Abstract
Prenatal alcohol exposure (PAE) alters adult neurogenesis and the neurogenic response to stress in male rats. As the effects of stress on neurogenesis are sexually dimorphic, the present study investigated the effects of PAE on adult hippocampal neurogenesis under both non-stressed and stressed conditions in female rats. Pregnant females were assigned to one of three prenatal treatments: 1) Alcohol (PAE) - liquid alcohol (ethanol) diet ad libitum (36% ethanol-derived calories); 2) Pair-fed - isocaloric liquid diet, with maltose-dextrin substituted for ethanol, in the amount consumed by a PAE partner (g/kg body wt/day of gestation); and 3) Control - lab chow ad libitum. Female offspring were assigned to either non-stressed (undisturbed) or stressed (repeated restraint stress for 9 days) conditions. On day 10, all rats were injected with bromodeoxyuridine (BrdU) and perfused either 24 hours (cell proliferation) or 3 weeks (cell survival) later. We found that PAE did not significantly alter cell proliferation or survival, whereas females from the Pair-fed condition exhibited elevated levels of cell survival compared to Control females. Importantly, however, the proportion of both new neurons and new glial cells in the hippocampal dentate gyrus was reduced in PAE compared to Control females. Exposure to stress did not alter neurogenesis in any of the prenatal treatment groups. In summary, compared to females from the Control condition, prenatal dietary restriction enhanced the survival of new neurons, whereas PAE altered the differentiation of newly produced cells in the adult dentate gyrus. Alterations in hippocampal neurogenesis following PAE may contribute to learning and memory deficits seen in individuals with fetal alcohol spectrum disorders.
Introduction
Alcohol consumed by the mother during pregnancy reaches the fetus through the placental barrier and can parallel blood levels reached in the mother (Guerri and Sanchis, 1995), thus affecting the brain of the developing fetus. The broad range of problems resulting from alcohol exposure in utero is referred to as the fetal alcohol spectrum disorders (FASD). Neurodevelopmental deficits associated with FASD can include microcephaly, cognitive deficits (e.g., executive function, learning and memory), attentional difficulties, hyperactivity and motor problems (Rasmussen, 2005; Spohr et al., 2007; Streissguth and O'Malley, 2000). Indeed, FASD is a leading known and preventable cause of learning disabilities (Streissguth et al., 1996). Importantly, facial dysmorphologies and growth deficiencies associated with FASD may become less prominent with time, while perturbations to the CNS can persist long into adulthood (Rasmussen, 2005; Spohr et al., 2007); thus FASD is often called the ‘invisible disability’. Mental health problems such as anxiety, depression and substance use problems are also common in individuals with FASD, and are often referred to as “secondary disabilities”. However, recent data indicating that neurobiological alterations may underlie these problems (Hellemans et al., 2009; O'Connor et al., 2006; Olson et al., 2007) suggest that these may be, at least partially, primary disabilities. Of particular relevance to this issue are neurobiological changes in the activity and regulation of the hypothalamic-pituitary-adrenal (HPA) axis.
The HPA axis is a major component of the stress system, and is altered by prenatal alcohol exposure (PAE) (for review see (Weinberg et al., 2008)). Specifically, both human and animal studies show that PAE re-programs the fetal HPA axis such that HPA tone is increased throughout life (Weinberg et al., 2008). Furthermore, sex differences in HPA reactivity and regulation are seen in both humans with FASD and animal models of PAE. Female rats typically show greater HPA responsiveness to acute or short-term stressors whereas male rats often show greater responsiveness to chronic or prolonged stressors, although stressor type, intensity and hormonal endpoints measured can all influence the pattern of response observed (Weinberg et al., 2008). Similarly, sex differences in HPA, autonomic and behavioral reactivity to stressors have been demonstrated in 5-7 month old male and female infants following prenatal alcohol exposure (Haley et al., 2006). Further PAE alters the development of the hypothalamic-pituitary-gonadal (HPG) axis (Esquifino et al., 1986; McGivern et al., 1998) as well as HPG-HPA interactions (Lan et al., 2006; Sliwowska et al., 2008) in affected offspring, which may mediate, at least partly, the sexual dimorphic responses observed. Thus the neurobiological mechanisms underlying sex differences in stress reactivity following PAE are complex and require further study.
The hippocampus exhibits a large degree of neural plasticity, which is modulated by exposure to stress (Galea et al., 2008; McEwen, 2008). Adult neurogenesis, the production of new neurons in the hippocampal dentate gyrus, includes cell proliferation (generation of new cells), cell survival (survival of these newly generated cells), and cell differentiation (acquiring a phenotype, typically either neuronal or glial). Adult neurogenesis exists in most mammalian species and is affected by both stress (Kuipers et al., 2006; Mirescu and Gould, 2006; Shors et al., 2007; Westenbroek et al., 2004) and PAE (Choi et al., 2005; Klintsova et al., 2007; Redila et al., 2006; Sliwowska et al., 2010). Intriguingly alterations in adult hippocampal neurogenesis following stress depend on duration of stress, type of stressor, sex of subject (Mirescu and Gould, 2006; Pawluski et al., 2009) and stressor controllability (Shors et al., 2007).
Importantly, the neurogenic response of the hippocampal dentate gyrus to stress is sexually dimorphic (for review see (Galea, 2008). Adult male rats exposed to stress often show reduced cell proliferation and survival in the dentate gyrus, while females generally show no significant change in cell proliferation (Falconer and Galea, 2003; Shors et al., 2007; Westenbroek et al., 2004) but either increased or decreased levels of cell survival in response to stress (Kuipers et al., 2006; Westenbroek et al., 2004). Moreover social housing conditions were found to eliminate alterations in cell survival in both males and females (Westenbroek et al., 2004). These findings suggest the possibility of sex differences and environmental conditions in the effects of stress on hippocampal neurogenesis.
PAE alters several aspects of neurogenesis within the hippocampal dentate gyrus in rodents (Choi et al., 2005; Klintsova et al., 2007; Livy et al., 2003; Redila et al., 2006; Sliwowska et al., 2010). In male rats, PAE does not appear to affect cell proliferation (Sliwowska et al., 2010) but has enduring effects on hippocampal neurogenesis, with a reduction in the number of newly produced neurons in PAE compared to Control offspring (Klintsova et al., 2007; Redila et al., 2006; Sliwowska et al., 2010). However sex differences in these effects have not been thoroughly explored. One study examining both male and female mice did not find an effect of PAE on cell survival, but the normal neurogenic response to an enriched environment was altered in both sexes (Choi et al., 2005), suggesting that neurogenesis may be altered in females as well as males following PAE. Our previous study found that the normal stress-induced decrease in cell survival observed in Pair-fed and Control males was lost following PAE (Sliwowska et al., 2010). Thus it appears that PAE alters the ability of immature neurons in male rats to respond to certain stimuli. However it remains unknown whether PAE alters the ability of new neurons to respond to stress in females.
