Abstract
Right from birth, the lymphatics play a crucial role in dietary functions. A majority of the lipid absorbed from the newborn’s lipid-rich diet enters the blood circulation through the lymphatic system, which transports triglyceride-loaded particles known as chylomicrons from the villi of the small intestine to the venous circulation near the heart. In light of the significance of this role, as well as the fact that lipid transport from the gut was one of the earliest discovered functions of the lymphatic vasculature, it is surprising that so little is known about how chylomicrons initially gain access to the lymphatic vessel. This review will focus on the current mechanisms thought to be important in this process and highlight important questions that need to be answered in the future.
Keywords: chylomicon, lymphatic, lipid, lymph, transcellular, paracellular
Introduction
The lymphatics have been observed to play an essential role in lipid transport for many years, as early work on the lymphatic system in the mid-1600s relied on this function to observe them, since the vessels could be easily identified from the white, lipid-rich fluid that filled them after a meal.1 However, our understanding of the molecular mechanisms that regulate the functional transport of lipid by the lymphatics is significantly behind our knowledge of other lymphatic functions such as fluid balance and lymphatic involvement in cancer metastasis. Nearly all dietary lipid is transported in chylomicrons from the gut to the blood through the lymphatic system by entering specialized lymphatic vessels, referred to as lacteals, in the villi of the intestine (Fig. 1). In doing so, the lymphatic vasculature allows postprandial lipid to be available for storage and energy throughout the body before it arrives at the liver.2 This unique property of the lymphatics has received recent attention from the pharmaceutical community, as it would be advantageous for an orally delivered drug to enter the lymphatic system utilizing similar mechanisms.3,4 However, since the mechanisms regulating uptake into lacteals remain unknown, most of the work in this area has focused on targeting the enterocyte to incorporate the drug into a chylomicron, to then be carried into the circulation.5 A more complete understanding of how chylomicrons enter into the lacteal would thus not only enhance our knowledge of the fundamental mechanisms that initiate lymphatic-lipid transport but could also provide new strategies for targeting lymphatics with orally delivered drugs or vaccines. While lymphatic involvement in lipid transport and adipose tissue has received recent attention in other reviews,6–8 this review will focus on our current understanding of how chylomicrons enter into the lacteals after secretion by enterocytes and will highlight some important areas of future research.
Figure 1.
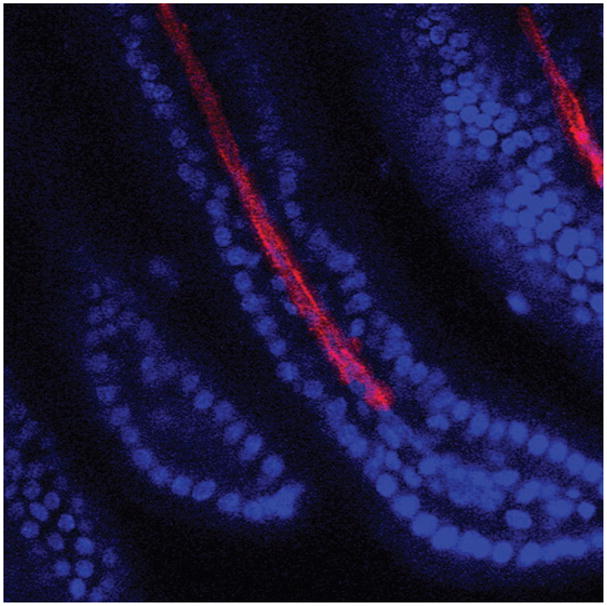
Optical slice taken on a confocal microscope of a whole-mount mouse intestine showing a villi with a lymphatic vessel running down the center. Cell nuclei are stained with DAPI (blue) and LEC are stained with LYVE-1 (red).
