Abstract
The ability to focus one’s attention underlies success in many everyday tasks, but voluntary attention cannot be sustained for extended periods of time. In the laboratory, sustained-attention failure is manifest as a decline in perceptual sensitivity with increasing time on task, known as the vigilance decrement. We investigated improvements in sustained attention with training (~5 hr/day for 3 months), which consisted of meditation practice that involved sustained selective attention on a chosen stimulus (e.g., the participant’s breath). Participants were randomly assigned either to receive training first (n = 30) or to serve as waiting-list controls and receive training second (n = 30). Training produced improvements in visual discrimination that were linked to increases in perceptual sensitivity and improved vigilance during sustained visual attention. Consistent with the resource model of vigilance, these results suggest that perceptual improvements can reduce the resource demand imposed by target discrimination and thus make it easier to sustain voluntary attention.
Keywords: sustained attention, meditation, training, vigilance, discrimination, threshold
The ability to focus one’s attention while resisting distraction is critical for adaptive, goal-directed behavior. Voluntary attention—guided by goals, previous knowledge, or explicit instructions—can be directed to spatial locations (e.g., Posner, 1980) and moments in time (e.g., Correa, Lupiáñez, Madrid, & Tudela, 2006) to improve behavioral accuracy and efficiency. However, voluntary attention is limited and cannot be sustained indefinitely. Research on sustained attention consistently demonstrates reduced target-discrimination accuracy (perceptual sensitivity) with increased time on task, a phenomenon known as the vigilance decrement (for a review, see Parasuraman, 1986). Despite this evidence, few attempts have been made to use training to improve healthy adults’ sustained attention. Studies have demonstrated improvements in performance on vigilance tasks following repetitive practice (e.g., Parasuraman & Giambra, 1991), but no study has yet identified a general training regimen for reducing the vigilance decrement. Similarly, training-related improvements in other domains are often limited to the training task administered. However, research shows that some forms of training, such as practice on action video games, can improve performance on untrained attention tasks, suggesting that training may lead to the transfer of learned skills to new situations (for a review, see Green & Bavelier, 2008). The study we report here investigated whether meditation training could yield improvements in voluntary sustained attention.
The attentional-resource model attributes the vigilance decrement to the exhaustion of limited information processing resources (for a recent review, see Warm, Parasuraman, & Mat-thews, 2008). Specifically, factors known to increase information processing demands, such as perceptually difficult targets (Nuechterlein, Parasuraman, & Jiang, 1983) or working memory load (Parasuraman, 1979), cause rapid and reliable decrements in perceptual sensitivity. When the total task demand (the combined resource demands of multiple aspects of a task) is high, vigilance decrements are large (See, Howe, Warm, & Dember, 1995). Attentional cues can mitigate the impact of resource demands during vigilance tasks. Hitchcock and his colleagues (2003) found that both accuracy and cerebral blood flow (a measure of resource consumption) declined less over time when a warning cue reliably (e.g., 80% of the time) rather than unreliably (e.g., 40% of the time) predicted the occurrence of the upcoming target. Similarly, other kinds of attentional cues have been shown to improve vigilance (for a review, see MacLean et al., 2009). Less is known about how training might affect vigilance.
Historical accounts from the Buddhist contemplative tradition describe meditation practices (Shamatha) that are designed to improve sustained attention (see Buddhaghosa, 1979 trans.). Shamatha practitioners learn to stabilize their attention on a chosen stimulus, such as the tactile sensation of breathing, and enhance the perceived detail of that stimulus in order to cultivate non-task-specific skill in regulating and controlling voluntary attention (Wallace, 1999). Practitioners use introspection to monitor their quality of attention, recognize when attention has wandered, and guide attention back to the chosen stimulus. This metacognitive or meta-attentive aspect of Shamatha training may support the transfer of meditation skills to other domains (Wallace & Shapiro, 2006) and lead to the improvements in perception and attention that are reported in practitioners’ daily lives (Wallace, 1999, p. 185).
The present study tested this claim empirically. Longitudinal research on meditation training (1–3 months of full-time daily practice) in nonexperts suggests that meditation improves temporal attention (Slagter et al., 2007) and attentional alerting (1-month group in Jha, Krompinger, & Baime, 2007). Longitudinal research has also suggested that mindfulness meditation training may enhance visual discrimination (Brown, Forte, & Dysart, 1984). Cross-sectional studies show that long-term meditation practitioners have superior sustained-attention skills in comparison with meditation-naive control participants (Brefczynski-Lewis, Lutz, Schaefer, Levinson, & Davidson, 2007; Valentine & Sweet, 1999). However, longitudinal improvements in sustained attention with meditation training have not yet been demonstrated.
We examined the effects of Shamatha training on 60 participants who were divided randomly into two groups: one that began training at a first retreat (n = 30) and one that served as a waiting-list control and attended a second retreat (n = 30). Retreat participants lived in a remote mountain setting (Shambhala Mountain Center, Red Feather Lakes, CO) for 3 months and received meditation instruction from B.A. Wallace in Shamatha, as well as complementary meditation practices that use imagery and concentration to develop compassion and kindness toward others (Wallace, 2006). Each day, participants attended two sessions that included group meditation and discussion, practiced in solitude (M = 5 hr/day of Shamatha, 45 min/day of complementary practices), and had weekly individual meetings with the instructor.
