cell lines and inhibit growth of xenografts that were resistant to inositol(1,3,4,5,6)P5. This molecule also shows increased specificity for PDK1 and may also inhibit mTOR [107].
Figure 4.
Representative lipid-based antagonists of PIP3–PH domain interactions.
Another inhibitor, the orally bioavailable alkylphospholipid perifosine (Keryx Biopharmaceuticals), has a similar structure to naturally occurring phospholipids. Perifosine was shown to inhibit the growth of PC-3 prostrate carcinoma cells that have mutated PTEN and high activation of the Akt pathway. It also inhibited Akt phosphorylation at Thr-308 and Ser-473 and the translocation of Akt to the plasma membrane. A constitutively active myr-Akt mutant was able overcome the inhibition of Akt phosphorylation and membrane translocation. Perifosine had no direct effect on the activity of PI3K, PDK1 or Akt itself [108,109]. Perifosine is currently in Phase III clinical trials to treat colorectal cancer and multiple myeloma.
Another group of lipid-modeled Akt inhibitors comprises the PtdIns ether-lipid analogs [110]. Meuillet et al. reported using D-3-deoxy-phosphatidyl-myo-inositols (DPIs), which cannot be phosphorylated at the 3-position of the inositol ring, to inhibit Akt activation. Both DPI, and the ether lipid derivative of DPI, D-3-deoxy-phosphatidyl-myo-inositol ether lipid (DPIEL) were able to bind directly to the PH domain of Akt in an in vitro binding assay. DPI and DPIEL were also able to inhibit Akt kinase activity in an in vitro assay. DPIEL displayed the highest activity in inhibiting Akt signaling and inducing apoptosis in NIH3T3 cells treated with PDGF, likely through direct binding to the Akt PH domain [110]. Several compounds related to DPIEL were produced that also inhibited activation of Akt and its substrates and were able to induce apoptosis in cancer cell lines with high Akt activity [111,112]. PX-316 was shown to be effective in preclinical xenograft models [113].
Nonlipid antagonists
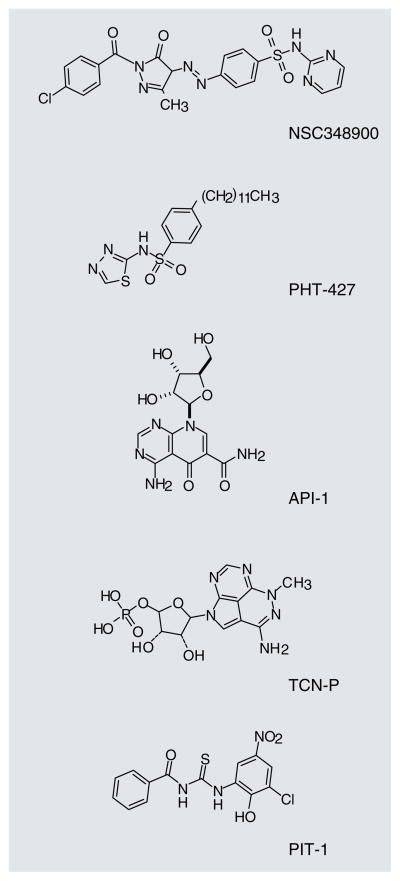
The lipid-based antagonists described previously demonstrate that molecules designed to inhibit PH domain interactions with phospholipids could indeed inhibit PI3K-dependent signaling. However, the in vivo activities of the lipid inhibitors have not been described with the exception of perifosine [109] and to a lesser extent PX-316 [113]. Nonlipid PIP3–PH domain antagonists are also being studied (Figure 5). The crystal structure of the Akt1 PH domain bound to the PIP3 inositol headgroup, myoinositol(1,3,4,5)P4, was published in 2002 [114]. Mahadevan et al. used an in silico library screen to identify small molecules that selectively bind the PH domain of Akt1 [115]. The docking of the lead compound, NSC348900, into the PH domain structure for Akt1 showed that NSC348900 interacted with the PH domain through hydrogen bonding with two arginines that were also shown to be important for a strong interaction with inositol(1,3,4,5)P4 (structures of NSC348900 and other inhibitors described in this section, are shown in Figure 5). NSC348900 and some of its analogs were tested for binding specificity towards the Akt1 PH domain relative to the PDK1 or IRS PH domains. The six compounds tested had varying binding affinities for each of the PH domains. This class of PH domain antagonists was able to inhibit Akt phos-phorylation and induce apoptosis in the HT-29 cancer cell line, but was unable to inhibit tumor growth in vivo, likely due to limited bioavailability [115]. Further in silico modeling and optimization resulted in a molecule, PHT-427. PHT-427 inhibited Akt phosphorylation through binding Akt and PDK1 PH domains, induced apoptosis, and was able to inhibit pancreatic tumor BxPC-3 xenograft growth following intraperitoneal injection [116]. It was recently shown that PHT-427 was able to inhibit the growth of human tumor xenografts in mice after oral administration [117].
