Abstract
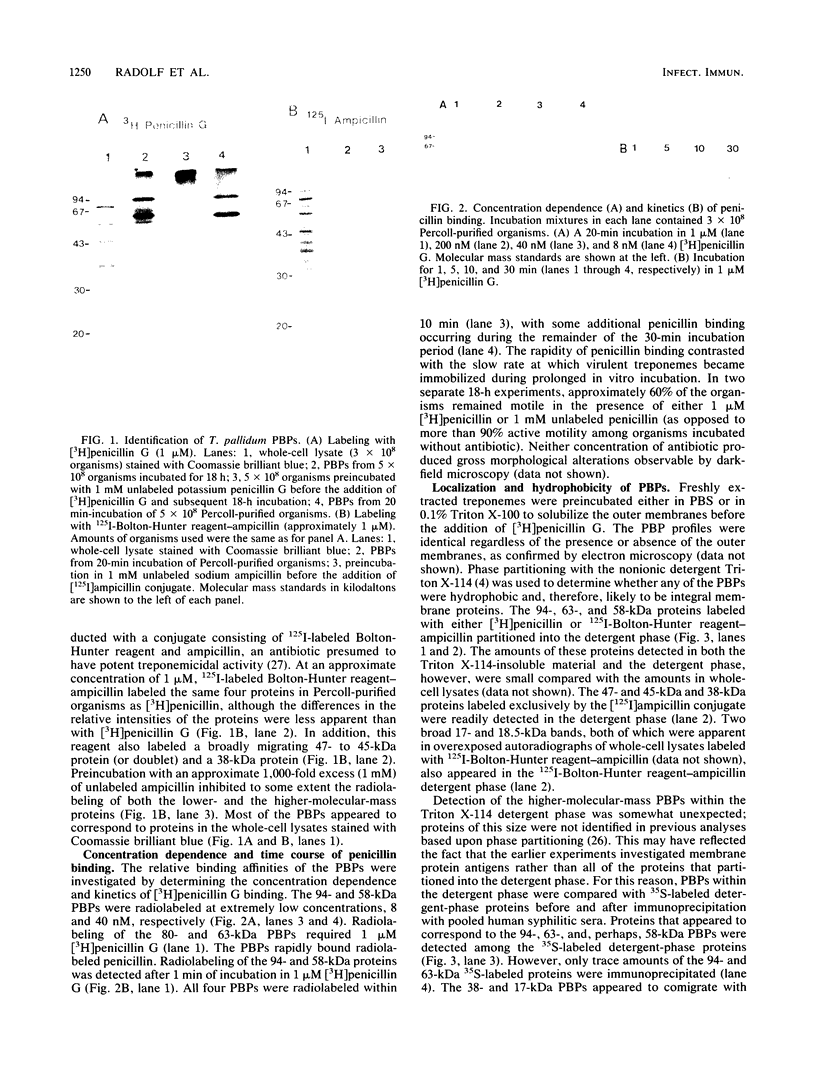
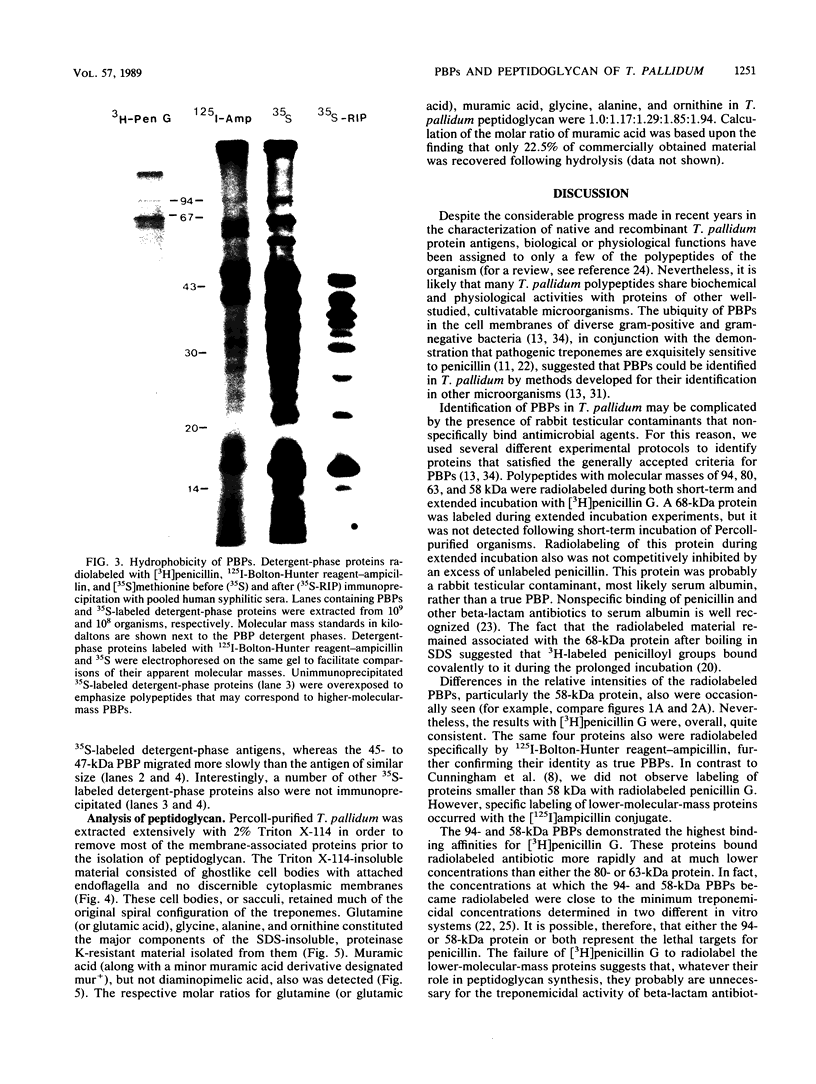
Penicillin-binding proteins (PBPs) of Treponema pallidum subsp. pallidum (T. pallidum) were characterized by using [3H]penicillin G and a conjugate consisting of ampicillin and 125I-labeled Bolton-Hunter reagent. Both antibiotics specifically radiolabeled proteins with molecular masses of 94, 80, 63, and 58 kilodaltons (kDa); 125I-labeled Bolton-Hunter reagent-ampicillin also radiolabeled several polypeptides with lower molecular masses. The 94- and 58-kDa proteins demonstrated the highest binding affinities for [3H]penicillin G and were radiolabeled at concentrations of 8 and 40 nM, respectively. Radiolabeling of PBPs was detectable after 1 min of incubation in 1 microM [3H]penicillin G and was nearly maximal within 10 min. The rapidity of penicillin binding contrasted with the observation that only 40% of virulent treponemes became immobilized during prolonged incubation in vitro with a much higher concentration (1 mM) of unlabeled penicillin. Two lines of evidence indicated that most, if not all, of the PBPs are integral cytoplasmic membrane proteins: (i) preincubation of organisms in 0.1% Triton X-100 solubilized nearly all of the outer membranes but did not affect radiolabeling of PBPs, and (ii) except for the 80-kDa protein, the PBPs partitioned into the detergent phase following extraction with the nonionic detergent Triton X-114. The presence of peptidoglycan in T. pallidum was confirmed by the detection of muramic acid in the sodium dodecyl sulfate-insoluble, proteinase K-resistant residue obtained from Triton X-114-extracted organisms.