Lymphatic uptake
The lymphatic vasculature has been known for some time to have the capacity to take up and transport particulate that is too large to enter the blood circulation. This unique property of the lymphatics has been utilized to image the lymphatic vasculature and assess function by either injecting labeled molecules, such as albumin,9 dextran,10,11 nanoparticles,12 or other large proteins,13 or by tracking immune cells that have entered the lymphatic vessels.14–16 Recent advances in molecular imaging have shown that initial lymphatics have a unique junctional structure that enables them to efficiently take up fluid and large proteins from the interstitial spaces.17,18 In addition, early transmission electron microscopy (TEM) work has shown that lymphatic endothelial cells (LEC) of initial lymphatics have anchoring filaments that attach the vessel to the tissue space and assist in opening up the junctions to allow for fluid entry.19 While quantifying the uptake of various tracers has proved useful in understanding the size selectivity and functionality of the lymphatic vasculature in various tissues, such methods are not very helpful for studying lacteal uptake, as the tracer must first get past the barrier provided by the epithelial cells of the intestine. Recently, investigators have reported the use of an orally delivered, fluorescently labeled, fatty acid analogue, Bodipy C16, to image lymphatic uptake from the intestine.20,21 However, the extent to which such a technique can provide a quantitative measurement of lacteal function remains to be seen. Thus, our current understanding of the mechanisms involved in lymphatic uptake into the central lacteal of the villus is dependent entirely on observations made from TEM images taken of fixed, postprandial intestinal tissue sections.
Chylomicron uptake: transcellular versus paracellular
When comparing most lymphatic and gastrointestinal physiology texts that go into the details of chylomicron uptake in the lacteal, the general consensus is that paracellular transport through the opening of intercellular gaps is the primary mechanism through which chylomicrons enter the lumen.1,22,23 However, a closer look at the literature reveals a large degree of uncertainty regarding this issue. TEM images of a lacteal containing chylomicrons were first reported by Palay.24 While the focus of this work was on the internal structure of the lipid-absorbing enterocyte, the authors displayed an image of a lacteal in which chylomicrons could be seen in the lumen as well as half a dozen chylomicrons entering between two overlapping junctions. Shortly thereafter, two different papers reported numerous TEM images demonstrating potential mechanisms of chylomicron entry into the lacteal.25,26 In these works, both open junctions containing chylomicron particles, as well as numerous endothelial vesicles with chylomicrons inside them were demonstrated. In hypothesizing which mechanism of chylomicron entry is more important, Casley-Smith acknowledged that it is difficult to know for certain the extent that chylomicrons can cross the vessel wall in vesicles. In summarizing his findings he stated that, “It would seem then that it is the open junctions which give the lacteals their great permeability. Thus, it is likely that much material passes through the patent lacteal junctions rather than through the endothelial cells.” Several other reports concurred with Casley-Smith’s original findings, being unable to rule out the importance of transcytosis, but at the same time concluding that paracellular transport was probably of primary importance.27–32
However, a few years later, Dobbins challenged this idea by demonstrating numerous vesicles containing chylomicrons and showing that the majority of the junctions between cells remained tightly closed.33,34 While he had shown in earlier studies that very few junctions are open in the lacteal35 even when the vessel was extremely distended in a person with intestinal lymphangiectasia, a common characteristic of the disease,36 this was the first study showing quantitative data on the distribution of junctions and vesicles in over 500 TEM images of chylomicron-containing lacteals.34 Since only 6 of the 149 junctions identified in the lacteals of mice and guinea pigs were open (having a gap greater than 40 Å) and since vesicles occupied approximately 15% of the cytoplasmic area within the cell, Dobbins concluded that transcellular transport must be of primary importance in the uptake of chylomicrons into the lacteal. Dobbins suggested that numerous gaps between junctions demonstrated in previous studies might in fact have been artificially created