Figure 5.
Representative nonlipid antagonists of PIP3–PH domain interactions.
Another small-molecule inhibitor of PH domains was recently identified in a cell-based screen designed to discover inhibitors of Akt-dependent growth. Kim et al. characterized a small-molecule inhibitor of Akt that is neither an ATP competitor nor an Akt substrate mimetic [118]. They identified API-1 as a selective Akt inhibitor that binds to the PH domain and inhibits membrane translocation. API-1 does not affect PI3K, PDK1, mTORC2, other AGC kinases or other signaling molecules. The authors went on to show that API-1 was able to inhibit the growth of two types of tumors with hyperactivated Akt (OVCAR3 and PANC-1) in mice, but it was unable to inhibit the growth of two types of tumors with lower levels of Akt activation.
Triciribine (TCN) phosphate, the active metabolite of the clinical candidate TCN, also works by binding the PH domain of Akt. This molecule was found to bind Akt PH domain with sub-micromolar affinity, and to inhibit plasma membrane translocation and Akt phosphorylation at Thr-308 and Ser-473, resulting in the inhibition of proliferation and induction of apoptosis [119]. Phase I clinical trial results for TCN have been recently published, demonstrating promising activity of the molecule in blocking Akt activity in patient tumors [120].
Recent work by our group described a class of molecules that selectively inhibits PIP3–PH domain interactions, but not PIP2–PH domain binding, called PITenins [121]. We used a high-throughput fluorescence polarization binding assay to test the ability of 50,000 compounds to inhibit the interaction between the PH domain of human Akt1 and fluorescently labeled PIP3. One molecule, PIT-1, inhibited this interaction with an IC50 value of approximately 31 μM in this in vitro assay. Furthermore, PIT-1 inhibited translocation of a GFP-fused Akt1 PH domain to the plasma membrane in response to PDGF stimulation and blocked PI3K-PDK1-Akt-dependent phosphorylation events in cellular assays, leading to the induction of metabolic stress and apoptosis. We also found that a dimethyl analog of PIT-1 (DM-PIT-1) was able to inhibit 4T1 breast cancer growth in Balb/c mice [121]. Importantly, we have found that chemical modifications of PIT-1 result in significant changes in the selectivity of the molecule towards target PH domains, resulting in more potent and selective inhibition of Akt PH domain.
Future perspective
The PI3K signaling pathway is a central regulator of many important cellular functions. PI3K signaling influences cell death and survival, cell growth, protein synthesis and vesicular trafficking. Dysregulation of the PI3K signaling pathway contributes to many human diseases including cancer. Currently, significant work is being undertaken to identify pathway inhibitors that are effective in fighting tumor growth, angiogenesis and metastasis.
In this article we have discussed multiple approaches to PI3K pathway modulation. PI3K, Akt and mTOR inhibitors are currently being tested in clinical trials. Development of isoform-specific PI3K inhibitors may permit increased selectivity and decreased toxicity over more broadly acting pathway inhibitors. In the same fashion, isoform-selective Akt kinase inhibitors will likely prove to be important research and clinical tools. Currently, there are no inhibitors that differentially target Akt1 versus Akt2. mTOR has long been recognized as a key regulator of cell growth, and several inhibitors that selectively target mTORC1 have been approved for human use. However, according to published data, these molecules display limited activity in many forms of human cancer. Recent appreciation of the complexity of mTOR signaling has motivated the development of inhibitors of the mTOR kinase activity, targeting both mTORC1 and mTORC2. Some of these molecules are now in the early stages of clinical development. Dual inhibition of PI3K and mTOR, has emerged as another promising strategy targeting both upstream PI3K-dependent steps in growth factor-dependent signaling and downstream mTORC1 and mTORC2 activity. Initial clinical testing of these pathway inhibitors has been positive. Future work will determine whether broad PI3K pathway inhibition is both safe and efficacious. Interestingly, several reports suggested that dual PI3K-mTOR pathway inhibitors act as cytostatic agents in multiple cancer types [51,55], which will require use of combination therapies to achieve tumor regression. Although it remains to be clinically verified, targeting downstream molecules that more directly regulate cell death, such as Akt or PDK1, may be more cytotoxic, for example as shown using knockdown of PDK1 in PI3K-dependent tumors [31] or by genetic deletion of all three Akt isoforms [28]. Therefore, it may represent an interesting alternative direction for developing single-agent anticancer therapies.
