Abstract
Objective
GH and IGFs have mitogenic properties, causing speculation that GH treatment could increase risk of malignancy. While studies in GH-treated childhood cancer survivors have suggested a slight increase in second neoplasms, studies in GH-treated adults have been equivocal.
Design
Incidence of de novo and second cancers was evaluated in 6840 GH-treated and 940 non GH-treated adult patients in the Hypopituitary Control and Complications Study pharmacoepidemiological database.
Methods
Evident cancer cases were evaluated in the main analysis, with sensitivity analyses including probable and possible cancers. Standardized incidence ratios (SIRs) for cancers were calculated using Surveillance, Epidemiology and End Results for the USA and GLOBOCAN for all other countries.
Results
During the mean follow-up of 3.7 years/GH-treated patient, 142 evident cancer cases were identified, giving an overall SIR of 0.88 (95% confidence interval (CI) 0.74–1.04); 95% CIs included the value of 1.0 for each country examined. The SIR for GH-treated patients from the USA (71 cases) was 0.94 (95% CI 0.73–1.18), and for non GH-treated patients from the USA (27 cases) was 1.16 (95% CI 0.76–1.69). For GH-treated patients from the USA aged <35 years, the SIR (six cases) was 3.79 (1.39–8.26), with SIR not elevated for all other age categories; SIR for patients from the USA with childhood onset (CO) GH deficiency (GHD) was 2.74 (95% CI 1.18–5.41). The SIR for colorectal cancer in GH-treated patients (11 cases) was 0.60 (95% CI 0.30–1.08).
Conclusions
With relatively short follow-up, the overall primary cancer risk in 6840 patients receiving GH as adults was not increased. Elevated SIRs were found for subgroups in the USA cohort defined by age <35 years or CO GHD.
Introduction
A risk of malignancies has been speculated in GH-treated patients, based on the mitogenic and proliferative properties of GH and insulin-like growth factors (IGFs), demonstrated by both experimental and epidemiological analyses (1–5). Previous surveillance data from the Hypopituitary Control and Complications Study (HypoCCS) and the Kabi International Metabolic Surveillance (KIMS) study have suggested that adult GH replacement is safe (6, 7). However, a potential association between GH treatment of children and adults and an increased risk for malignancy has remained an important topic of debate.
Earlier studies suggested that GH treatment was not associated with increased tumor recurrence or second neoplasms (8–12). However, data from the 5-year survivors of childhood cancer enrolled in the Childhood Cancer Survivor Study indicated an increased, though low, risk of second neoplasms in GH-treated childhood cancer survivors compared with non GH-treated cancer survivors (13, 14). The relative risk (95% confidence interval (CI)) of second neoplasm for GH-treated versus non GH-treated patients was 2.15 (1.3–3.5), with meningiomas being the most common second neoplasm in GH-treated patients (14). Some studies of non GH-treated hypopituitary adults have suggested an increased cancer risk (15–17), but others have not (18), whereas for GH-treated cohorts, the results have been equally variable (17, 19–21). Swerdlow et al. (19) found that the overall cancer risk in 1849 British adults who received pituitary-derived GH as a child or young adult was not significantly increased compared with the general population, but the occurrence of colorectal cancer was elevated, with a standardized incidence ratio (SIR; 95% CI) of 7.9 (1.0–28.7) based on two cases. A large retrospective study in 6107 adults from the USA treated in childhood with pituitary-derived GH reported no increased mortality from de novo tumors, colon cancer, or Hodgkin lymphoma (20), and a prospective study of 289 GH-treated Swedish hypopituitary patients indicated no increased risk for overall or colorectal cancer (17).
We undertook this study to determine the risk of cancer in a large cohort of adult GH-deficient patients enrolled in HypoCCS, a prospective, international observational study. To our knowledge, this is the first reported prospective evaluation of the risk of primary cancer in a large cohort of adult patients receiving GH therapy during adulthood for GH deficiency (GHD).