because of the delicate process of creating tissue sections for TEM imaging. The controversy over the two mechanisms of transport continued through the next decade, with some supporting Dobbins’ hypothesis that a transcellular mechanism is the primary route of uptake into the initial lymphatics of the gut.37–40 However, Collan demonstrated through TEM images that while numerous junctions were tightly connected and overlapping (which he referred to as “complicated joint areas”), there appeared to be junctions interspersed that contained a “simple joint area” between the junctions, which he suggested would be the primary entry point for chylomicrons.41 Others suggested junctional entry of chylomicrons through the tip of the villus,42–44 and still others have continued to recognize the uncertainty regarding the relative importance of the two mechanisms.2
Chylomicron transport
After initial entry into the lacteal, the chylomicrons must be transported through the lymphatic system to the blood. This appears to be done in two stages. Flow starts through the initial lymphatics, which lack the smooth muscle found on collecting lymphatics, by utilizing the peristaltic motion of the intestinal wall, first described by Florey.45 Collan later showed through TEM images that there resided muscle cells within close proximity of the lacteal, which he suggested could provide some pumping activity to the villus.46 Lymph formation, which is increased through an increase in the intraluminal pressure in the gut, also directly correlates with lymph flow through the initial lymphatics and can provide a driving force to promote lymph flow.47 Once in the initial lymphatics, it was recognized early on that the intrinsic contractility of the collecting lymphatics was essential in driving lymph flow.45,48 This pumping activity is responsive to changes in mechanical loads such as wall shear stress49,50 and transmural pressure.49–52 However, it is currently unknown how these mechano-sensitive features of the lymphatic vasculature are utilized in optimizing postprandial lymph transport and how such a phenomenon correlates with the observed increase in contraction frequency that occurs after olive oil administration.53
Models of lacteal uptake
One of the most impactful techniques that has been essential to our understanding of lipid absorption in the gut is the lymph fistula model. By isolating and collecting lymph before lipid reaches the blood, investigators have been able to uncover many of the mechanisms regulating chylomicron assembly54 as well as show the importance of lymph flow on lipid absorption.55 However, the challenge associated with using this technique to study lymphatic uptake of lipid is that, while a general idea of the kinetics of the process of lipid transport by lymphatics can be deduced, it is difficult to separate out certain effects of the lymphatic vasculature (e.g., pump function, lacteal permeability, LEC vesicular uptake) from that of the enterocyte. We recently reported on a tissue-engineered model of the lacteal in which chylomicron uptake across the lymphatic endothelium could be visualized and quantified.21 By supplementing the media supplied to an enterocyte cell model with oleic acid, a fluorescently labeled fatty acid, and a bile salt, we were able to get the cell to synthesize and secrete fluorescently labeled lipoproteins, which could then be used to track and quantify lipid transport across the lymphatic endothelium. Through the use of confocal and TEM imaging we demonstrated transcytosis of lipoproteins across the LEC and showed that the model recapitulated the desired characteristics of in vivo lipid transport in the small intestine. One of the advantages of this model is that the contributions to lipid transport of the enterocyte and the lymphatic can be separated, something that is difficult to do with the in vivo lymph fistula model. This in vitro model will prove useful in determining the relative importance of vesicular-driven transport of chylomicrons into the lacteal and the molecular mechanisms behind this process. In another recent paper, a tissue preparation system was discussed in which a loop of an isolated small intestine along with the blood and lymphatic vessels that support it could be cannulated and kept functioning.56 Such a model could be used to determine the effect of hemodynamics on lacteal function by providing a means to directly control and monitor hemodynamic parameters independently (e.g., arterial pressure, venous pressure, interstitial fluid pressure, lymphatic pressure) while at the same time controlling the contents of the small intestine and the rate at which they are delivered.