Outcomes were assessed at three points during each retreat: before the start of the retreat (pretraining), halfway through the retreat (midtraining), and at the end of the retreat (post-training)—using a test of sustained visual attention that produced significant decrements in perceptual sensitivity before training. We predicted that meditation training would reduce this decrement under some but not all circumstances. Specifically, we hypothesized that improvements in participants’ vigilance would be related to the resource demands of the assigned task. For example, training-related improvements in baseline perception could improve vigilance by reducing the resource demands associated with discriminating difficult targets (Nuechterlein et al., 1983; Parasuraman & Mouloua, 1987). To characterize the relations among perception, resource demand, and vigilance, we analyzed (a) discrimination (visual threshold for line-length differences), (b) average sensitivity (during sustained attention), and (c) vigilance (decrement in perceptual sensitivity over time).
Method
Participants
Volunteers were recruited using magazine and online advertisements. We selected participants between 21 and 70 years old who were (a) willing to be assigned to either retreat, (b) familiar with intensive meditation practice (at least three 5-day retreats), and (c) willing to abstain from recreational drugs and tobacco 3 months prior to and during the study, as well as abstain from alcohol use during the study. All selected participants had normal or corrected-to-normal vision and hearing, and no known neurological or Axis I psychiatric impairments (Mini International Neuropsychiatric Interview screen; Sheehan et al., 1998). After selection (∼50% inclusion rate), participants were assigned to either the first retreat group or the waiting-list control group through stratified (age, sex, handedness, ethnicity, and meditation experience) random assignment (starting N for Retreat 1 = 30 per group; starting N for Retreat 2 = 29). Retreat and waiting-list control participants were matched on the basis of demographic factors and psychological characteristics (see Table 1).1 Three months after the end of Retreat 1, waiting-list control participants began training and underwent assessment in Retreat 2. Our final analyses of average sensitivity and vigilance included only participants with complete data (Retreat 1: n = 59; Retreat 2: n = 27).
Table 1.
Group Matching on Demographic and Psychological Variables
| Measure | Retreat 1 group | Waiting-list control group | All participants | Between-group difference (degrees of freedom) |
|---|---|---|---|---|
| Age at study entry (years) | 49 (23–69) | 46 (22–65) | 48 | 0.79 (58) |
| Sex | 14 male, 16 female | 14 male, 16 female | 28 male, 32 female | — |
| Handedness | 29 R, 1 L | 28 R, 2 L | 57 R, 3 L | — |
| Education | 5.2 (1–6) | 4.9 (3–6) | 5.07 | 1.09 (58) |
| Nonverbal IQ | 10.8 (4.0–17.0) | 11.2 (6.0–17.0) | 11.0 | 0.47 (58) |
| Meditation experience | ||||
| Number of retreats | 13 (2–50) | 15 (2–100) | 14 | 0.58 (58) |
| Mean meditation (minutes/day) | 56 (8.5–180) | 54 (12.8–155) | 55 | 0.18 (51) |
| Lifetime meditation practice (hours) | 2,549 (250–9,500) | 2,668 (200–15,000) | 2,610 | 0.16 (57) |
| BFI | ||||
| Openness | 5.5 (3.8–7.0) | 5.6 (4.0–7.0) | 5.57 | 0.45 (56) |
| Conscientiousness | 5.2 (2.4–6.8) | 5.0 (2.3–6.9) | 5.10 | 0.73 (56) |
| Neuroticism | 3.2 (1.2–5.2) | 3.2 (1.7–5.1) | 3.25 | 0.04 (56) |
| Extraversion | 4.2 (2.7–6.7) | 4.3 (2.6–6.5) | 4.27 | 0.67 (56) |
| Agreeableness | 5.2 (3.3–7.0) | 5.3 (3.5–6.8) | 5.25 | 0.25 (56) |
| STAI | 1.7 (1.0–2.3) | 1.8 (1.0–2.7) | 1.77 | 1.06 (56) |
| CES-D | 1.6 (1.0–4.9) | 1.8 (1.0–2.9) | 1.71 | 0.96 (54) |
| Well-being | 5.6 (4.1–6.6) | 5.4 (4.5–6.6) | 5.54 | 0.91 (56) |
Note: The table presents mean values (with ranges in parentheses) for participants who completed all assessments during the first retreat (n = 30 in each group). Between-group t tests (far right column) showed no significant differences between the retreat and waiting-list control groups on any of the demographic or psychological measures at the pretraining assessment (ps > .05). Hand dominance (L = left-handed; R = right-handed) was assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Educational achievement was scored on the following scale: 1 = less than high school diploma; 2 = high school diploma; 3 = some college; 4 = college degree; 5 = some graduate study; 6 = graduate degree. The measure of nonverbal IQ was the total number of correct items on a timed version of half of the Raven’s Progressive Matrices (Raven, Court, & Raven, 1988; the second half was administered at the posttraining assessment). Meditation experience variables included the total number of meditation retreats lasting at least 5 consecutive days, average daily minutes of formal meditation practice, and total number of lifetime hours of formal meditation practice. Questionnaire measures included the Big Five Inventory (BFI; John, Donahue, & Kentle, 1991), the State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977), and the Psychological Well-Being Scale (Ryff, 1989). Scores on all questionnaire measures are based on a 7-point Likert-type scale.