The present study was designed to fill this gap in our knowledge of the effects of PAE and stress on female offspring. We examined the effects of PAE and repeated restraint stress in adulthood on cell proliferation, survival and differentiation in the dentate gyrus of the hippocampus in adult female rats. Based on previous findings in PAE males, and the reported sex differences in both stress responsiveness and neurogenesis in normal male and female rats, we hypothesized that PAE would alter cell proliferation, survival and differentiation in female rats, and that stress would likely have different effects in females from those observed previously in males. Finally, based on previous findings in male PAE rats, we hypothesized that PAE might also eliminate the normal neurogenic response to stress in females.
Materials and methods
1. Breeding of animals
Adult virgin female (250-275 grams (g); n=32) and male (275-300g; n=18) Sprague-Dawley rats were obtained from Charles River Laboratories (St Constant, PQ, Canada). Rats were group-housed by sex and maintained on 12:12 hour light/dark cycle (lights on 0600 hr). The colony room temperature was held constant (21-22°C). Animals were given ad libitum access to water and standard rat chow (Jamieson's Pet Food Distributors Ltd, Delta, BC, Canada) during recovery from shipping and adaptation to the colony room. One to two weeks following arrival, each female was paired with a male in a stainless steel suspended cage (25 × 18 × 18 cm), with wired mesh front and floor. Wax paper was placed under the cages and checked daily for the presence of vaginal plugs, which indicated day 1 of gestation (GD 1). Once a vaginal plug was found, the female was singly housed in a clear polycarbonate cages lined with bedding and was assigned to experimental group. All animal use and care procedures were in accordance with the National Institutes of Health guidelines, and were approved by the University of British Columbia Animal Care Committee.
2. Prenatal Treatments
On the first day of pregnancy (gestation day 1 [GD 1]), females were rehoused in polycarbonate cages (24 × 16 × 46 cm) with pine shavings as bedding, and randomly assigned to one of three groups: 1) Alcohol-exposed (PAE) - liquid ethanol diet, ad libitum (n = 9 dams); 2) Pair-fed (PF) - liquid control diet, with maltose dextrin isocalorically substituted for ethanol, in the amount consumed by a PAE partner (g/kg/body wt/day of gestation), which controls for the reduced food intake typical in ethanol-consuming dams (n = 8 dams), 3) Control (C), lab chow, ad libitum (n = 10 dams). Animals were weighed and weight- matched so that each ethanol-treated dam would have a weight-matched PF control partner. All dams had ad libitum access to water throughout gestation and lactation.
Ethanol was administered to pregnant females throughout gestation using liquid diets formulated in our laboratory to provide optimal nutrition, with 36% of the total calories derived from ethanol (Dyets Inc. Bethlehem, PA, USA). Commercial 95% ethyl grain alcohol was used (Commercial Alcohol Inc., ON, Canada, catalog no. P210EAAN). Maltose-dextrin was isocalorically substituted for ethanol in the liquid control diet (Pair-fed group). Fresh diets were presented daily at 1600-1700 hr. This feeding schedule permits maintenance of relatively normal corticoid circadian rhythms in the Pair-fed dams, as the corticoid rhythm in animals receiving a reduced ration, such as Pair-fed animals, re-entrains to the time of feeding (Gallo and Weinberg, 1981; Krieger, 1974). Every day bottles from the previous day were removed and weighed to determine the amount of diet consumed. Experimental diets were provided to dams until GD 21, at which time they were replaced with standard laboratory chow ad libitum. The pregnant females were handled only on GD 1, 7, 14 and 21 for routine cage changing and weighing.
On postnatal day 1 (PND 1), pups were weighed and all litters were culled to 10 (5 females and 5 males when possible) to control for any confounding effects of litter size or sex ratio. Dams and pups were weighed on PND 1, 8, 15 and 22. Pups were weaned on PND 22 and group-housed by litter and sex, with ad libitum access to standard rat chow and water. On PND 35, offspring were pair-housed until the onset of testing at PND 60-65.
3. Blood Ethanol Concentration (BEC) Measurements
To determinate the maximal or near maximal BEC achieved by ethanol-treated dams, tail blood samples were taken on GD 15, approximately 2-3 hours after the presentation of the PAE diet, to coincide with the end of a major eating bout. Blood samples were allowed to coagulate for 2 hours at room temperature and then spun down at 3000 r.p.m. for 20 minutes at 40°C. Serum was collected and stored at − 20°C until the time of assay. Blood ethanol concentrations were measured using Pointe Scientific Inc. Alcohol Reagent Set (Lincoln Park, Mi, USA; minimum detectable concentration of ethanol of 2 mg/dl).
4. Restraint Stress
The effects of repeated restraint stress on the proliferation and survival of new cells were examined. All subjects were pair-housed until day 1 of restraint stress, at which time all animals were individually housed. Individual housing was chosen, as past studies have demonstrated that alterations in cell survival following stress in males and females may be lost or attenuated, respectively, if animals are group housed (Westenbroek et al., 2004). Control, Pair-fed, and PAE females in the non-stressed condition remained undisturbed in their home cages other than weekly cage changing. Animals in the stressed condition were exposed to repeated restraint stress in polyvinyl chloride restraint tubes for 1 hour daily for nine consecutive days (1200-1300). Restraint tubes were 5.5 × 20 centimeters (cm) (inner diameter × length). The front cap of the tube had four holes 1 cm apart to allow for ventilation and the end cap had a 1.5 cm diameter opening for the tail. Although there are physical components to restraint stress, it is primarily a psychological stressor and causes no pain or injury (Briski and Gillen, 2001). Animals were 60-65 days of age at the start of restraint stress.
5. BrdU Injection and Perfusion
On day 10, 24 hours following the last session of restraint stress, females in both the undisturbed (non-stressed) and stressed conditions were injected intraperitoneally (i.p.) with 200 mg/kg bromodeoxyuridine (BrdU; a DNA synthesis marker). At 24 hours (proliferation group) or 3 weeks (survival group) after BrdU injection, animals were anesthetized with a lethal dose of chloral hydrate (35% wt/vol, 1 ml per 100 grams body weight, i.p.) and perfused transcardially with 0.9% saline for 10 minutes, followed by 4% paraformaldehyde (PFA) for 20 minutes, using perfusion pumps (Welch Pumps, VWR, Canada). Brains were removed and stored in paraformaldehyde for 4 hours after perfusion and then transferred into 20% sucrose solution at 4°C until saturation. Coronal sections (40 μm) were obtained throughout the entire extent of the hippocampus using a microtome cryostat (HM 505E; MICROM International GmbH, Germany).