Lacteal dysfunction
While our fundamental understanding of chylomicron uptake by lymphatics is still premature, there are several noteworthy studies of late that have reported disease models of the intestinal lymphatics and the resulting consequences to lipid absorption in the gut. In a seminal paper, Harvey described a mouse model of leaky intestinal lymphatics in a heterozygous mouse model effecting a gene important to lymphatic lineage commitment (Prox1+/−), which resulted in adult onset obesity due to the adipogenic nature of the chyle leaking from the vessel.20 Whether lymphatic dysfunction is an underlying cause of certain clinical cases of regional abdominal obesity remains to be seen; however, the lymphatics have been implicated in chyle leakage in other diseases such as intestinal lymphangiectasia.57 Several other genes have also been indicated in maintaining a functional lymphatic network for lipid absorption in the gut and while the underlying mechanisms causing the dysfunction are different in each report, the primary manifestation of the dysfunction is a phenotype of abnormal lipid transport and metabolism.58–60 In wild-type animals, lacteal morphology has been shown to vary dramatically with dietary status, as the lacteal drastically retracts into the lamina propria during fasting, and can be quickly restored (within 3 days) upon refeeding.61 Finally, Van Dyck reported on a transcription factor, whose functions remain largely unknown, that when deleted, results in a mouse that dies of postnatal starvation.62 Interestingly, starvation was not due to poor enterocyte absorption of lipid or chylomicron synthesis, but rather was a result of the accumulation of chylomicrons in the lamina propria due to their not being transported properly into the lacteal. Taken together, these data all indicate that lymphatic involvement in lipid transport is much more active than previously thought, and is not merely the passive draining of fluid and chylomicrons from the gut through large gaps between cells.
Future directions
With the advances in lymphatic molecular biology and imaging techniques, it is an exciting time to be involved in lymphatic research. The emergence of recent reports implicating the lymphatic vasculature in certain lipid disorders highlights the importance of understanding the fundamental biology that regulates lipid transport by lymphatics. Fundamental to this process is the initial uptake of chylomicrons secreted by enterocytes into the lumen of the lacteal, yet it is still unclear exactly how these rather large particles rapidly gain access to the lymphatic vessel. Hopefully these new tools and molecular approaches will allow insight into this significant process in the near future.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Yoffey JM. Lymphatics, Lymph, and the Lymphomyeloid Complex. Academic Press; London: 1970. [Google Scholar]
- 2.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–G726. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- 3.Porter CJH, Charman WN. Uptake of drugs into the intestinal lymphatics after oral administration. Adv Drug Deliv Rev. 1997;25:71–89. [Google Scholar]
- 4.Trevaskis NL, et al. Intestinal lymphatic transport enhances the postprandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm Res. 2009;26:1486–1495. doi: 10.1007/s11095-009-9860-z. [DOI] [PubMed] [Google Scholar]
- 5.Porter CJH, et al. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 6.Rutkowski JM, et al. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey NL. The link between lymphatic function and adipose biology. Ann NY Acad Sci. 2008;1131:82–88. doi: 10.1196/annals.1413.007. [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab. 2010;21:480–487. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song L, et al. High-speed dynamic 3d photoacoustic imaging of sentinel lymph node in a murine model using an ultrasound array. Med Phys. 2009;36:3724–3729. doi: 10.1118/1.3168598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellor RH, et al. Enhanced cutaneous lymphatic network in the forearms of women with postmastectomy oedema. J Vasc Res. 2000;37:501–512. doi: 10.1159/000054083. [DOI] [PubMed] [Google Scholar]
- 11.Swartz MA, et al. Transport in lymphatic capillaries. Part 1 Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996;39:H324–H329. doi: 10.1152/ajpheart.1996.270.1.H324. [DOI] [PubMed] [Google Scholar]