All study and task details were approved by the institutional review board of the University of California, Davis. Participants gave informed consent and were debriefed after their training. Participants paid for their own room and board during the retreat (∼$5,300) but were paid $20 per hour during data collection. Waiting-list control participants were flown to the retreat center to be tested during Retreat 1 for purposes of comparison; their travel expenses at these times were covered.
Measures
On-site testing
Testing sessions were conducted in two laboratories in the building where participants lived and meditated. Each laboratory included a sound-attenuated, darkened testing room and an adjacent control room. Presentation software (Neu-robehavioral Systems, available from http://www.neurobs.com) was used to deliver stimuli on an LCD monitor (Viewsonic VX-922). At each of the three assessments in Retreat 1, waiting-list control participants arrived 3 days (range = 65–75 hr) before testing for acclimatization.
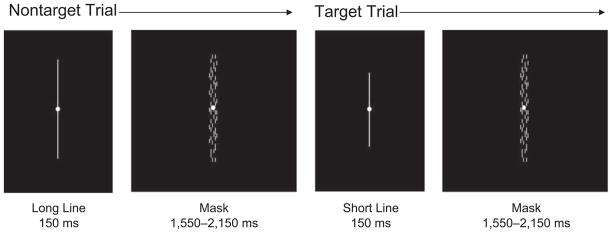
At each assessment, participants completed the threshold procedure (∼10 min) followed by the sustained-attention task (32 min), and also completed the threshold procedure a second time in Retreat 1. In both tasks, participants saw a single vertical line appear at the center of the screen; the line could be either long (frequent nontarget) or short (rare target). The task instructions emphasized the importance of speed and accuracy in responding to the short line (see Fig. 1) by pressing the left mouse button.
Fig 1.
Stimuli and timing for the threshold procedure and sustained-attention task. Single lines (light gray, 40.29 cd/m2) were presented at the center of the screen against a black background (0.35 cd/m2) while participants fixated on a small yellow dot (shown in white) from a viewing distance of 57 cm. In the threshold procedure, long nontarget lines (4.82°) were presented 70% of the time, and short target lines (range = 2.76°-4.78°) were presented 30% of the time. In the sustained-attention task, target frequency was reduced to 10% of stimuli. Each stimulus was presented for 150 ms, and a mask was presented during the variable interstimulus interval of 1,550 to 2,150 ms. The instructions emphasized the importance of speed and accuracy in responding to the short target lines.
In the threshold procedure, the length of the short target line varied according to a parameter estimation (PEST) algorithm (Taylor & Creelman, 1967), which determined the short line length that participants could correctly detect at a given level of accuracy (e.g., 75%). Participants received auditory feedback: a ding when they detected a target correctly and a whoosh when they missed a target or responded to a nontarget. We defined discrimination as the difference in visual angle between the threshold-level short line and the long line, with smaller threshold values representing a greater degree of discrimination. In Retreat 1, all participants performed the threshold procedure again after completing the sustained-attention task, so we could assess short-term changes in discrimination.
In Retreat 1, we set the length of the short target line in the sustained-attention task to each participant’s threshold at each assessment, an approach we used previously (MacLean et al., 2009). We used an 85% accuracy threshold at the pretraining assessment of Retreat 1 and a 75% accuracy threshold at all other assessments. Because the testing level was not the same across all assessments, we conducted separate analyses of group differences in outcome measures at each assessment in Retreat 1.
Follow-up testing
Approximately 5 months after each retreat, participants completed a follow-up assessment of discrimination, which was conducted via laptop computers sent to participants’ homes. Participants received instructions for setting viewing distance and ambient lighting. The threshold procedure at follow-up matched the laboratory procedure exactly.
Analysis
The nonparametric index of perceptual sensitivity, A’ (Stanislaw & Todorov, 1999), was calculated from hit (correct response to a target) and false alarm (incorrect response to a nontarget) rates for each of eight contiguous 120-trial blocks during the sustained-attention task. The decline in A’ was largest during the first half of the task, and thus we defined improvements in vigilance as positive changes in the slope of A’ during the first four blocks. We analyzed changes in slope using hierarchical linear models, which produced estimates of the fixed effects at the group level (these estimates are similar to those produced using standard regression models), as well as estimates of the random effects at the individual level (i.e., variability around fixed-effects estimates). We conducted the analyses of vigilance in SAS Version 9.1 (Littell, Miliken, Stoup, & Wolfinger, 1996).