6. BrdU immunohistochemistry
Tissue was processed to reveal BrdU labeling by applying solutions to series of every 10th section from the anterior-posterior extent of the hippocampus of each rat (n=4-8 per group). All incubations were performed at room temperature and on a shaker unless stated otherwise. Brain sections were rinsed (3× 10 minutes) in TBS (0.1 M tris-phosphate buffer in 0.9% saline; pH 7.4) to equilibrate tissue. Sections were incubated in 0.6% H202 for 30 minutes and then rinsed again in TBS (3 × 10 minutes). Next, DNA was denatured by applying 2 N HCl for 30 minutes at 37°C, then the tissue was incubated in 0.1 M borate buffer for 10 minutes, rinsed in TBS (3 × 10 minutes) and blocked with 3% normal horse serum (NHS) in TBS + 0.1% Triton-X for 30 minutes. Next tissue was incubated in mouse monoclonal antibody against BrdU (1:200 + 3% NHS + TBS + 0.1% Triton-X; Sigma, Oakville, Ontario, Canada) at 4°C for 24 hours. The following day, the tissue was rinsed in TBS (3 × 10 minutes) and incubated in mouse secondary antibody IgG Biotinylated (1:100 + 3% NHS + TBS + 0.1% Triton-X; Vector Laboratories, Burlington, Ontario, Canada) for 4 hours. Following another rinse in TBS (3 × 10 minutes) sections were incubated in avidin-biotin conjugate (ABC, Elite Kit; 1:50; Vector Laboratories, Burlington, Ontario, Canada) for 1.5 hours and rinsed again in TBS (3 × 10 minutes). Next, sections were reacted for 5 minutes in 0.02% diaminobenzidine (Sigma-Oakville, Ontario, Canada) with 20 μl H202. Finally, sections were rinsed in TBS (3 × 10 minutes) and mounted on slides, dried overnight, and then counterstained with Cresyl Violet acetate (Acros through Fisher Scientific, New Jersey, NY, USA), dehydrated in graded ethanol, cleared with Xylene and cover-slipped with Permount (Fisher Scientific, New Jersey, NY, USA).
7. Fluorescent double-staining with neuronal nuclei (NeuN, a neuron-specific nuclear protein) and BrdU immunohistochemistry
Ten sections per rat (n=4-8 per group) from an adjacent series of hippocampus slices as those used above were processed for BrdU and NeuN (neuronal marker) immunohistochemistry to assess the phenotype of BrdU-ir cells. Sections were rinsed (1 × 20 minutes and 3 × 10 minutes) in TBS followed by incubation in 2 N HCl for 30 minutes at 37°C. Sections were rinsed in borate buffer (1 × 10 minutes) and then rinsed in TBS (3 × 10 minutes). Sections were blocked in 3% normal donkey serum (NDS) in TBS + 0.3% Triton-X, (Vector Laboratories, Burlington, Ontario, Canada) for 30 minutes followed by incubation with mouse anti-NeuN (1:200, Chemicon, Temecula, California, USA) and rabbit anti-BrdU (1:250, Chemicon, Temecula, California, USA) in 3% NDS in 0.3% Triton-X for 20 hr at room temperature. The next day, tissue was rinsed in TBS (3× 10 min.) and then blocked in 3% NDS in TBS + 0.3% Triton-X for 30 min., followed by incubation in donkey anti-mouse Alexa 488 antibody with donkey anti-rabbit Alexa 647 for 3.5 hours (1:200; Invitrogen, Burlington, Ontario Canada). Sections were then rinsed in TBS (3 × 10 minutes) and mounted on microscope slides and cover-slipped with 2.5% PVA-DABCO (Sigma, Oakville, Ontario, Canada) and stored in the dark at 4°C.
8. Fluorescent double-staining for glial fibrillary acidic protein (GFAP, a protein expressed by astrocytes) and BrdU immunohistochemistry
Ten sections per rat (n=4-8 per group) from an adjacent series of hippocampus slices as those used above were processed for BrdU and GFAP (glial marker) immunohistochemistry to assess the phenotype of BrdU-ir cells. Sections were rinsed in TBS overnight. The next day sections were rinsed in TBS (3 × 10 minutes) and blocked in TBS with 0.3% Triton X (TBS+) and 5% Normal Goat Serum (TBS++) for 35 minutes. Sections were then incubated overnight with mouse anti-GFAP (1:750; Chemicon, Temecula, California, USA) in TBS+ at room temperature. Sections were rinsed in TBS (3 × 10 minutes) followed by incubation in the dark at 4°C for 22 hours with donkey anti-mouse red cyanine dye (Cy3) (1:200; Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA). The next day, tissue was fixed in PFA (4%) for 30 minutes, and rinsed in 0.9% NaCl (2 × 15 minutes) followed by incubation in 2M HCl for 30 minutes at 37°C. Sections were then rinsed in TBS (2 × 10 minutes) and blocked in TBS++ for 25 minutes followed by incubation overnight at 5°C in rat anti-BrdU (1:500; Serotec, Raleigh, NC, USA). Sections were then rinsed in TBS (3 × 10 minutes) and incubated for 24 hours in fluorophore conjugated anti-rat Alexa 488 (1:500; Invitrogen, Burlington, Ontario, Canada). The next day sections were rinsed the in TBS (3 × 10 minutes) and then mounted on microscope slides, cover-slipped with 2.5% PVA-DABCO (Sigma, Oakville, Ontario, Canada) and stored in the dark at 4°C.
9. Determination of Stage of Estrous Cycle
Stage of estrous cycle was determined at the time of perfusion in all animals as described in (Marcondes et al., 2002). Once females were anesthetized, vaginal lavage was performed using a blunt tipped disposable pipette and 0.3 ml physiological saline. The material collected was then applied to a slide and air-dried overnight. Slides were then stained with Toluidine blue (1%) and analyzed under a light microscope (40×) to determine the stage of the estrous cycle: 1) proestrus (70% round nucleated epithelial cells); 2) estrus (70% un-nucleated cornified cells); 3) diestrus I (nucleated and un-nucleated leukocytes); and 4) diestrus II (primarily leukocytes).