As we have discussed, the downstream function of much of the PIP3-dependent signaling pathway is unknown. PH-domain specific PIP3 interactions are beginning to be evaluated and it has been shown that a specific subset of PH domain-containing proteins are regulated by PIP3 formation. Targeting PIP3–PH domain binding provides an interesting tool for dissecting PIP3 signaling and for better understanding the role of different aspects of PI3K signaling in the regulation of tumorigenesis. This strategy may provide new targets for developing specific cancer therapeutics against particular components of the PI3K signaling pathway deregulated in certain tumors. Given the wealth of available data regarding the structures of different PH domains, identification of selective PIP3–PH domain antagonists is likely to be soon achieved and may present a new therapeutic direction for targeting PI3K signaling.
Executive summary.
Phosphoinositide 3-kinase (PI3K) signaling pathway regulates a diverse set of cellular proteins that play a critical role in many normal cellular functions.
Abnormal regulation of the PI3K pathway is linked to cancer and many other diseases including inflammation, allergies, cardiovascular disease and metabolic disease.
Small-molecule inhibitors are being developed to inhibit this pathway. These include PI3K inhibitors, mammalian target of rapamycin inhibitors, dual PI3K mammalian target of rapamycin inhibitors, isoform-specific PI3K inhibitors and Akt inhibitors. Many of these molecules are in clinical trials.
Akt-independent signaling is an important, yet sometimes overlooked, aspect of PI3K signaling. This signaling occurs through phosphatidylinositol-(3,4,5)-triphosphate (PIP3)/pleckstrin homology (PH) domain interactions.
Inhibition of PH domain interactions with PIP3 provides a mechanism to selectively inhibit target proteins. Both lipid and nonlipid small-molecule PIP3–PH domain inhibitors are being developed.
Key Term
- AGC kinases
Family of approximately 60 protein kinases that are related to cAMP-dependent protein kinase 1 (protein kinase [PK] A), cGMP-dependent protein kinase (PKG) and PKC. Family members include Akt, SGK and S6K
- SGK kinase
Serum and glucocorticoid kinase is a serine/threonine kinase that has three isoforms. These kinases are homologous to Akt and play a role in cell survival, growth, proliferation and migration
- Fluorescence polarization
Method for detecting molecular interactions in solution. It is based on the size-dependent emission of polarized light by a fluorophore when it is excited by polarized light
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
The authors laboratory has received funding from the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Martin TF. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- 3.Toker A. Phosphoinositides and signal transduction. Cell Mol Life Sci. 2002;59(5):761–779. doi: 10.1007/s00018-002-8465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 5.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 6.Stephens LR, Jackson TR, Hawkins PT. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993;1179(1):27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 7.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274(13):8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 8.Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol. 2010;6(7):507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19(6):223–230. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24(52):7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 11.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29(5):233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Mora A, Komander D, van Aalten DMF, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15(2):161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Currie RA, Walker KS, Gray A, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt. 3):575–583. [PMC free article] [PubMed] [Google Scholar]
- 14.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 15.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 16.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 17•.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. Comprehensive review of the Akt signaling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 19.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380(Pt. 2):297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010;17(1):93–102. doi: 10.1038/cdd.2009.68. [DOI] [PubMed] [Google Scholar]
- 21.Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3β pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem. 2008;283(22):15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285(19):14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2(4):339–345. [PubMed] [Google Scholar]
- 24.Danial NN, Walensky LD, Zhang C-Y, et al. Dual role of proapoptotic BAD in insulin secretion and βcell survival. Nat Med. 2008;14(2):144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matheny RW, Jr, Adamo ML. Current perspectives on Akt Akt-ivation and Akt-ions. Exp Biol Med (Maywood) 2009;234(11):1264–1270. doi: 10.3181/0904-MR-138. [DOI] [PubMed] [Google Scholar]