Results
Retreat 1
Discrimination
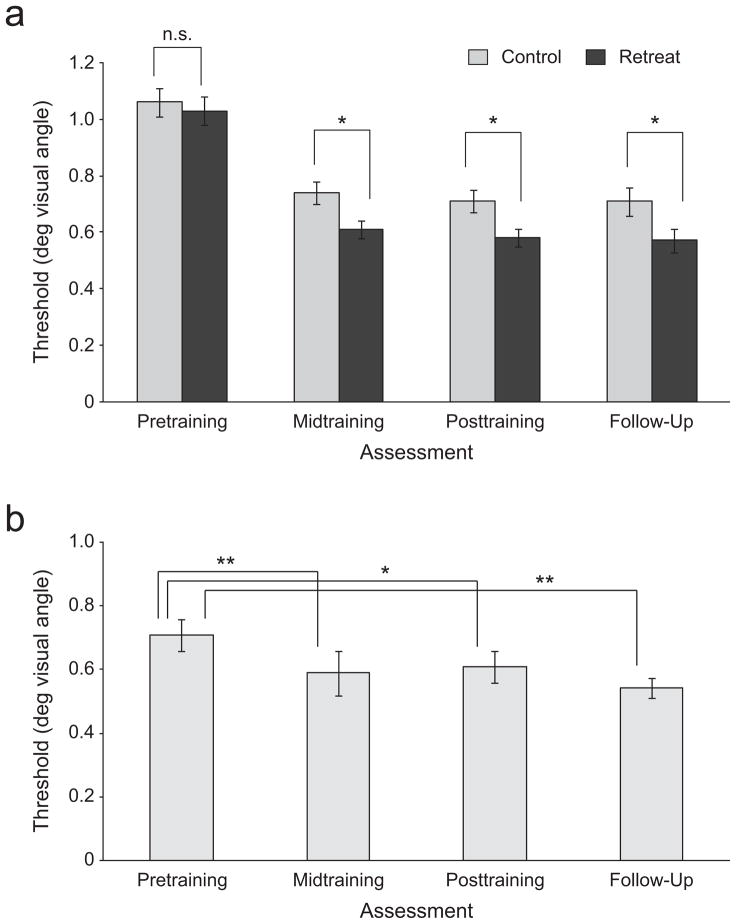
We tested group differences in threshold at each assessment using analysis of variance (ANOVA) with SPSS Version 17.0. Each ANOVA included group (retreat vs. control) as the between-subjects factor and task exposure (threshold tested before vs. after the sustained-attention task) as the within-subjects factor. At the pretraining assessment point, we found no significant differences between groups, F(1, 58) = 0.32, p = .57. Thus, at the beginning of training, the groups were matched in discrimination ability. We found significantly lower thresholds in the retreat group than in the control group at midtraining assessment, F(1, 58) = 4.80, p = 0.32, ηp2 = .08, and posttraining assessment, F(1, 58) = 5.80, p = .019 032, ηp2 = .09. These results indicate training-specific improvements in discrimination (see Fig. 2a).
Fig 2.
Improvements in discrimination in (a) Retreat 1 and (b) Retreat 2. Discrimination threshold is plotted as a function of the time of assessment (pretraining, midtraining, posttraining, and follow-up). Error bars correspond to ±1 SEM; brackets indicate statistical comparisons (*p < .05, **p < .01).
We found neither a significant effect of task exposure nor a significant interaction of task exposure and group at any assessment point (p > .41 at pretraining, p > .08 at midtraining, p > .31 at posttraining). Thus, there was no short-term change in threshold during any assessment (M = 0.79° before the sustained-attention task vs. M = 0.78° after the sustained-attention task). Therefore, we did not repeat the threshold procedure after the sustained-attention task in Retreat 2.
Average sensitivity
We found no significant group differences in average A’ (across all eight blocks) at pretraining (M = .95 for both groups, p = .61), midtraining (M = .90 for both groups, p = .83), or posttraining (M = .89 for retreat and .90 for control, p = .22) assessment points. These results indicate that the groups were matched in their overall performance on the sustained-attention task.
Vigilance
Using hierarchical linear models, we modeled changes in vigilance as a function of the fixed effects of block (slope) and group, and their interaction. We included random effects on the intercept to allow for individual differences in initial performance (A’ during the first block). At all assessments, the best-fitting model of performance over time predicted significant declines in A’ across blocks for all participants, regardless of their group membership (p < .0001; see Table 2, where slope estimates indicate amount of decrease in sensitivity per block).
Table 2.
Parameter Estimates From Models of Vigilance for Retreat 1
| Model and parameter | Estimate | Test statistic | BIC |
|---|---|---|---|
| Pretraining | –902 | ||
| Fixed effects | |||
| β0 (intercept) | 0.972 | t = 212** | |
| β1 (slope) | –0.006 | t = 3.94*** | |
| Random effects | |||
| σ02 (intercept) | 0.001 | z = 4.06*** | |
| σe2 (residual variance) | 0.001 | z = 9.41*** | |
| Midtraining | –759 | ||
| Fixed effects | |||
| β0 (intercept) | 0.939 | t = 142*** | |
| β1 (slope) | –0.012 | t = 5.37*** | |
| Random effects | |||
| σ02 (intercept) | 0.001 | z = 4.39*** | |
| σe2 (residual variance) | 0.001 | z = 9.41*** | |
| Posttraining | –765 | ||
| Fixed effects | |||
| β0 (intercept) | 0.936 | t = 149** | |
| β1 (slope) | –0.013 | t = 6.12*** | |
| Random effects | |||
| σ02 (intercept) | 0.001 | z = 4.20*** | |
| σe2 (residual variance) | 0.001 | z = 9.41*** | |
Note: Full maximum likelihood estimates are reported for the best-fitting models of change in perceptual sensitivity during sustained performance (n = 59 at all assessments). Slope estimates refer to the amount of decrease in sensitivity per block (four blocks). BIC is the Bayesian information criterion; smaller (more negative) values indicate a better model fit. At each assessment, slope was centered to the first block of the sustained-attention task (Block 1 = 0). In all cases, the simpler models (shown here) were better fits than models that included group and interaction effects (not shown; BIC = –898 for pretraining, –751 for midtraining, and –759 for posttraining).
p < .01.
p < .001.