10. Quantification of data
Slides were coded prior to analysis so that experimenters were blind to experimental condition. Total number of BrdU-ir cells in the granule cell layer (GCL), which included the subgranular zone (SGZ), defined as a 50 μm band between the granule cell layer and the hilus of the dentate gyrus, was counted throughout the entire series of coronal sections through the rostral-caudal extent of the hippocampus. BrdU-ir cells were counted in the hilus and compared to counts in the granule cell region for a number of reasons: 1) to determine whether any effects were due to generalized effects on blood brain permeability; 2) progeny from progenitor cells in the hilus give rise to a population of cells that are mainly glial cells, whereas progeny from progenitor cells in the subgranular zone give rise to cells that are mainly neurons (Cameron et al., 1993); and 3) new neurons in the hilus are considered ectopic (McCloskey et al., 2006; Scharfman et al., 2007). We counted only those BrdU-ir cells in which the nucleus was completely filled with 3,3′-Diaminobenzidine brown product or had a punctuate appearance. BrdU-ir cells in the uppermost plane of focus were not counted. All BrdU-ir cells were counted under a 100× objective using an E600 microscope (Nikon). The total number of BrdU-ir cells was estimated by multiplying the total number by ten, as previously described (Eadie et al., 2005; Kronenberg et al., 2003; Sliwowska et al., 2010).
For the phenotyping of cells, fifty randomly-chosen BrdU-ir cells in each of ten sections per brain (n=5-8 per group) were analyzed to determine if they were double-labeled with either the neuron protein marker (NeuN/BrdU-ir) or a glial protein marker (GFAP/BrdU-ir). A subset of double-labeled cells found under epifluorescence was also examined under a confocal microscope (BioRad 200) to confirm double labeling for both NeuN (Figure 1A) and GFAP (Figure 1B). The data were presented as proportion of double-labeled cells per all analyzed BrdU-ir cells (as in (Pawluski and Galea, 2007). Volume of the GCL+SGZ and hilus were calculated using Cavalieri's principle by summing the total area and multiplying it by 400μm (area was calculated using ImageJ software on digitized images collected under 200× magnification).
Figure 1.
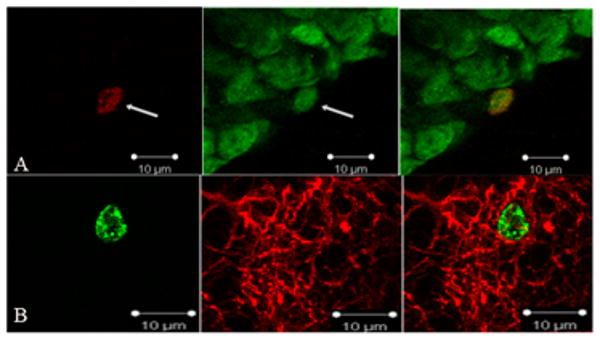
Photomicrograph images of representative labeling of BrdU/NeuN-ir and BrdU/GFAP-ir cells in the dentate gryus. Panel A shows a neuron labeled with NeuN and BrdU at 1000× magnification. Panel B shows an astrocyte labeled with GFAP and BrdU at 1000× magnification.
11. Statistical Analyses
Developmental data were analyzed using repeated measures analyses of variance (ANOVA) with prenatal group (Control, Pair-fed, PAE) as the between-subjects factor, and day of gestation or lactation as the within-subjects factor for the dams, or postnatal day as the within-subjects factor for the female offspring. A repeated-measures ANOVA was used for all hippocampal measurements (i.e. volume, BrdU-ir cells, and phenotyping) with prenatal group and stress condition (undisturbed, stressed) as between-subject factors and region (i.e. GCL, hilus) as the within-subject factor. Stage of estrus was used as a covariate for cell proliferation, as proestrus, when estradiol levels are high, affects cell proliferation (Tanapat et al., 1999)). A priori hypotheses utilized a Bonferroni correction, while post-hoc analyses utilized Newman-Keuls paired comparisons. All statistical analyses were performed using Statistica 6.1 software (StatSoft, Inc).
Results
Developmental Data
Blood ethanol concentration (BEC)
The mean BEC for ethanol-consuming dams was 144.36 ± 31.32 mg/dl, measured ∼2-3 hours after lights off, consistent with previous studies that have employed the same breeding and feeding protocols (Lan et al., 2006; Sliwowska et al., 2008). It has been shown that BECs of this level induce biological and behavioral alterations in the offspring (Weinberg et al., 2008).
Dam body weights during gestation and lactation
The present study utilized female littermates of male offspring used in a previous study (Sliwowska et al., 2010). However, maternal weights are reported here as three additional dams and their litters were included in the present study. Analysis of maternal weight throughout pregnancy revealed a significant interaction between prenatal treatment group and day (F(14, 203)=16.81, p<0.001), with PAE and PF dams weighing less than C dams on GD 21 (ps < 0.016), but no differences among groups during lactation (ps > 0.41; Table 1).
Table 1.
| Pregnancy outcome variables | Prenatal Treatment Group | ||
|---|---|---|---|
| Control | Pair-Fed | PAE | |
| Number of pregnant dams | 15 | 8 | 9 |
| Maternal death | 0 | 0 | 1 |
| Perinatal death | 0 | 0 | 4 |
| Length of gestation (days) | 22.8±0.2 | 22.9±0.2 | 22.9±0.3 |
| Dam weight (grams) | |||
| Gestational Day 1 | 264.1±2.3 | 269.9±2.7 | 267.9±2.3 |
| Gestational Day 7 | 294.5±4.2 | 268.8±3.7 | 276.9±2.9 |
| Gestational Day 14 | 329.5±5.7 | 292.2±4.6 | 303.0±6.4 |
| Gestational Day 21 | 429.9±7.3 | 363.0±9.0* | 372.9±10.9* |
| Lactational Day 1 | 328.9±6.3 | 299.8±7.7 | 302.0±6.4 |
| Lactational Day 8 | 345.7±4.2 | 337.5±7.5 | 341.3±5.8 |
| Lactational Day 15 | 363.2±4.6 | 355.9±7.9 | 259.2±7.5 |
| Lactational Day 22 | 341.6±5.3 | 341.6±5.3 | 338.7±5.2 |
| Litter size | 15.8±0.3 | 16.1±0.5 | 15.3±0.7 |
| Offspring weight (grams) | |||
| Postnatal Day 1 | 6.4±0.1# | 5.69±0.1 | 5.6±0.2 |
| Postnatal Day 8 | 16.9±0.5 | 16.0±0.3 | 14.7±0.4 |
| Postnatal Day 15 | 32.8±0.7 | 31.2±0.8 | 29.9±0.6 |
| Postnatal Day 22 | 52.3±1.5 | 48.6±1.5* | 44.6±0.9* |
Developmental Data. Mean ± SEM. For dam weight data: *On Gestational Day 21: PAE=Pair-fed<Control (ps < 0.016). For offspring (females only) weight data: #Control > Pair-fed=PAE (ps < 0.01). *On Postnatal Day 22, PAE<Pair-fed<Control (ps < 0.01). PAE - animals prenatally exposed to alcohol.