- 12.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 13.O’mahony S, et al. Imaging of lymphatic vessels in breast cancer-related lymphedema: intradermal versus subcutaneous injection of 99mtc-immunoglobulin. AJR Am J Roentgenol. 2006;186:1349–1355. doi: 10.2214/AJR.04.1341. [DOI] [PubMed] [Google Scholar]
- 14.Dixon JB, et al. Measuring microlymphatic flow using fast video microscopy. J Biomed Opt. 2005;10:064016, 1–7. doi: 10.1117/1.2135791. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JB, et al. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JB, et al. Image correlation algorithm for measuring lymphocyte velocity and diameter changes in contracting microlymphatics. Ann Biomed Eng. 2007;35:387–396. doi: 10.1007/s10439-006-9225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trzewik J, et al. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 2001;15:1711–1717. doi: 10.1096/fj.01-0067com. [DOI] [PubMed] [Google Scholar]
- 18.Baluk P, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leak LV. Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res. 1970;2:361–391. doi: 10.1016/0026-2862(70)90031-2. [DOI] [PubMed] [Google Scholar]
- 20.Harvey NL, et al. Lymphatic vascular defects promoted by prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 21.Dixon JB, et al. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol Bioeng. 2009;103:1224–1235. doi: 10.1002/bit.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmonds WJ. Absorption of lipids. In: Guyton AC, editor. Gastrointestinal Physiology. Butterworths & Co Ltd; London: 1974. [Google Scholar]
- 23.Barrowman JA. Physiology of the Gastro-Intestinal Lymphatic System. Cambridge University Press; Cambridge: 1978. General physiological considerations; pp. 31–81. [PubMed] [Google Scholar]
- 24.Palay SL, Karlin LJ. An electron microscopic study of the intestinal villus. Ii The pathway of fat absorption. J Biophys Biochem Cyt. 1959;5:373–384. doi: 10.1083/jcb.5.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp M, et al. An electron microscopic study of the central lacteal in the intestinal villus of the cat. Z Zellforsch Mikrosk Anat. 1962;57:475–486. [PubMed] [Google Scholar]
- 26.Casley-Smith JR. Identification of chylomicra and lipoproteins in tissue sections and their passage into jejunal lacteals. J Cell Biol. 1962;15:259–277. doi: 10.1083/jcb.15.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladman AJ, et al. A morphological study of fat transport in the normal human jejunum. Am J Anat. 1963;112:389–419. doi: 10.1002/aja.1001120307. [DOI] [PubMed] [Google Scholar]
- 28.Freeman JA, Geer JC. Intestinal fat and iron transport, goblet cell mucus secretion, and cellular changes in protein deficiency observed with the electron microscope. Am J Dig Dis. 1965;10:1005–1025. doi: 10.1007/BF02233375. [DOI] [PubMed] [Google Scholar]
- 29.Rubin CE. Electron microscopic studies of triglyceride absorption in man. Gastroenterology. 1966;50:65–77. [PubMed] [Google Scholar]
- 30.Casley-Smith JR. An electron microscopic study of injured and abnormally permeable lympatics. Ann NY Acad Sci. 1964;116:803–830. doi: 10.1111/j.1749-6632.1964.tb52547.x. [DOI] [PubMed] [Google Scholar]
- 31.Tytgat GN, et al. Synthesis and transport of lipoprotein particles by intestinal absorptive cells in man. J Clin Invest. 1971;50:2065–2078. doi: 10.1172/JCI106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collan Y, Kalima TV. The lymphatic pump of the intestinal villus of the rat. Scand J Gastroenterol. 1970;5:187–196. [PubMed] [Google Scholar]
- 33.Dobbins WO, Rollins EL. Intestinal mucosal lymphatic permeability—an electron microscopic study of endothelial vesicles and cell junctions. J Ultrastr Res. 1970;33:29–59. doi: 10.1016/s0022-5320(70)90117-6. [DOI] [PubMed] [Google Scholar]
- 34.Dobbins WO. Intestinal mucosal lacteal in transport of macromolecules and chylomicrons. Am J Clin Nutr. 1971;24:77–90. doi: 10.1093/ajcn/24.1.77. [DOI] [PubMed] [Google Scholar]
- 35.Dobbins WO. The intestinal mucosal lymphatic in man. A light and electron microscopic study. Gastroenterology. 1966;51:994–1003. [PubMed] [Google Scholar]
- 36.Dobbins WO. Electron microscopic study of the intestinal mucosa in intestinal lymphangiectasia. Gastroenterology. 1966;51:1004–1017. [PubMed] [Google Scholar]