Discussion
Taken together, these results suggest that although retreat participants may have improved in their ability to sustain attention, our setting of line length (based on threshold at each assessment) precluded observation of such improvements as reflected in measured vigilance. This is because of how we set the target line lengths to equate the demand imposed by the sustained-attention task across the different participant groups and successive assessments.
Retreat 2
Our aim in Retreat 2 was to examine how vigilance changed with training when target parameters were held constant. Participants completed the threshold procedure before the sustained-attention task at each assessment and were therefore unaware of a design modification. However, we fixed the length of the target line of the sustained-attention task for each individual to the threshold value that was achieved at the beginning of training, so that task demand could decrease systematically as training progressed and a participant’s discrimination improved.
Discrimination
We used repeated measures ANOVA to test within-subjects change in threshold across the five assessments (midtraining and posttraining assessments of Retreat 1, and pre-, mid- and posttraining assessments of Retreat 2) at which the 75% threshold level had been used on the sustained-attention task. The effect of assessment was significant, F(4, 25) = 4.05, p = .011, ηp2 = .39, a result indicating improvement in participants’ discrimination over time. Comparisons between pairs of assessments confirmed that discrimination improved significantly from pretraining to midtraining during Retreat 2, F(1, 28) = 8.27, p = 0.008, ηp2 = .23, but there was no additional improvement from midtraining to posttraining, F(1, 28) = 0.45, p = .50 (see Fig. 2b). No significant changes in discrimination were observed during the nontraining period (including the midtraining and posttraining assessments in Retreat 1 and the pretraining assessment in Retreat 2; all ps > .21). These findings replicate the training-specific changes in discrimination observed in Retreat 1.2
Average sensitivity
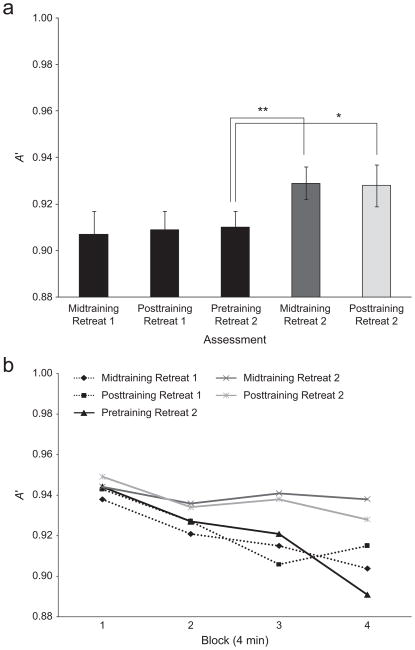
A repeated measures ANOVA on within-subjects change in average A’ in the sustained-attention task revealed a significant effect of assessment, F(4, 23) = 3.26, p = .03, ηp2 = .36. Average A’ improved significantly from pretraining to midtraining during Retreat 2, F(1, 26) = 8.38, p = .008, ηp2 = .24, but there was no additional improvement from midtraining to posttraining, F(1, 26) = 0.004, p = .95 (see Fig. 3a). No significant changes in average A’ were observed across the nontraining assessments (all ps > .83). The observed increases in average A’ during training suggest reductions in total task demand due to improved discrimination.
Fig 3.
Improvements in vigilance and average sensitivity in Retreat 2 participants. In (a), A’ for the sustained-attention task is plotted as a function of the time of assessment. Results for each assessment are averaged across eight blocks (total duration of 32 min per assessment). In (b), A’ for the same task is plotted as a function of block (four contiguous 4-min blocks, 120 trials/block), separately for the same five assessment points shown in (a). Retreat 2 participants were assessed during Retreat 1 but did not begin training until Retreat 2. Error bars correspond to ±1 SEM; brackets indicate statistical comparisons (*p < .05, **p < .01).
Vigilance
Using hierarchical linear models, we modeled changes in vigilance as a function of the fixed effects of block (slope) and assessment, and their interaction. As in analyzing the data from Retreat 1, we included random effects on the intercept. The model of vigilance during the nontraining period revealed a significant effect of block, β = –0.009, p = .008, but no significant effect of either assessment or the interaction of assessment and block (all ps > .32; see Table 3, where slope estimates indicate amount of decrease in sensitivity per block), confirming that the vigilance decrement did not change with simple task practice before training. We then modeled within-subjects change in vigilance during the training period, to test our prediction that training would reduce the vigilance decrement. The model revealed a significant effect of block, β = –0.013, p < .0001, and a nonsignificant effect of assessment (p = .94). A significant interaction between block and assessment (β = 0.005, p = .012; see Table 3) indicated that vigilance improved with training, as we predicted. As can be seen in Fig. 3b, participants showed significant reductions in the vigilance decrement (i.e., less negative slope) across the first half of the sustained-attention task during the training period of Retreat 2.
Table 3.