Female offspring body weights
Analysis of female pup body weights on their day of birth (PND 1) revealed a main effect of prenatal treatment group (F(2,29)=9.49, p < 0.001), with PAE and PF pups weighing significantly less than C pups (ps < 0.01; Table 1). Analysis of weight gain across the preweaning period revealed a significant interaction between prenatal treatment group and postnatal day (F(4,56)=7.15, p < 0.001). No significant differences in weight among prenatal treatment groups were observed on PND 8 or 15 (p > 0.10), but PAE and PF females weighed less than C females on PND 22 (PAE<PF<C; ps < 0.01).
Experimental Data
An attenuation of weight gain was observed in rats exposed to restraint stress, regardless of prenatal group
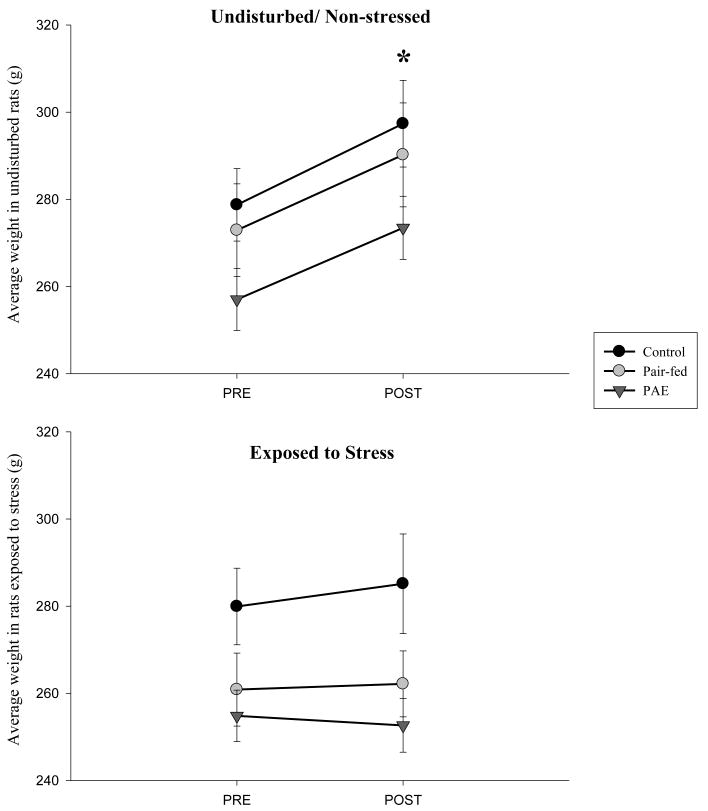
In adult offspring, body weight was recorded over the nine day stress period, both in animals exposed to daily restraint stress and in those that remained undisturbed. There were main effects of prenatal group (F(2,62) = 4.52, p = 0.015) and day F(1,62)=35.8, p < 0.001), and a significant day by stress interaction (F(1,62)=25.69, p < 0.001). Post-hoc tests revealed that PAE females weighed less than their C counterparts (p = 0.01), while PF females did not differ from either C or PAE females (ps > 0.09). Across prenatal groups, animals that remained undisturbed displayed significant weight gain (ps < 0.001), whereas animals exposed to stress showed no significant change in weight over the nine day period (ps > 0.18), suggesting an attenuation of normal weight gain in all animals exposed to stress (see Figure 2). The absence of a significant stress by prenatal group interaction (p = 0.69) indicates that all females exposed to stress were similarly affected, regardless of prenatal treatment.
Figure 2.
Body weights (grams) pre- and post-stress treatment. A significant day by stress condition interaction (F(1,62)=25.69, p < 0.001), indicated that across prenatal groups, females that remained undisturbed displayed significant weight gain (post-stress > pre-stress weights, ps < 0.001), whereas animals exposed to stress showed no significant change in weight over the nine day period (ps > 0.18).
No significant differences in dentate gyrus volume were observed between stress treatments or among prenatal groups
As expected, the volume of the hilus was greater than that of the GCL for all animals at both the 24 hour (F(1,30)=686.10, p < 0.001) and 3 week (F(1,29)=304.99, p < 0.001) post-BrdU administration time points. No significant differences in region volumes were observed among prenatal groups at either 24 hours (F(2,30)=2.08, p = 0.14) or 3 weeks (F(2,29)=2.05, p = 0.14). Nevertheless, region volume was controlled for in all analyses by analyzing density rather than total cell counts, as previous studies reported reduced hippocampal volume following alcohol exposure in utero (Livy et al., 2003; Mattson et al., 2001; Riikonen et al., 1999).
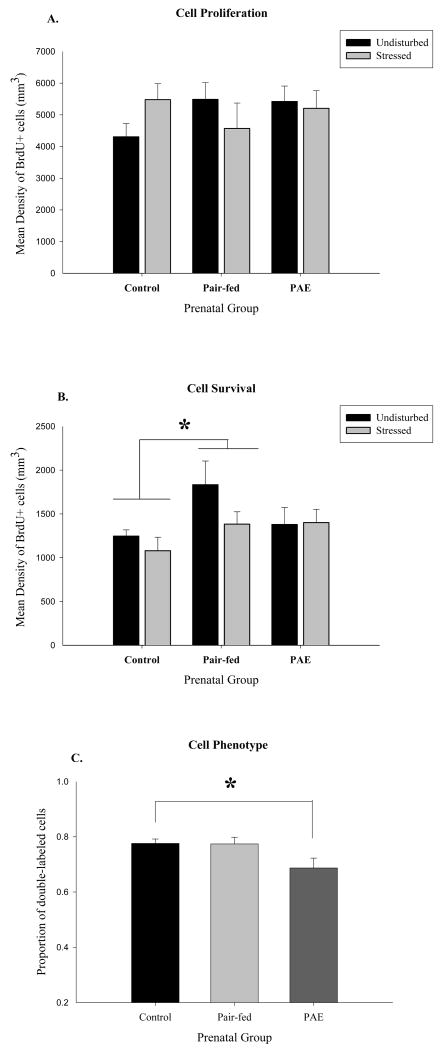
No significant differences in cell proliferation were observed among Control, Pair-fed and Prenatal alcohol exposed females
As expected, there was a significant main effect of region, with greater cell proliferation occurring in the GCL compared to the hilus (F(1, 28)= 319.57, p < 0.001) but no other significant main effects or interactions (i.e. prenatal group by stress treatment by hippocampus region; all ps > 0.19). A priori we hypothesized that PAE females would show reduced cell proliferation and/or survival under non-stressed conditions compared to PF and C females, and that following stress, PAE would attenuate any stress-induced change in cell proliferation or survival. We found that both under non-stressed conditions and following stress, differences in cell proliferation among prenatal groups failed to reach significance (ps ≤ 0.05, but Bonferroni correction requires p ≤ 0.016). These results were not significantly altered by controlling for stage of estrus, likely because of the small number of subjects in proestrus.