- 26.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66(8):3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 27.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38(3):333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliopoulos D, Polytarchou C, Hatziapostolou M, et al. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2(92):ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67(1):167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 30•.Park WS, Heo WD, Whalen JH, et al. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol Cell. 2008;30(3):381–392. doi: 10.1016/j.molcel.2008.04.008. Describes the development of a model system that was used to predict which pleckstrin homology (PH) domain-containing proteins are regulated by PIP3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Vasudevan KM, Barbie DA, Davies MA, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16(1):21–32. doi: 10.1016/j.ccr.2009.04.012. Demonstrates that phosphoinositide 3-kinase may promote cancer through either AKT-dependent or AKT-independent mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawes AT, Edelstein-Keshet L. Phosphoinositides and Rho proteins spatially regulate actin polymerization to initiate and maintain directed movement in a one-dimensional model of a motile cell. Biophys J. 2007;92(3):744–768. doi: 10.1529/biophysj.106.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121(5):551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533(3):190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch E, Ciraolo E, Ghigo A, Costa C. Taming the PI3K team to hold inflammation and cancer at bay. Pharmacol Ther. 2008;118(2):192–205. doi: 10.1016/j.pharmthera.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82(2):250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 37.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167(3):399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. Thorough review of the role of the phosphoinositide 3-kinase pathway in cancer and the development of therapeutic inhibitors of this pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandelker D, Gabelli SB, Schmidt-Kittler O, et al. A frequent kinase domain mutation that changes the interaction between PI3Kα and the membrane. Proc Natl Acad Sci USA. 2009;106(40):16996–17001. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 41.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784(1):159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Cleary JM, Shapiro GI. Development of phosphoinositide-3 kinase pathway inhibitors for advanced cancer. Curr Oncol Rep. 2010;12(2):87–94. doi: 10.1007/s11912-010-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3(7):763–772. [PubMed] [Google Scholar]
- 44.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269(7):5241–5248. [PubMed] [Google Scholar]
- 45.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68(1):206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 46.Calvo E, Edelman G, Baselga J, et al. A Phase I dose-escalation study of the safety, pharmacokinetics and pharmacodynamics of XL147, a novel PI3K inhibitor administered orally to patients with advanced solid tumors. Ejc Suppl. 2008;6(12):69–69. [Google Scholar]
- 47.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 48.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raynaud FI, Eccles S, Clarke PA, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67(12):5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 51.Workman P, Clarke PA, Raynaud FI, Van Montfort RLM. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;70(6):2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9(5):341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 54.Wagner AJ, Von Hoff DH, LoRusso PM, et al. A first-in-human Phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol. 2009;27(15):3501. [Google Scholar]
- 55.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 56.Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68(22):9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 58.Knight SD, Adams ND, Burgess JL, et al. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. Acs Med Chem Lett. 2010;1(1):39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkatesan AM, Dehnhardt CM, Delos Santos E, et al. Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem. 2010;53(6):2636–2645. doi: 10.1021/jm901830p. [DOI] [PubMed] [Google Scholar]
- 60.Cheng HM, Bagrodia S, Bailey S, et al. Discovery of the highly potent PI3K/mTOR dual inhibitor PF-04691502 through structure based drug design. MedChemComm. 2010;1(2):139–144. [Google Scholar]
- 61.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110β catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13(3):169–172. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- 62.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274(16):10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 63.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell. 2006;125(4):733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase δ in neutrophil directional movement. J Immunol. 2003;170(5):2647–2654. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- 65.Billottet C, Grandage VL, Gale RE, et al. A selective inhibitor of the p110δ isoform of PI 3-kinase inhibits AML cell proliferation and survival and increases the cytotoxic effects of VP16. Oncogene. 2006;25(50):6648–6659. doi: 10.1038/sj.onc.1209670. [DOI] [PubMed] [Google Scholar]