Parameter Estimates From Models of Vigilance for Retreat 2
| Model and parameter | Estimate | Test statistic | BIC |
|---|---|---|---|
| Nontraining | -1,001 | ||
| Fixed effects | |||
| β0 (intercept) | 0.935 | t = 111*** | |
| β1 (slope) | -0.009 | t = 2.67** | |
| β2 (assessment) | 0.005 | t = 0.91 | |
| β3 (Slope × Assessment) | -0.003 | t = 1.00 | |
| Random effects | |||
| σ02 (intercept) | 0.001 | z = 2.80** | |
| σ02 (residual variance) | 0.002 | z = 12.2*** | |
| Training | -1,165 | ||
| Fixed effects | |||
| β0 (intercept) | 0.944 | t = 119*** | |
| β1 (slope) | -0.013 | t = 4.81*** | |
| β2 (assessment) | 0.001 | t = 0.07 | |
| β3 (Slope × Assessment) | 0.005 | t = 2.52* | |
| Random effects | |||
| σ02(intercept) | 0.001 | z = 3.32** | |
| σ02(residual variance) | 0.001 | z = 12.2*** | |
Note: Full maximum likelihood estimates are reported for models of change in perceptual sensitivity across blocks (slope) and assessments (n = 27 participants in all models). BIC is the Bayesian information criterion; smaller (more negative) values indicate a better model fit. Slope was centered to the first block of the sustained-attention task (Block 1 = 0), and assessment was centered to the first assessment in the model.
p < .05.
p < .01.
p < .001.
Follow-up
Discrimination
We investigated the stability of discrimination improvements at a follow-up assessment conducted approximately 5 months after each retreat. To examine results for Retreat 1, we compared the performance of Retreat 1 participants at follow-up (n = 29) with the performance of the waiting-list control group at the beginning of Retreat 2 (n = 29). We found that Retreat 1 participants’ thresholds were significantly lower than control participants’ thresholds, F(1, 56) = 4.58, p = .037, ηp2 = .08, confirming the maintenance of discrimination improvements after completion of training (see Fig. 2a). Similarly, participants in the control group exhibited significantly lower thresholds at their follow-up (n = 27) than at the beginning of their training in Retreat 2, F(1, 26) = 16.9, p < .0001, ηp2= .39 (see Fig. 2b). These results demonstrate enduring changes in participants’ discrimination after training.
Daily meditation
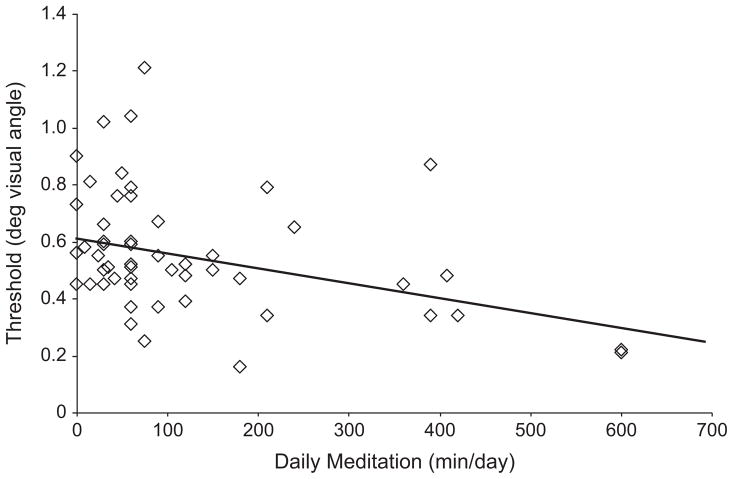
We explored whether the maintenance of discrimination improvements following training might be related to the amount of postretreat daily meditation by combining data from participants from both retreats (n = 54 with complete meditation reports and threshold data). We confirmed through t tests that there were no significant differences between the two retreat groups in either the amount of time devoted to daily meditation following the retreat (129 min/day vs. 112 min/day), t(52) = 0.41, p = .68, or discrimination thresholds at follow-up (0.56º vs. 0.55°), t(52) = 0.20, p = .84. The amount of time spent in daily meditation significantly predicted follow-up threshold, r = –.36, p = .007. This result indicates a correlation between the long-term stability of training-induced discrimination improvement and the maintenance of regular, but less intensive, meditation practice (see Fig. 4).3
Fig 4.
Discrimination threshold as a function of the average amount of time spent in daily meditation at follow-up (n = 54). The graph shows the experimental data and a regression line fit to those data.
Discussion
In a longitudinal study of long-term daily meditation, we found reliable improvements in visual discrimination in two retreat groups. This finding is consistent with a previous report of increased visual sensitivity following intensive meditation training (Brown, Forte, & Dysart, 1984) and suggests that mental training of attention on nonvisual perceptions (e.g., sensations of breathing and mental events) generalizes to improved visual perception of task-relevant stimuli. We also observed improvements in vigilance that were related to enhanced perception.