Specific effects of pair-feeding on cell survival
As expected, a significant main effect of region was observed for cell survival, as evidenced by the density of BrdU+ labeled cells 3 weeks after BrdU administration (F(1, 29)=341.86, p < 0.001; see Figure 3B), with GCL > hilus. There was a significant main effect of prenatal group (F(2, 29) = 3.47, p = 0.04). Post-hoc analyses indicated that under basal conditions, PF females had greater levels of cell survival than C females (p = 0.002), but not compared to PAE females (p = 0.07).
Figure 3.
A. Density of BrdU-ir cells in the granule cell layer (GCL) 24 hours after BrdU administration (n = 4-7 per group). No significant differences in cell proliferation among groups. B. Density of BrdU-ir labeled cells measured 3 weeks after BrdU administration (n = 4-8 per group). Within the GCL, Pair-fed > Control (p = 0.03). C. Phenotype of surviving cells (n = 50 cells/rat). The proportion of double-labeled (NeuN/BrdU or GFAP/BrdU) cells, collapsed across stress condition, was reduced in rats prenatally exposed to alcohol (PAE < Control (p = 0.02)).
Reduced proportion of new neurons and glial cells in females prenatally exposed to alcohol
The phenotype of the surviving cells was assessed using the proportion of double-labeled NeuN/BrdU+ and GFAP/BrdU+ cells (see Table 2 for exact values and Figure 3C). The majority of BrdU+ cells were neurons as indicated by a significant main effect of cell type, F(1,25) = 794.16, p < 0.001 (collapsed across stress condition: C=77.6%; PF=77.4%; PAE=68.7%). There was also a significant main effect of prenatal group (F(2,25) = 3.36, p = 0.05). Interestingly, post-hoc analyses revealed that there were reduced numbers of double-labeled cells (new neurons or new glia) overall in PAE compared to C rats (p = 0.02) but not compared to PF rats (p = 0.07).
Table 2.
| New Cell Phenotype | Prenatal Group | ||
|---|---|---|---|
| Control | Pair-fed | PAE | |
| NeuN (neuronal) | .77±.02 | .77±.02 | .68±.03 |
| GFAP (glial) | .23±.03 | .26±.02 | .20±.01 |
Proportion of co-labeled (NeuN/BrdU+ and GFAP/BrdU+) cells.
Discussion
In the present study we found that prenatal alcohol exposure did not significantly alter cell proliferation or survival, but altered differentiation of the surviving cells 3 weeks after BrdU administration. Specifically, prenatal alcohol exposure reduced the proportion of both new neurons and new glial cells in the hippocampal dentate gyrus compared to those in Control females. To our knowledge these data provide the first demonstration that prenatal alcohol exposure can reduce neurogenesis and gliogenesis in the adult female hippocampus by altering differentiation of the newly produced surviving cells. Intriguingly Pair-fed females had increased levels of cell survival compared to Control females, demonstrating a unique effect of the pair-feeding treatment in itself. Additionally, under our conditions, repeated restraint stress does not appear to alter either cell survival or differentiation in adult female rats.
There were no differences in cell proliferation across stress condition or prenatal group
In the present study, no significant differences in levels of cell proliferation were observed among the three prenatal treatments. Our finding in Control females is generally consistent with previous studies finding no significant effect of either acute (Falconer and Galea, 2003; Shors et al., 2007) or chronic (Westenbroek et al., 2004) stress on cell proliferation in female rats. However, the type and duration of the stress paradigm, as well as the timing and amount of BrdU administration differs among these studies, which makes it difficult to compare them directly. For example, these previous studies used either one day of exposure to predator odor or footshock stress (Falconer and Galea, 2003; Shors et al., 2007) or 3 weeks (Westenbroek et al., 2004) of exposure to footshock stress, while in the present study we used nine days of restraint stress. It is likely that the type and duration of stress significantly affect whether or not exposure to a stressor will alter cell proliferation in the dentate gyrus of adult females. The issue of how stress may affect neurogenesis in females, and how these effects may differ from those in males warrants further investigation.
Prior exposure to repeated stress did not alter cell survival in female rats
In the present study we found that the levels of cell survival three weeks after the cessation of stress did not differ between the stressed and undisturbed conditions. Past studies examining 3 weeks of stress (foot-shock) have found both increases and decreases in cell survival in individually housed female Wistar rats (Kuipers et al., 2006; Westenbroek et al., 2004). The conflicting findings between these two past studies are likely attributable to differences in experimental paradigms. When animals were sacrificed 6 days post-BrdU administration and BrdU was given in the middle of the stress period (days 13-16 of a 21 day stress regimen) a decrease in the number of BrdU-ir cells was observed (Kuipers et al., 2006), whereas an increase in cell survival was found two weeks following BrdU administration when BrdU was given early during the stress period (Westenbroek et al., 2004). Thus it appears that cell survival is differentially affected depending on when BrdU is given in relation to the stress regimen: when BrdU is given early during the stress there is an increase in cell survival, while when BrdU is given later on, there is a decrease in cell survival. Our study examined the effect of repeated stress three weeks after BrdU injection, when BrdU was given on the last day of the 9 day restraint regimen. We found that levels of cell survival did not differ among groups in the stress condition. This adds to previous findings, suggesting that the neurogenic response to stress depends on stressor type and duration in addition to timing of BrdU administration.
Pair-fed females show enhanced cell survival
In the present study, Pair-fed females overall had elevated levels of cell survival compared to Control females. Although pair-feeding is the accepted control for separating nutritional effects from those of alcohol, it is actually a treatment in itself (Weinberg, 1984). Unlike alcohol consuming dams who consume their diet ad libitum, pair-fed dams are given a reduced ration of food (matched to the reduced intake of the ethanol-consuming dams), and typically consume their entire ration of diet within hours of presentation, thus remaining without food for many hours until the next feeding. Indeed, it has been shown that for animals receiving a reduced ration of food, circadian rhythms may re-entrain to the feeding time rather than the light cycle, as food becomes the most salient cue in the environment (Krieger, 1974; Gallo & Weinberg, 1981). As pair-fed dams are getting less food than they would eat if given the same diet ad libitum, it is likely that they are hungry, which could introduce an element of stress for these animals, and an element of prenatal stress for their fetuses. The possibility that mild prenatal stress may contribute to the differential outcome observed in Pair-fed animals requires further investigation. Of note, the control diet used for pair-feeding is identical to the alcohol diet, with maltose-dextrin, which contains glucose units, isocalorically substituted for ethanol. The effect of additional glucose on the developing offspring, specifically relating to adult neurogenesis, remains unknown.