Full text
PDF

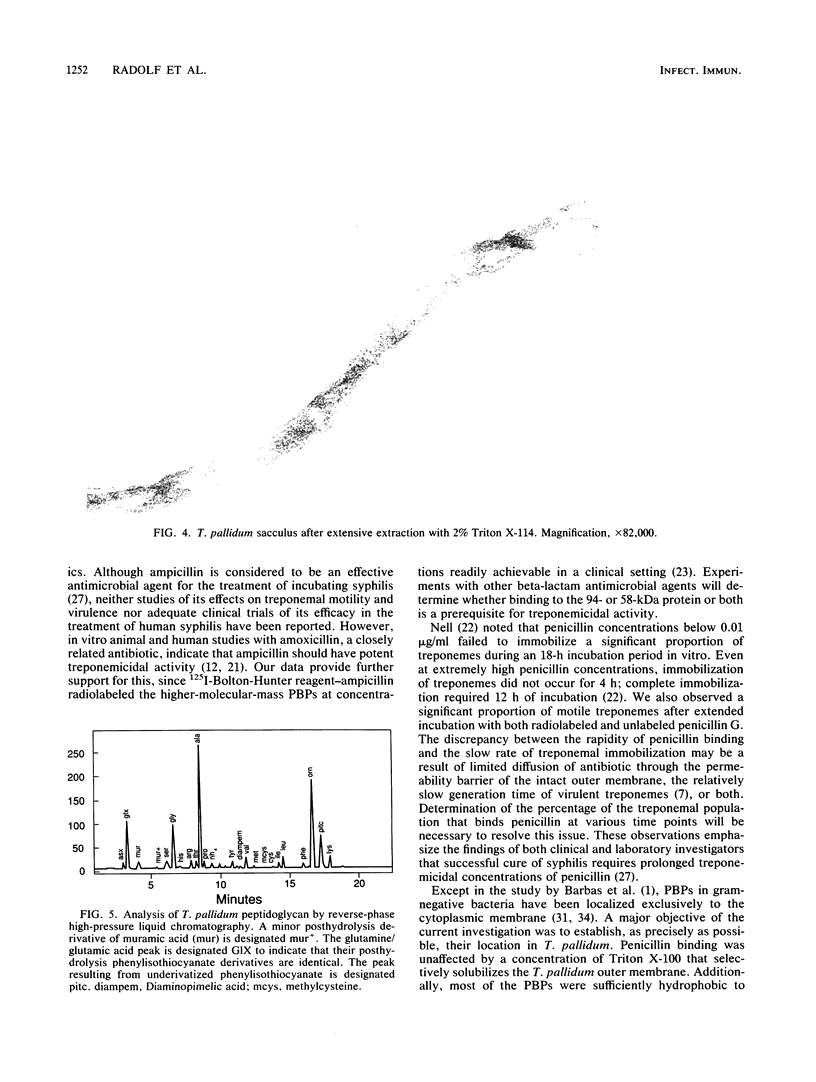


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Amano K., Hackstadt T., Perry L., Caldwell H. D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982 Jul;151(1):420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. M., Miller J. N., Lovett M. A. Identification of Treponema pallidum penicillin-binding proteins. J Bacteriol. 1987 Nov;169(11):5298–5300. doi: 10.1128/jb.169.11.5298-5300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. M., Walker E. M., Miller J. N., Lovett M. A. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988 Dec;170(12):5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H., FLEISCHMAN R., MUSSELMAN A. D. The effective concentrations of penicillin in vitro and in vivo for streptococci, pneumococci, and Treponema pallidum. J Bacteriol. 1950 May;59(5):625–643. doi: 10.1128/jb.59.5.625-643.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber W. R., Bos J. D., Rietra P. J., Fass H., Van Eijk R. V. Treponemicidal levels of amoxicillin in cerebrospinal fluid after oral administration. Sex Transm Dis. 1983 Jul-Sep;10(3):148–150. doi: 10.1097/00007435-198307000-00011. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Cimarusti C. M., Sykes R. B. Binding of monobactams to penicillin-binding proteins of Escherichia coli and Staphylococcus aureus: relation to antibacterial activity. Antimicrob Agents Chemother. 1983 Jan;23(1):98–104. doi: 10.1128/aac.23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Holt S. C., Canale-Parola E. Peptidoglycan of free-living anaerobic spirochetes. J Bacteriol. 