Vigilance research studies show that task factors such as perceptually difficult targets and working memory load increase information processing resource demands, leading to poorer performance on these tasks over time (Warm et al., 2008). Our findings suggest that training-related improvements in perception can decrease resource demands and thus improve vigilance. The differences between the results of Retreats 1 and 2 illustrate the important relations among task features, resource demand, and vigilance. We observed no training-related changes in vigilance when we adjusted target length according to individual threshold to match the retreat and control groups on overall performance (Retreat 1). However, when we held target length constant (Retreat 2), we found that improvements in target discrimination ability led to better overall performance (increases in average A’) and improvements in vigilance. These results suggest that training-related improvements in perception reduced the resources required to discriminate an unchanging target, which in turn increased the resources available for sustaining voluntary attention. This implies that a limited central resource is tapped by both increased perceptual and increased attentional demands. However, we did not find compelling evidence to suggest that our meditation training led to direct beneficial changes either in the ability to sustain attention or in the qualitative nature and efficiency of metacognitive processes, even though such changes would be expected to occur eventually if the traditional literature of Shamatha meditation (Wallace, 1999) is correct.
Line discrimination relies on several stages of information processing that could be affected by training, from early-stage perceptual processing to later-stage decision making and response execution. Because our dependent measures took into account both hit and false alarm rates, it is likely that the observed improvements in threshold and perceptual sensitivity reflect improvements in perceptual encoding (Macmillan & Creelman, 2005). This interpretation is supported by electrophysiological studies that have linked improvements in perceptual sensitivity to early-stage perceptual processing (e.g., Luck et al., 1994). It is important to note that we found no significant group differences in reaction time (median correct reaction time) or response bias (See, Warm, Dember, & Howe, 1997) and no changes in either of these performance indices during training in either retreat (all ps > .15), which suggests that we can largely rule out a decision- or response-stage account of our results.
In the line discrimination task, an accurate working memory representation of the nontarget supports the perceptual identification of the rare target when it occurs. Using experimental stimuli similar to ours, Parasuraman and Mouloua (1987) demonstrated that both perceptual manipulations (changing the length difference between the target and nontar-get lines) and working memory manipulations (requiring a comparison between the currently visible target and a nontar-get line held in memory) modulated decrements in perceptual sensitivity. During meditation, practitioners receive extensive experience in attending to the representations of sensory experience and observing how these representations change from one moment to the next. Thus, a core aspect of meditation training involves maintaining and accessing information in working memory from decaying sensory traces. It is therefore possible that training-related improvements in the precision of visual working memory representations (Zhang & Luck, 2008) contributed to the observed changes in vigilance.
Interpretations of previous meditation findings have been complicated by possible alternate explanations, such as preexisting group differences (in cross-sectional designs) and self-selection bias (when assignment to training is not random). Although our use of a longitudinal, waiting-list controlled design eliminated these particular confounds, factors other than meditation may still have contributed to our results. First, specific features of the training environment, such as the secluded high-altitude retreat setting and regular interactions with a committed teacher, could have influenced performance. However, changes in perception and vigilance are unlikely to occur at an altitude of 2,500 m (Virues-Ortega, Buela-Casal, Garrido, & Alcazar, 2004). In addition, the persistence of discrimination improvements after the completion of formal training in our study indicates that retreat-specific factors were not necessary for superior discrimination in participants. Moreover, the significant correlation between follow-up threshold and daily meditation suggests that discrimination ability was directly related to meditation and not to incidental factors.
Changes in personal motivation during training also could have played a role in improved vigilance (e.g., Tomporowski & Tinsley, 1996). Before data collection, all participants had committed $5,300 toward room and board for the retreat, and they were all interested in meditation, the project’s scientific goals, and the instructor’s teachings. Given our study’s strongly motivated retreat and control participants, and the stability of the control group’s data during nontraining assessments, we speculate that motivation did not differentially contribute to results for the two groups. However, we acknowledge the importance of empirically assessing the contribution of motivation in future training studies, for example, by comparing meditation training and active control treatments provided by the same teacher in the same environment.
Our results add to a growing body of evidence that meditation training can improve aspects of attention (Lutz, Slagter, Dunne, & Davidson, 2008), while specifically suggesting that the enhanced sustained-attention ability that has been linked to long-term meditation practice (Brefczynski-Lewis et al., 2007; Valentine & Sweet, 1999) most likely reflects plasticity in the adult brain. Our findings also add to reports of training-induced improvements in other core cognitive processes, such as working memory capacity and nonverbal intelligence (Jaeggi, Buschkuehl, Jonides, & Perrig, 2008; Olesen, Westerberg, & Klingberg, 2004). Together, these findings suggest that it is possible to produce general improvements in mental function that can benefit daily activities.
Acknowledgments
We thank Allen Kanner for conducting the psychological screening, Shiri Lavy for performing the group stratification, Ewa Wojciulik for contributing preliminary design ideas, and David Meyer and two anonymous reviewers for providing thoughtful comments on previous versions of this manuscript. We also thank the Shambhala Mountain Center, Clifford D. Saron’s lab staff, Noelle Blalock and Center for Mind and Brain administrative staff, and our participants.
Funding
This work was funded by Fetzer Institute Grant 2191 to Clifford D. Saron, and by gifts from the Hershey Family, Chade-Meng Tan, Yoga Research and Education, Mental Insight Foundations, the Santa Barbara Institute for Consciousness Studies, the Baumann Institute, and anonymous and individual donors, all to Clifford D. Saron. Funding also included a National Science Foundation predoctoral fellowship to Katherine A. MacLean and a postdoctoral fellowship from the Social Sciences and Humanities Research Council of Canada to Baljinder K. Sahdra.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Age, sex, nonverbal IQ, and previous meditation experience were not significant predictors of training-related changes in discrimination, average sensitivity, or vigilance in either retreat (all ps > .16).
