Abstract
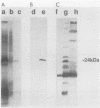
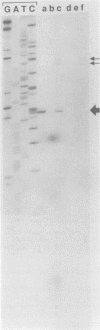
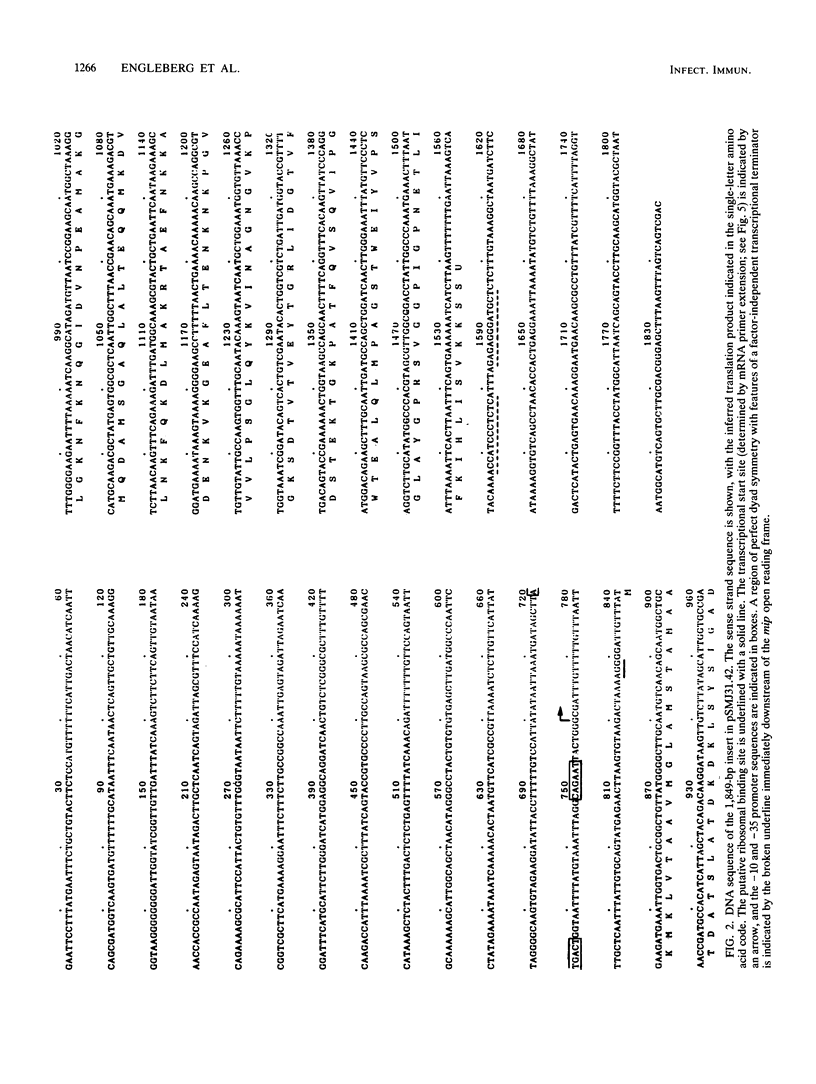
In a previous study, a 24-kilodalton (kDa) protein surface antigen of Legionella pneumophila was cloned into Escherichia coli and found to be expressed on the host cell surface. Subsequently, a site-directed mutation in this gene (designated mip) in L. pneumophila was found to impair the capacity of this bacterium to initiate intracellular infection in human macrophages. The work presented here indicates that the antigenic gene product is distinct from the 24- to 29-kDa major outer membrane protein of L. pneumophila. In addition, the antigen was identified as a highly basic protein on two-dimensional nonequilibrium polyacrylamide gels and on two-dimensional monoclonal antibody immunoblots. When the DNA fragment encoding this protein was sequenced, a long open reading frame of 699 base pairs was identified within a region to which antigen expression was previously mapped. mip mRNA isolated from both L. pneumophila and transformed E. coli had the same 5' end, as determined by primer extension analysis, indicating that the same promoter sequences are used in both species. A likely factor-independent transcriptional terminator was found 20 residues downstream of the stop codon, suggesting that mip is encoded on a monocistronic message. The inferred polypeptide began with a possible 20- to 24-residue signal sequence, and, as predicted by two-dimensional electrophoresis, had a molecular weight of 24,868 and was a potent polycation with an estimated pI of 9.8.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Barrera O., Swanson J. Antigens that are similar in apparent molecular weight to gonococcal outer membrane protein III. J Clin Microbiol. 1987 Jul;25(7):1155–1158. doi: 10.1128/jcm.25.7.1155-1158.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler C. A., Street E. D., Hatch T. P., Hoffman P. S. Disulfide-bonded outer membrane proteins in the genus Legionella. Infect Immun. 1985 Apr;48(1):14–18. doi: 10.1128/iai.48.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cianciotto N. P., Eisenstein B. I., Mody C. H., Toews G. B., Engleberg N. C. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989 Apr;57(4):1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierkauf F. A., Beukers H., Deierkauf M., Riemersma J. C. Phygocytosis by rabbit polymorphonuclear leukocytes: the effect of albumin and polyamino acids on latex uptake. J Cell Physiol. 1977 Aug;92(2):169–175. doi: 10.1002/jcp.1040920205. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H. Comparative study of selective media for isolation of Legionella pneumophila from potable water. J Clin Microbiol. 1982 Oct;16(4):697–699. doi: 10.1128/jcm.16.4.697-699.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret W., Ruckdeschel G. Membrane proteins of legionellaceae. I. Membrane proteins of different strains and serogroups of Legionella pneumophila. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Jul;259(4):433–445. doi: 10.1016/s0176-6724(85)80075-4. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Engleberg N. C. Applied molecular genetics: new tools for microbiologists and clinicians. J Infect Dis. 1986 Mar;153(3):416–430. doi: 10.1093/infdis/153.3.416. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Drutz D. J., Eisenstein B. I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984 May;44(2):222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Dixon D., Eisenstein B. I. Antibodies isolated by using cloned surface antigens recognize antigenically related components of Legionella pneumophila and other Legionella species. J Immunol. 1986 Feb 15;136(4):1415–1417. [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley C. E., Cohen M. L., Halter J., Meyer R. D. Nosocomial Legionnaires' disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann Intern Med. 1979 Apr;90(4):583–586. doi: 10.7326/0003-4819-90-4-583. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hindahl M. S., Iglewski B. H. Isolation and characterization of the Legionella pneumophila outer membrane. J Bacteriol. 1984 Jul;159(1):107–113. doi: 10.1128/jb.159.1.107-113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984 Jan;36(1):27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pearlman E., Engleberg N. C., Eisenstein B. I. Identification of protein antigens of Legionella pneumophila serogroup 1. Infect Immun. 1985 Jan;47(1):74–79. doi: 10.1128/iai.47.1.74-79.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L., Ehrenfeucht A. Use of the 'Perceptron' algorithm to distinguish translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2997–3011. doi: 10.1093/nar/10.9.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Antimicrobial specificity of leukocyte lysosomal cationic proteins. Science. 1966 Nov 25;154(3752):1049–1051. doi: 10.1126/science.154.3752.1049. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]