Abstract
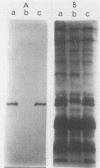
To investigate the pathogenesis of Legionnaires disease at a molecular level, we mutated by directed allelic exchange a gene encoding a Legionella pneumophila-specific 24,000-dalton (Da) surface protein. Southern hybridization and immunoblot analyses demonstrated that the predicted DNA rearrangement occurred in L. pneumophila with a specific loss of 24-kDa antigen expression. Compared with its isogenic parent, the mutant was significantly impaired in its ability to infect transformed U937 cells, a human macrophagelike cell line; i.e., the bacterial inoculum of the mutant strain that was required to initiate infection of the macrophage monolayer was ca. 80-fold greater than that of the isogenic parent strain. The mutant strain regained full infectivity on reintroduction of a cloned 24-kDa protein gene, indicating that the reduced infectivity was due specifically to the mutation in that gene. Compared with the parent strain, the mutant strain was recovered at titers that were ca. 10-fold lower shortly after infection, but it exhibited a similar intracellular growth rate over the next 40 h, indicating that the mutant was defective in its ability to initiate macrophage infection rather than in its ability to replicate intracellularly. When opsonized, the mutant strain was still significantly less infectious than the parent strain, despite equivalent macrophage association, suggesting that the mutant was not merely missing a ligand for macrophage attachment. The mutant also exhibited reduced infectivity in explanted human alveolar macrophages, demonstrating the relevance of the U937 cell model for analyzing this mutant phenotype. These results represent the first identification of a cloned L. pneumophila gene that is necessary for optimal intracellular infection; we designate this gene mip, for macrophage infectivity potentiator.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baine W. B., Rasheed J. K., Mackel D. C., Bopp C. A., Wells J. G., Kaufmann A. F. Exotoxin activity associated with the Legionnaires disease bacterium. J Clin Microbiol. 1979 Mar;9(3):453–456. doi: 10.1128/jcm.9.3.453-456.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A plasmid cloning vector for the direct selection of strains carrying recombinant plasmids. Gene. 1981 Oct;15(1):99–102. doi: 10.1016/0378-1119(81)90108-6. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Engleberg N. C. Applied molecular genetics: new tools for microbiologists and clinicians. J Infect Dis. 1986 Mar;153(3):416–430. doi: 10.1093/infdis/153.3.416. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Carter C., Weber D. R., Cianciotto N. P., Eisenstein B. I. DNA sequence of mip, a Legionella pneumophila gene associated with macrophage infectivity. Infect Immun. 1989 Apr;57(4):1263–1270. doi: 10.1128/iai.57.4.1263-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Cianciotto N., Smith J., Eisenstein B. I. Transfer and maintenance of small, mobilizable plasmids with ColE1 replication origins in Legionella pneumophila. Plasmid. 1988 Jul;20(1):83–91. doi: 10.1016/0147-619x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Drutz D. J., Eisenstein B. I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984 May;44(2):222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Dixon D., Eisenstein B. I. Antibodies isolated by using cloned surface antigens recognize antigenically related components of Legionella pneumophila and other Legionella species. J Immunol. 1986 Feb 15;136(4):1415–1417. [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Lochner J. E., Bigley R. H., Iglewski B. H. The effects of Legionella pneumophila toxin on oxidative processes and bacterial killing of human polymorphonuclear leukocytes. J Infect Dis. 1982 Sep;146(3):328–334. doi: 10.1093/infdis/146.3.328. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Blake M., Niles W. D., Horwitz M. A. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J Bacteriol. 1985 Apr;162(1):85–91. doi: 10.1128/jb.162.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Hindahl M. S., Iglewski B. H. Cloning and expression of a common Legionella outer membrane antigen in Escherichia coli. Microb Pathog. 1987 Feb;2(2):91–99. doi: 10.1016/0882-4010(87)90101-x. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987 Nov 1;166(5):1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner J. E., Bigley R. H., Iglewski B. H. Defective triggering of polymorphonuclear leukocyte oxidative metabolism by Legionella pneumophila toxin. J Infect Dis. 1985 Jan;151(1):42–46. doi: 10.1093/infdis/151.1.42. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Chen J. X., Shuman H. A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988 Jun;56(6):1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Kiely G. M., Bender R. A. Transposon Tn5 encodes streptomycin resistance in nonenteric bacteria. J Bacteriol. 1984 Jul;159(1):388–389. doi: 10.1128/jb.159.1.388-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Engleberg N. C., Eisenstein B. I. Identification of protein antigens of Legionella pneumophila serogroup 1. Infect Immun. 1985 Jan;47(1):74–79. doi: 10.1128/iai.47.1.74-79.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Jiwa A. H., Engleberg N. C., Eisenstein B. I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988 Aug;5(2):87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Dunn M. M., Guzzetta P., Nunez G., Stastny P., Lipscomb M. F. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol. 1984 Jan;132(1):181–186. [PubMed] [Google Scholar]
- Tsang V. C., Wilson B. C., Peralta J. M. Quantitative, single-tube, kinetic-dependent enzyme-linked immunosorbent assay (k-ELISA). Methods Enzymol. 1983;92:391–403. doi: 10.1016/0076-6879(83)92033-5. [DOI] [PubMed] [Google Scholar]
- Winn W. C., Jr Legionnaires disease: historical perspective. Clin Microbiol Rev. 1988 Jan;1(1):60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson E. A., Guiney D. G., Jr Conjugal transfer of bacterial chromosomes mediated by the RK2 plasmid transfer origin cloned into transposon Tn5. J Bacteriol. 1984 Oct;160(1):451–453. doi: 10.1128/jb.160.1.451-453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]