Improvements in participants’ discrimination remained significant after controlling for visual acuity (M = 20/20, T2a vision screener available from Titmus Optical, Chester, VA; Retreat 1: group difference at the posttraining assessment, p = .009; Retreat 2: effect of assessment, p = .018).
A similar analysis showed that daily meditation during the retreat (M = 368 min/day, SD = 88 min/day) did not significantly predict discrimination threshold, average sensitivity, or vigilance at the post-training assessment (p > .51), suggesting that average meditation throughout the reported range was effective for producing training-related changes in performance.
References
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences, USA. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Forte M, Dysart M. Visual sensitivity and mindfulness meditation. Perceptual and Motor Skills. 1984;58:775–784. doi: 10.2466/pms.1984.58.3.775. [DOI] [PubMed] [Google Scholar]
- Buddhaghosa . In: The path of purification. Nanamoli B, editor. Kandy, Sri Lanka: Buddhist Publication Society; 1979. [Google Scholar]
- Correa Á, Lupiáñez J, Madrid E, Tudela P. Temporal attention enhances early visual processing: A review and new evidence from event-related potentials. Brain Research. 2006;1076:116–128. doi: 10.1016/j.brainres.2005.11.074. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Exercising your brain: A review of human brain plasticity and training-induced learning. Psychology and Aging. 2008;23:692–701. doi: 10.1037/a0014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock EM, Warm JS, Matthews G, Dember WN, Shear PK, Tripp LD, et al. Automation cueing modulates cerebral blood flow and vigilance in a simulated air traffic control task. Theoretical Issues in Ergonomic Science. 2003;4:89–112. [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences, USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The “Big Five” Inventory—Versions 4a and 54. Berkeley: University of California, Institute of Personality Assessment and Research; 1991. [Google Scholar]
- Littell RC, Miliken GA, Stoup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Aichele SR, Bridwell DA, Mangun GR, Wojciulik E, Saron CD. Interactions between endogenous and exogenous attention during vigilance. Attention, Perception, & Psychophysics. 2009;71:1042–1058. doi: 10.3758/APP.71.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. 2. Mahwah, NJ: Erlbaum; 2005. [Google Scholar]
- Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: Image degradation produces rapid sensitivity decrement over time. Science. 1983;220:327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. Memory load and event rate control sensitivity decrements in sustained attention. Science. 1979;205:924–927. doi: 10.1126/science.472714. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. Vigilance, monitoring and search. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of perception and human performance: Vol. 2. Cognitive processes and performance. Oxford, England: Wiley; 1986. pp. 43–1–43–39. [Google Scholar]
- Parasuraman R, Giambra L. Skill development in vigilance: Effects of event rate and age. Psychology and Aging. 1991;6:155–169. doi: 10.1037//0882-7974.6.2.155. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Mouloua M. Interaction of signal discriminability and task type in vigilance decrement. Perception & Psychophysics. 1987;41:17–22. doi: 10.3758/bf03208208. [DOI] [PubMed] [Google Scholar]
- Posner M. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–35. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s Progressive Matrices and Vocabulary Scales (Section 4) London: H.K. Lewis; 1988. [Google Scholar]
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology. 1989;57:1069–1081. [Google Scholar]
- See JE, Howe SR, Warm JS, Dember WN. Meta-analysis of the sensitivity decrement in vigilance. Psychological Bulletin. 1995;117:230–249. [Google Scholar]
- See JE, Warm JS, Dember WN, Howe SR. Vigilance and signal detection theory: An empirical evaluation of five measures of response bias. Human Factors. 1997;39:14–29. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Creelman CD. PEST: Efficient estimates on probability functions. The Journal of the Acoustical Society of America. 1967;41:782–787. [Google Scholar]
- Tomporowski PD, Tinsley VF. Effects of memory demand and motivation on sustained attention in young and older adults. American Journal of Psychology. 1996;109:187–204. [PubMed] [Google Scholar]
- Valentine ER, Sweet PLG. Meditation and attention: A comparison of the effects of concentrative and mindfulness meditation on sustained attention. Mental Health, Religion & Culture. 1999;2:59–70. [Google Scholar]
- Virues-Ortega J, Buela-Casal G, Garrido E, Alcazar B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychological Review. 2004;14:197–224. doi: 10.1007/s11065-004-8159-4. [DOI] [PubMed] [Google Scholar]
- Wallace BA. The Buddhist tradition of Samatha: Methods for refining and examining consciousness. Journal of Consciousness Studies. 1999;6:175–187. [Google Scholar]
- Wallace BA. The attention revolution. Boston: Wisdom; 2006. [Google Scholar]
- Wallace BA, Shapiro SL. Mental balance and well-being: Building bridges between Buddhism and Western psychology. American Psychology. 2006;61:690–701. doi: 10.1037/0003-066X.61.7.690. [DOI] [PubMed] [Google Scholar]
- Warm JS, Parasuraman R, Matthews G. Vigilance requires hard mental work and is stressful. Human Factors. 2008;50:433–441. doi: 10.1518/001872008X312152. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008;453:233–235. doi: 10.1038/nature06860. [DOI] [PMC free article] [PubMed] [Google Scholar]