Methods
Study design and study population
The primary objective of HypoCCS is to determine long-term safety of GH replacement (Humatrope; Eli Lilly and Company, Indianapolis, IN, USA) in adults with GHD (7, 22). All patients had established diagnoses of adult GHD, either alone or with other pituitary hormone deficiencies, determined by clinical history and/or biochemical testing. As HypoCCS is a non-interventional study, the diagnostic approach and treatment decisions were determined by the investigating physician but should be made in accordance with the Humatrope package insert for that country. Patients were ineligible for HypoCCS if they had unresolved or unstable conditions, listed as contraindications or precautions for GH therapy, including evidence or suspicion of active malignancy or evidence of ongoing pituitary or other intracranial tumor activity.
HypoCCS is conducted in accordance with the Declaration of Helsinki guidelines and all applicable regulatory requirements in the participating countries. Ethical review board approval and written consent from all patients for data collection, electronic processing, and publication were obtained in accordance with the national laws. HypoCCS is registered with ClinicalTrials.gov, number NCT 01088399. The patients for this analysis were recruited from Austria, Belgium, Canada, Czech Republic, Denmark, France, Germany, Hungary, Iceland, Italy, Norway, Spain, Sweden, the Netherlands, the UK, and the USA.
Between 1996 and July 2008 (the cutoff date for this analysis), the HypoCCS database included 7785 patients with at least one follow-up visit; 6840 were GH-treated during HypoCCS participation, 940 were non GH-treated, and five had unknown treatment status (excluded from analysis). Of the 6840 GH-treated patients, 5522 (81%) were reported to have adult onset (AO) GHD and 1299 (19%) childhood onset (CO) GHD.
Follow-up time was calculated per patient from date of first visit in HypoCCS until the date of the last available follow-up visit, date of completion of the study, date of the reported cancer, or date of death, whichever was the latest occurrence. It should be noted that the first HypoCCS visit date may not have reflected the exact start of GH treatment.
Primary cancer case selection
Preliminary review for potential cancer cases
HypoCCS collects data on adverse events, whether they are considered GH related by participating investigators. All adverse events in the database were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 10.0 (http://www.meddramsso.com (accessed 26 November 2010)). The HypoCCS database was systematically reviewed using the Malignancies Standardized MedDRA Query (SMQ) to select events potentially related to malignant disease. The ‘Malignancies SMQ’ is a surveillance tool containing categories of events that only indicates the possibility of cancer; thus, the preliminary list contained many non-cancers and related conditions/therapies, as well as recurrences of previous malignancies. Where adverse event terms were vague or related to procedures, requests for clarification were sent to participating investigators.
HypoCCS also prospectively collects information on new neoplastic conditions, using specific checkboxes on the case report forms. Such entries were reviewed to include any potential cases not reported as adverse events. In addition, serious adverse event (SAE) reports in the Lilly pharmacovigilance database were included if not already present in the preliminary listing. SAE reports were reviewed for all cases where death was reported.
Selection of incident cancer cases
Only incident invasive cancer events (primary site only) diagnosed after the date of enrollment in HypoCCS were included in this analysis. Potential cancer cases were categorized as de novo (included), second (included), and recurrence (excluded). De novo was defined as the first occurrence of neoplasm in a patient with no history of neoplasm, and second as development, by an independent oncogenic event, of a different neoplasm type in a patient with a previous neoplasm. The list of potential incident cases was reviewed by three authors (W W W, D M G, and L L R) who independently scored cases, as follows: 1, evident cancer case; 2, high index of suspicion for a cancer case; 3, moderate index of suspicion for a cancer case; 4, highly unlikely to reflect a cancer case; and 5, not a cancer case. Events recorded as procedures or laboratory findings potentially associated with cancer were scored according to the classification. Individual scores were averaged; where any individual score differed by two or more from other reviewers, the case was discussed and consensus reached. Cases with an average score of 1.0 were defined as ‘evident cancer cases’ for the main analysis. Sensitivity analyses were performed to account for non-specific event terms (mass, lesion, lump, and tumor), procedures (elevated prostate specific antigen (PSA), abnormal pap smear, and breast biopsy), and investigations recorded without supporting information; cases with an average score of <2.5 were defined as ‘probable cancer cases’ for ‘sensitivity analysis level 1’ and those with an average score of <3.5 were defined as ‘possible cancer cases’ for ‘sensitivity analysis level 2’. In the event of a death where no involvement of cancer was indicated, but cause could not be confirmed (20 cases through the course of the study since 1996, including eight with presumed or likely morbidity provided by the investigator), the case was not included in the main or sensitivity analyses, but the follow-up time was included in total patient-years.