- 66.Flinn W, Byrd J, Furman R, et al. Preliminary evidence of clinical activity in a Phase I study of Cal-101, a potent selective inhibitor of the P110δ isoform of phosphatidylinositol 3-kinase, in patient with B-cell malignancies. Haematol-Hematol J. 2009;94:303–303. [Google Scholar]
- 67.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27(41):5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 68.Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther. 2007;6(8):2139–2148. doi: 10.1158/1535-7163.MCT-07-0120. [DOI] [PubMed] [Google Scholar]
- 69.LoPiccolo J, Granville CA, Gills JJ, Dennis PA. Targeting Akt in cancer therapy. Anticancer Drugs. 2007;18(8):861–874. doi: 10.1097/CAD.0b013e3280cc2c6f. [DOI] [PubMed] [Google Scholar]
- 70.Rhodes N, Heerding DA, Duckett DR, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68(7):2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 71.Lindsley CW, Zhao Z, Leister WH, et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15(3):761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Wu WI, Voegtli WC, Sturgis HL, Dizon FP, Vigers GPA, Brandhuber BJ. Crystal structure of human AKT1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS One. 2010;5(9):e12913. doi: 10.1371/journal.pone.0012913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 74.Voliva CF, Pecchi S, Burger M, et al. Biological characterization of NVP-BKM120, a novel inhibitor of phosphoinosotide 3-kinase in Phase I/II clinical trials. Presented at: The 101st American Association for Cancer Research Congress; WA, DC, USA. 20 April 2010. [Google Scholar]
- 75.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5(8):671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 76.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized Phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22(5):909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 77.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7(2):e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284(12):8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Q, Chang JW, Wang J, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl) benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53(19):7146–7155. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chresta CM, Davies BR, Hickson I, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70(1):288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 81.Carayol N, Vakana E, Sassano A, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci USA. 2010;107(28):12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peifer C, Alessi DR. Small-molecule inhibitors of PDK1. ChemMedChem. 2008;3(12):1810–1838. doi: 10.1002/cmdc.200800195. [DOI] [PubMed] [Google Scholar]
- 83.Stockman BJ, Kothe M, Kohls D, et al. Identification of allosteric PIF-pocket ligands for PDK1 using NMR-based fragment screening and 1H-15N TROSY experiments. Chem Biol Drug Des. 2009;73(2):179–188. doi: 10.1111/j.1747-0285.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 84.Bobkova EV, Weber MJ, Xu Z, et al. Discovery of PDK1 kinase inhibitors with a novel mechanism of action by ultrahigh throughput screening. J Biol Chem. 2010;285(24):18838–18846. doi: 10.1074/jbc.M109.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lemmon MA, Ferguson KM. Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem Soc Trans. 2001;29(Pt. 4):377–384. doi: 10.1042/bst0290377. [DOI] [PubMed] [Google Scholar]
- 86.Haslam RJ, Koide HB, Hemmings BA. Pleckstrin domain homology. Nature. 1993;363(6427):309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- 87.Mayer BJ, Ren R, Clark KL, Baltimore D. A putative modular domain present in diverse signaling proteins. Cell. 1993;73(4):629–630. doi: 10.1016/0092-8674(93)90244-k. [DOI] [PubMed] [Google Scholar]
- 88•.DiNitto JP, Lambright DG. Membrane and juxtamembrane targeting by PH and PTB domains. Biochim Biophys Acta. 2006;1761(8):850–867. doi: 10.1016/j.bbalip.2006.04.008. Provides a helpful overview of PH domain structures. [DOI] [PubMed] [Google Scholar]
- 89.Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans. 2004;32(Pt. 5):707–711. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 90.Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371(6493):168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 91.Yu JW, Mendrola JM, Audhya A, et al. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell. 2004;13(5):677–688. doi: 10.1016/s1097-2765(04)00083-8. [DOI] [PubMed] [Google Scholar]
- 92.Isakoff SJ, Cardozo T, Andreev J, et al. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 1998;17(18):5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferguson KM, Kavran JM, Sankaran VG, et al. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol Cell. 2000;6(2):373–384. doi: 10.1016/s1097-2765(00)00037-x. [DOI] [PubMed] [Google Scholar]
- 94.Kontos CD, Stauffer TP, Yang WP, et al. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18(7):4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8(6):343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 96.Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5β. Dev Cell. 2006;11(1):21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Takabayashi T, Xie MJ, Takeuchi S, et al. LL5β directs the translocation of filamin A and SHIP2 to sites of phosphatidylinositol 3,4,5-triphosphate (PtdIns(3,4,5)P3) accumulation, and PtdIns(3,4,5)P3 localization is mutually modified by co-recruited SHIP2. J Biol Chem. 2010;285(21):16155–16165. doi: 10.1074/jbc.M109.081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plantard L, Arjonen A, Lock JG, Nurani G, Ivaska J, Stromblad S. PtdIns(3,4,5)P3 is a regulator of myosin-X localization and filopodia formation. J Cell Sci. 2010;123(Pt. 20):3525–3534. doi: 10.1242/jcs.069609. [DOI] [PubMed] [Google Scholar]