1973 Jul;115(1):426–435. doi: 10.1128/jb.115.1.426-435.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafaye P., Lapresle C. Fixation of penicilloyl groups to albumin and appearance of anti-penicilloyl antibodies in penicillin-treated patients. J Clin Invest. 1988 Jul;82(1):7–12. doi: 10.1172/JCI113603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. E., Harrison S. M., Tramont E. C. Oral amoxycillin, an alternative treatment for neurosyphilis. Genitourin Med. 1985 Dec;61(6):359–362. doi: 10.1136/sti.61.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELL E. E. Comparative sensitivity of treponemes of syphilis, yaws, and bejel to penicillin in vitro, with observations on factors affecting its treponemicidal action. Am J Syph Gonorrhea Vener Dis. 1954 Mar;38(2):92–106. [PubMed] [Google Scholar]
- Norris S. J., Edmondson D. G. In vitro culture system to determine MICs and MBCs of antimicrobial agents against Treponema pallidum subsp. pallidum (Nichols strain). Antimicrob Agents Chemother. 1988 Jan;32(1):68–74. doi: 10.1128/aac.32.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F. Biopharmacology of syphilotherapy. J Am Vener Dis Assoc. 1976 Dec;3(2 Pt 2):109–127. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect Immun. 1985 Mar;47(3):799–807. doi: 10.1128/iai.47.3.799-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Wendel G. D., Jr, Stark B. J., Jamison R. B., Molina R. D., Sullivan T. J. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med. 1985 May 9;312(19):1229–1232. doi: 10.1056/NEJM198505093121905. [DOI] [PubMed] [Google Scholar]