Prenatal stress and dietary restriction are factors that can influence fetal programming of behavioral and physiological systems (Vieau et al., 2007; Weinberg, 1984; Welberg and Seckl, 2001) and may result in long-term alterations in the offspring. Our study is the first to show that, compared to the Control condition, prenatal dietary restriction increases neurogenesis in adult females. Interestingly, the present finding of increased cell survival in the Pair-fed condition is consistent with past studies showing that dietary restriction in adulthood increased neurogenesis in male mice and rats (Lee et al., 2002a; Lee et al., 2002b). It is important to note that the Pair-fed dams in our paradigm consume adequate nutrients for a healthy pregnancy (Weinberg, 1985), and therefore pair-feeding is more a model of mild prenatal dietary restriction rather than prenatal malnutrition.
Prenatal alcohol exposure reduced the proportion of newly produced neurons and glia in adult females
Prenatal alcohol exposure did not affect levels of BrdU-ir cell survival compared to levels in Control females. Importantly, however, when surviving cells were phenotyped, we found that rats prenatally exposed to alcohol exhibited a reduced proportion of newly produced neurons and astrocytes when compared to Control rats. Neurogenesis levels can be reduced in two ways, by reducing the total number of BrdU-ir cells which also express a neuronal phenotype or by reducing the proportion of BrdU-ir cells that express a neuronal phenotype. Past literature in PAE males showed reduced hippocampal neurogenesis via a reduction in BrdU-ir cell survival (Klintsova et al., 2007; Redila et al., 2006; Sliwowska et al., 2010). In contrast, we demonstrate that prenatal exposure to alcohol reduces the proportion of cells expressing a neuronal phenotype.
A number of factors have been shown to alter the level of neurogenesis following stress or early life insults. For example, Redila et al., (2006) showed that exercise rescues the decrease in neurogenesis in adult male rats prenatally exposed to alcohol, although levels of cell survival never reached those observed in controls. Whether rescue of reduced neuronal differentiation with prenatal alcohol exposure is possible in females remains to be determined. In addition, social housing condition has been shown to protect against the effects of stress on cell survival and thus possibly on neuronal differentiation. For example, stress was shown to increase BrdU labeling in individually-housed but not in socially-housed females, whereas stress decreased BrdU labeling in individually housed but not in socially-housed males. Thus alterations in BrdU labeling following stress may occur differentially in males and females and may be lost or attenuated, respectively, if animals are group housed (Westenbroek et al., 2004). In the present study, all animals were individually housed and we did not find differences in cell differentiation between the stressed and non-stressed conditions. It is possible that individual housing in itself results in some level of stress and thus may have masked any additional effects of the restraint stress experienced by animals in the stressed condition. Importantly, in females prenatally exposed to alcohol, the proportion of newly produced neurons and glia that survived at three weeks was reduced under both stressed and non-stressed conditions, suggesting a robust effect of prenatal alcohol exposure on neurogenesis, and in particular, on cell differentiation.
In adult rats prenatally exposed to alcohol (Singh et al., 2009), in vitro studies have shown a delay in the differentiation of cells into neurons in the hippocampus. Thus it is possible that the reduced proportion of double-labeled cells found in alcohol-exposed compared to Control females in the present study reflects a slower maturation or delayed developmental timeline of these new cells. If so, a neuronal (or glial) phenotype of surviving BrdU-ir cells may be expressed at a later time point (i.e., sometime after the 21 days of survival examined in the present study). Future studies should address this issue in order to determine whether there are reduced levels of new neurons and glia or if these cells are simply slower to mature in alcohol-exposed animals.
Sexually dimorphic effects of stress and prenatal alcohol exposure on the hippocampus
We have shown previously that intensity, duration and type of stressor can affect the hormonal pattern of response observed in males and females prenatally exposed to alcohol compared to each other and to their control counterparts (reviewed in (Weinberg et al., 2008). As noted, sexually dimorphic neurogenic responses to stress within the hippocampus are commonly observed in animals from standard prenatal conditions (Falconer and Galea, 2003; Galea et al., 1997; Shors et al., 2001; Westenbroek et al., 2004). Typically, males exhibit a stress-induced decrease in cell proliferation while females generally show no change (Falconer and Galea, 2003; Galea et al., 1997; Shors et al., 2007; Westenbroek et al., 2004). By contrast, levels of cell survival are typically decreased in males but can be increased or decreased in response to stress in females (Kuipers et al., 2006; Westenbroek et al., 2004). Despite significant differences in methodology between our studies and those cited above, including the introduction of prenatal treatments, we also observe striking sex differences in the neurogenic response to stress. Specifically, our work showed no effects of stress on cell proliferation in males (Sliwowska et al., 2010) or females (present study) from any prenatal treatment group. Interestingly, however, we found sexually dimorphic effects of both stress and prenatal alcohol exposure on hippocampal cell survival. Alcohol exposure had a suppressive effect on cell survival in males under non-stressed conditions, and the neurogenic response to repeated restraint stress was lost in males prenatally exposed to alcohol (Sliwowska et al., 2010). By contrast, in the present study, neither stress nor prenatal alcohol exposure altered BrdU-ir cell survival in females, but the proportion of newly produced neurons and glia was reduced in alcohol-exposed compared to Control females, regardless of stress condition. Past data demonstrate that prenatal alcohol exposure differentially affects HPA activity and regulation as well as associated brain regions, such as the hippocampus, in males and females (Weinberg et al., 2008). Thus, it is possible that sex differences in cell survival are mediated by sexually dimorphic effects of stress on HPA activity in males and females, and/or by altered HPA-gonadal interactions in PAE males and females following stress.
The alcohol-exposed hippocampus
Data from the present study support and extend those from previous studies using animal models showing that the hippocampus is particularly sensitive to the effects of prenatal alcohol exposure (Bonthius and West, 2006; Choi et al., 2005; Klintsova et al., 2007; Livy et al., 2003; Redila et al., 2006; Singh et al., 2009). Moreover, MRI studies have found a reduction in volume of the left hippocampus compared to the right, and that the extent of left hemisphere impairment was greater in the children with FAS than in un-exposed children, indicating teratogenic effects of alcohol on the hippocampus in the developing human fetus (Riikonen et al., 1999).