- 99.Hamaguchi N, Ihara S, Ohdaira T, et al. Pleckstrin-2 selectively interacts with phosphatidylinositol 3-kinase lipid products and regulates actin organization and cell spreading. Biochem Biophys Res Commun. 2007;361(2):270–275. doi: 10.1016/j.bbrc.2007.06.132. [DOI] [PubMed] [Google Scholar]
- 100.Qu X, Kawauchi-Kamata K, Miah SM, Hatani T, Yamamura H, Sada K. Tyrosine phosphorylation of adaptor protein 3BP2 induces T-cell receptor-mediated activation of transcription factor. Biochemistry. 2005;44(10):3891–3898. doi: 10.1021/bi048353o. [DOI] [PubMed] [Google Scholar]
- 101.Cullen PJ, Venkateswarlu K. Potential regulation of ADP-ribosylation factor 6 signalling by phosphatidylinositol 3,4,5-trisphosphate. Biochem Soc Trans. 1999;27(4):683–689. doi: 10.1042/bst0270683. [DOI] [PubMed] [Google Scholar]
- 102.Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 2010;16(12):1410–1416. doi: 10.2174/138161210791033950. [DOI] [PubMed] [Google Scholar]
- 103.Berrie CP, Falasca M. Patterns within protein/polyphosphoinositide interactions provide specific targets for therapeutic intervention. FASEB J. 2000;14(15):2618–2622. doi: 10.1096/fj.00-0096hyp. [DOI] [PubMed] [Google Scholar]
- 104.Razzini G, Berrie CP, Vignati S, et al. Novel functional PI 3-kinase antagonists inhibit cell growth and tumorigenicity in human cancer cell lines. FASEB J. 2000;14(9):1179–1187. doi: 10.1096/fasebj.14.9.1179. [DOI] [PubMed] [Google Scholar]
- 105.Piccolo E, Vignati S, Maffucci T, et al. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway. Oncogene. 2004;23(9):1754–1765. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- 106.Maffucci T, Piccolo E, Cumashi A, et al. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res. 2005;65(18):8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
- 107.Falasca M, Chiozzotto D, Godage HY, et al. A novel inhibitor of the PI3K/Akt pathway based on the structure of inositol 1,3,4,5,6-pentakisphosphate. Br J Cancer. 2010;102(1):104–114. doi: 10.1038/sj.bjc.6605408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2(11):1093–1103. [PubMed] [Google Scholar]
- 109.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11(2):102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meuillet EJ, Mahadevan D, Vankayalapati H, et al. Specific inhibition of the Akt1 pleckstrin homology domain by D-3-deoxy-phosphatidyl-myo-inositol analogues. Mol Cancer Ther. 2003;2(4):389–399. [PubMed] [Google Scholar]
- 111.Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125(5):1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- 112.Castillo SS, Brognard J, Petukhov PA, et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64(8):2782–2792. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 113.Meuillet EJ, Ihle N, Baker AF, et al. In vivo molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. Oncol Res. 2004;14(10):513–527. doi: 10.3727/0965040042380487. [DOI] [PubMed] [Google Scholar]
- 114.Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase B/Akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12(14):1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- 115.Mahadevan D, Powis G, Mash EA, et al. Discovery of a novel class of AKT pleckstrin homology domain inhibitors. Mol Cancer Ther. 2008;7(9):2621–2632. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moses SA, Ali MA, Zuohe S, et al. In vitro and in vivo activity of novel small-molecule inhibitors targeting the pleckstrin homology domain of protein kinase B/AKT. Cancer Res. 2009;69(12):5073–5081. doi: 10.1158/0008-5472.CAN-08-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meuillet EJ, Zuohe S, Lemos R, et al. Molecular pharmacology and antitumor activity of PHT-427, a novel Akt/phosphatidylinositide-dependent protein kinase 1 pleckstrin homology domain inhibitor. Mol Cancer Ther. 2010;9(3):706–717. doi: 10.1158/1535-7163.MCT-09-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim D, Sun M, He L, et al. A small molecule inhibits Akt through direct binding to Akt and preventing Akt membrane translocation. J Biol Chem. 2010;285(11):8383–8394. doi: 10.1074/jbc.M109.094060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119•.Berndt N, Yang H, Trinczek B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17(11):1795–1804. doi: 10.1038/cdd.2010.63. Characterizes a phosphatidylinositol-(3,4,5)-trisphosphate/PH domain inhibitor that is currently in clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garrett CR, Coppola D, Wenham RM, et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9479-2. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miao B, Skidan I, Yang J, et al. Small molecule inhibition of phosphatidylinositol-3,4,5-triphosphate (PIP3) binding to pleckstrin homology domains. Proc Natl Acad Sci USA. 2010;107(46):20126–20131. doi: 10.1073/pnas.1004522107. [DOI] [PMC free article] [PubMed] [Google Scholar]