SIR and 95% CI calculations
SIRs were calculated, by country, for both primary all-sites cancer and colorectal cancer, as the ratio between the number of cases observed in HypoCCS and the expected number of incident cases based on reference data. The expected case count was determined using country-, gender-, and age-specific cancer incidence rates for the general population in Europe and Canada (23), and gender-, race-, age-, and calendar year-specific cancer incidence rates for the general population in the USA (24), utilizing the corresponding number of patient-years in HypoCCS. Total observed and expected counts were obtained by sum of the strata. An estimate of total SIR was calculated using the pooled results from Europe/Canada and the USA.
Incidence rates for invasive cancers are available for all the participating countries in the GLOBOCAN data published by the International Agency for Research on Cancer (IARC (23)). GLOBOCAN 2002 was selected as the best reference because this represented the midpoint of the period of HypoCCS data collection. The USA contributed approximately half of the enrolled HypoCCS patients, and national incidence rates were available from the Surveillance, Epidemiology and End Results (SEER) program (24). Cancer cases in HypoCCS were defined according to the criteria of SEER (24), and thus excluded non-melanoma skin cancer and in-situ cancer except breast or bladder. While recognizing that in-situ breast and bladder cancers are not included in the GLOBOCAN registries (23), they were included in our analysis to provide a conservative assessment.
The observed number of cancer cases was assumed to follow a Poisson distribution, and 95% CIs were calculated using an exact method (25).
Results
Demographic factors at the start of GH therapy and details of GH treatment are provided in Table 1, for both GH-treated and non GH-treated patients from all countries. The mean±s.d. follow-up/patient for all countries was 3.7±2.9 years for GH-treated and 2.9±2.4 years for non GH-treated patients, while for patients from the USA, it was 3.3±2.6 and 3.0±2.7 years respectively.
Table 1.
Demographic and diagnostic factors at study entry and GH treatment for patients from all participating HypoCCS countries.
| GH-treated (n=6840) | Non GH-treated (n=940) | |
|---|---|---|
| Sex | ||
| Male | 3571 (52%) | 549 (58%) |
| Female | 3269 (48%) | 391 (42%) |
| GH deficiency onseta | ||
| Adult (AO) | 5522 (81%) | 813 (86%) |
| Childhood (CO) | 1299 (19%) | 127 (14%) |
| Cause of GH deficiency | ||
| Pituitary adenoma | 45% | 54% |
| Craniopharyngioma | 11% | 9% |
| Other intracranial tumor | 7% | 9% |
| Idiopathic | 17% | 9% |
| Other diagnoses | 20% | 19% |
| No. of pituitary hormone deficiencies other than GH | ||
| 0 | 16% | 13% |
| 1 | 17% | 17% |
| 2 | 18% | 20% |
| 3 | 34% | 39% |
| 4 | 15% | 11% |
| Median age at study entry (years; Q1, Q3) | 46.4 (34.1, 56.3) | 54.4 (42.2, 65.6) |