- 37.Azzali G. The passage of macrophage and lymphocytes from the interstitium across the lymphatic endothelium of rat lacteals. Cell Tissue Res. 1990;262:191–193. doi: 10.1007/BF00327761. [DOI] [PubMed] [Google Scholar]
- 38.Azzali G. The ultrastructural basis of lipid transport in the absorbing lymphatic vessel. J Submicr Cyt. 1982;14:45–54. [PubMed] [Google Scholar]
- 39.Azzali G. Transendothelial transport of lipids in the absorbing lymphatic vessel. Experientia. 1982;38:275–277. doi: 10.1007/BF01945110. [DOI] [PubMed] [Google Scholar]
- 40.Azzali G, et al. Ultrastructure of absorbing peripheral lymphatic vessel (alpa) in guinea pig Peyer’s patches. Microvasc Res. 2002;64:289–301. doi: 10.1006/mvre.2002.2428. [DOI] [PubMed] [Google Scholar]
- 41.Collan Y, Kalima TV. Topographic relations of lymphatic endothelial cells in the initial lymphatic of the intestinal villus. Lymphology. 1974;7:175–184. [PubMed] [Google Scholar]
- 42.Sabesin SM, Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- 43.Lee JS. Tissue fluid pressure, lymph pressure, and fluid transport in rat intestinal villi. Microvasc Res. 1986;31:170–183. doi: 10.1016/0026-2862(86)90032-4. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani O. Three-dimensional organization of lymphatics and its relationship to blood vessels in rat small intestine. Cell Tissue Res. 1987;248:365–374. doi: 10.1007/BF00218204. [DOI] [PubMed] [Google Scholar]
- 45.Florey H. Observations on the contractility of lacteals: Part II. J Physiol. 1927;63:1–18. doi: 10.1113/jphysiol.1927.sp002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collan Y, Kalima TV. The lymphatic pump of the intestinal villus of the rat. Scand J Gastroenterol. 1970;5:187–196. [PubMed] [Google Scholar]
- 47.Lee JS. Distension pressure on subserosal and mesenteric lymph pressures of rat jejunum. Am J Physiol. 1986;251:G611–G614. doi: 10.1152/ajpgi.1986.251.5.G611. [DOI] [PubMed] [Google Scholar]
- 48.Florey H. Observations on the contractility of lacteals: Part I. J Physiol. 1927;62:267–272. doi: 10.1113/jphysiol.1927.sp002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gashev AA, et al. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gashev AA, et al. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 51.Benoit J, et al. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257:H2059–H2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 52.Hargens A, Zweifach B. Contractile stimuli in collecting lymph vessels. Am J Physiol. 1977;233:H57–H65. doi: 10.1152/ajpheart.1977.233.1.H57. [DOI] [PubMed] [Google Scholar]
- 53.Miura S, et al. Increased lymphocyte transport by lipid absorption in rat mesenteric lymphatics. Am J Physiol. 1987;253:G596–G600. doi: 10.1152/ajpgi.1987.253.5.G596. [DOI] [PubMed] [Google Scholar]
- 54.Lo CM, et al. Why does the gut choose apolipoprotein b48 but not b100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294:G344–G352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 55.Tso P, et al. Role of lymph flow in intestinal chylomicron transport. Am J Physiol. 1985;249:G21–G28. doi: 10.1152/ajpgi.1985.249.1.G21. [DOI] [PubMed] [Google Scholar]
- 56.Lautenschläger I, et al. A model of the isolated perfused rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G304–G313. doi: 10.1152/ajpgi.00313.2009. [DOI] [PubMed] [Google Scholar]
- 57.Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann’s disease) Orphanet J Rare Dis. 2008;3:1–8. doi: 10.1186/1750-1172-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Backhed F, et al. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci USA. 2007;104:606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu J, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Investig. 2008;118:3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bazigou E, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habold C, et al. Morphological changes of the rat intestinal lining in relation to body stores depletion during fasting and after refeeding. Pflugers Arch Eur J Physiol. 2007;455:323–332. doi: 10.1007/s00424-007-0289-0. [DOI] [PubMed] [Google Scholar]
- 62.Van Dyck F, et al. Loss of the plagl2 transcription factor affects lacteal uptake of chylomicrons. Cell Metab. 2007;6:406–413. doi: 10.1016/j.cmet.2007.09.010. [DOI] [PubMed] [Google Scholar]