Adult neurogenesis in the hippocampus has been implicated in learning and memory (Epp et al., 2007; Leuner et al., 2006), and the observed alterations in neurogenesis with prenatal alcohol exposure may contribute to learning and memory deficits in humans with FASD. Children with FASD have altered performance in learning and memory tasks, and show impulsivity and lack of response inhibition, behaviors associated with the hippocampus (Coles, 2006; Riley et al., 2003). Consistent with the human data, prenatal alcohol exposure impairs spatial learning and memory and results in hyperactivity and deficits in response inhibition in animal models (Christie et al., 2005; Clements et al., 2005; Gabriel et al., 2002; Hamilton et al., 2003; Livy et al., 2003; Riley et al., 1979; Streissguth and Giunta, 1988). Together, these findings support the hypothesis that prenatal alcohol exposure results in long-lasting alterations in the hippocampus, and that these alterations may contribute to learning and memory deficits associated with dysfunction of the hippocampus. Further work is needed to elucidate the effects of prenatal alcohol exposure on neurobiological mechanisms influencing plasticity, to better understand when and how the alcohol-exposed hippocampus is altered. Increased knowledge of the effects of prenatal alcohol exposure and its underlying neurobiological mechanisms can inform better targeted behavioral and pharmacological interventions and treatments.
Acknowledgments
Supported by a grant from the UBC Human Early Learning Partnership to LAMG and JW, NIH/NIAAA AA007789 to JW, a University Graduate Fellowship to KAU and a grant from IMPART (CIHR) to KAU and JHS. We would like to acknowledge the assistance of the UBC BioImaging Centre for imaging NeuN/BrdU-ir co-labeled cells. The views presented in the paper are solely those of the authors and do not represent the policy of HELP or the Province of British Columbia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonthius DJ, West JR. Alcohol-Induced Neuronal Loss in Developing Rats: Increased Brain Damage with Binge Exposure. Alcoholism: Clinical and Experimental Research. 2006;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Briski K, Gillen E. Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res Bull. 2001;55:401–8. doi: 10.1016/s0361-9230(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29:2053–62. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–26. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Clements KM, Girard TA, Ellard CG, Wainwright PE. Short-term memory impairment and reduced hippocampal c-Fos expression in an animal model of fetal alcohol syndrome. Alcohol Clin Exp Res. 2005;29:1049–59. doi: 10.1097/01.alc.0000171040.82077.e. [DOI] [PubMed] [Google Scholar]
- Coles CD. Prenatal alcohol exposure and human development. In: Miller MW, editor. Brain development: normal processes and the effects of alcohol and nicotine. Oxford University Press; New York: 2006. [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–85. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology. 1986;44:483–7. doi: 10.1159/000124690. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975:22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Johnston S, Weinberg J. Prenatal ethanol exposure and spatial navigation: effects of postnatal handling and aging. Dev Psychobiol. 2002;40:345–57. doi: 10.1002/dev.10023. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–41. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62:247–60. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–18. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Guerri C, Sanchis R. Acetaldehyde and alcohol levels in pregnant rats and their fetuses. Pharmacol Biochem Behav. 1995;52:93–99. doi: 10.1016/0741-8329(85)90057-6. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hamilton D, Kodituwakku P, Sutherland R, Savage D. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143 doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–82. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Krieger D. Food and Water Restriction Shifts Corticosterone, Temperature, Activity and Brain Amine Periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–63. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuipers SD, Trentani A, Westenbroek C, Bramham CR, Korf J, Kema IP, Ter Horst GJ, Den Boer JA. Unique patterns of FOS, phospho-CREB and BrdU immunoreactivity in the female rat brain following chronic stress and citalopram treatment. Neuropharmacology. 2006;50:428–40. doi: 10.1016/j.neuropharm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–84. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002a;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002b;80:539–47. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–58. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–14. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–91. [PMC free article] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24:2203–10. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern RF, Handa RJ, Raum WJ. Ethanol exposure during the last week of gestation in the rat: inhibition of the prenatal testosterone surge in males without long-term alterations in sex behavior. Neurotoxicol Teratol. 1998;20:483–90. doi: 10.1016/s0892-0362(98)00009-9. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–8. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ, Frankel F, Paley B, Schonfeld AM, Carpenter E, Laugeson EA, Marquardt R. A controlled social skills training for children with fetal alcohol spectrum disorders. J Consult Clin Psychol. 2006;74:639–48. doi: 10.1037/0022-006X.74.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HC, Jirikowic T, Kartin D, Astley S. Responding to the Challenge of Early Intervention for Fetal Alcohol Spectrum Disorders. Infants & Young Children. 2007;20:172–189. [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–57. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29:1359–67. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–11. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev Med Child Neurol. 1999;41:652–9. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley E, Lochry E, Shapiro E. Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology. 1979;62:47–52. doi: 10.1007/BF00426034. [DOI] [PubMed] [Google Scholar]
- Riley EP, Guerri C, Calhoun F, Charness ME, Foroud TM, Li TK, Mattson SN, May PA, Warren KR. Prenatal Alcohol Exposure: Advancing Knowledge Through International Collaborations. Proceedings of an International Workshop on Fetal Alcohol Syndrome Alcoholism: Clinical & Experimental Research. 2003;27:118–135. doi: 10.1097/01.ALC.0000047351.03586.A3. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007;29:14–27. doi: 10.1159/000096208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–95. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Gupta S, Jiang Y, Younus M, Ramzan M. In vitro neurogenesis from neural progenitor cells isolated from the hippocampus region of the brain of adult rats exposed to ethanol during early development through their alcohol-drinking mothers. Alcohol & Alcoholism. 2009;44:185–198. doi: 10.1093/alcalc/agn109. [DOI] [PubMed] [Google Scholar]
- Sliwowska J, Barker J, Barha C, Lan N, Galea L, Weinberg J. Prenatal ethanol exposure attenuates stressed-induced suppression of hippocampal neurogenesis in adult male rats. Stress. 2010 doi: 10.3109/10253890903531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–23. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC. Fetal alcohol spectrum disorders in young adulthood. J Pediatr. 2007;150(2):175–9. doi: 10.1016/j.jpeds.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Fetal Alcohol & Drug Unit, Tech Rep 96-06. Seattle: University of Washington; 1996. Understanding the occurence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE). Final report to the Centers for Disease Control and Prevention (CDC) [Google Scholar]
- Streissguth AP, Giunta CT. Mental health and health needs of infants and preschool children with Fetal Alcohol Syndrome. International Journal of Family Psychiatry. 1988;9:29–47. [Google Scholar]
- Streissguth AP, O'Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–90. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32 1:S16–20. doi: 10.1016/j.psyneuen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol. 1984;6:261–269. [PubMed] [Google Scholar]
- Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcohol Clin Exp Res. 1985;9:49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L, Seckl J. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]