| Median starting GH dose (mg/day; Q1, Q3) | 0.30 (0.20, 0.46) | NA |
| Mean±s.d. follow-up time in study (years) | 3.7±2.9 | 2.9±2.4 |
| Total patient-years in study | 25 034 | 2688 |
19 GH-treated patients, where onset of GHD was unknown.
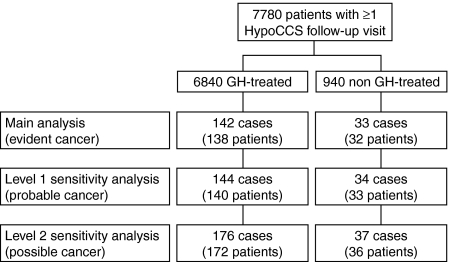
Following the case identification process, 142 cases in 138 GH-treated patients and 33 cases in 32 non GH-treated patients were included in the main analysis, with 176 cases in 172 GH-treated and 37 cases in 36 non GH-treated patients in the sensitivity analyses (Fig. 1). The most common cancer diagnoses from GH-treated patients in the main analysis were prostate cancer (n=24), breast cancer (n=16), malignant melanoma (n=15, including one case of lentigo maligna), colorectal cancer (n=11), lung cancer (n=11), thyroid cancer (n=9), and glioma (n=9, including specified cases of astrocytoma and glioblastoma multiforme).
Figure 1.
Adult hypopituitary patients reviewed for cancer case selection and inclusion in the main, level 1 and level 2 sensitivity analyses, according to GH treatment status as adults in the HypoCCS study. Note i) that an individual patient could have had more than one cancer event, with each event counted as a separate case for the incidence analysis; ii) case and patient counts are cumulative across analysis levels.
Of the 138 GH-treated patients with cancer cases included in the main analysis, 85 were male and 21 had a report of previous malignant disease (Table 2). Of the previous primary cancers, eight were intracranial tumors (astrocytoma, medulloblastoma, and dysgerminoma) or leukemia, of the type often occurring during childhood, potentially reflected in the mean age at onset of the second cancer of 30.6±11.2 years. The remaining 13 patients, with average age at onset of second cancer of 68.0±10.1 years, had primary cancers more typically associated with adulthood, including six patients with skin cancers and single cases of breast, cervical, lung, nasal, nasopharyngeal, and thyroid cancers, plus hemangiopericytoma (Table 2).
Table 2.
Second cancers in GH-treated patients included in the main analysis.
| Previous history of neoplastic disease | Second cancer observed during HypoCCS | Age at cancer onset (years)a | GHD onset type | Sex |
|---|---|---|---|---|
| Astrocytoma/oligoastrocytoma | Glioblastomab | 31.1 | AO | F |
| Astrocytoma | Thyroid cancer | 25.4 | CO | F |
| Astrocytoma | Uterine cancer | 53.7 | AO | F |
| Medulloblastoma | Glioblastoma | 20.2 | CO | M |
| Medulloblastoma | Papillary thyroid carcinoma | 34.9 | CO | M |
| Pineal dysgerminoma | Glioblastoma multiforme | 31.4 | CO | M |
| Acute lymphocytic leukemia | Malignant melanoma | 31.4 | CO | F |
| Lymphoblastic leukemia | Ewing sarcoma | 16.8 | CO | M |
| Basal cell carcinoma | Polycythemia vera | 65.9 | AO | M |
| Basal cell carcinoma and pituitary adenoma | Malignant melanoma | 84.0 | AO | M |
| Basal cell carcinoma and pituitary adenoma | Pancreatic islet cell cancer | 69.5 | AO | F |
| Basal cell carcinoma, skin cancer, and craniopharyngioma | Lentigo maligna | 81.9 | CO | M |
| Malignant melanoma | Malignant melanomab | 57.6 | AO | F |
| Squamous cell carcinoma and pituitary adenoma | Malignant melanoma | 64.7 | AO | M |
| Breast cancer and pituitary adenoma | Lung cancer | 73.1 | AO | F |
| Cervical cancer | Glioma | 61.3 | AO | F |
| Hemangiopericytoma | Lymphoma | 67.1 | AO | M |
| Lung cancer and pituitary adenoma | Colon adenocarcinoma | 77.3 | AO | M |
| Maxillary sinus cancer | Lung cancer | 60.4 | AO | F |
| Nasopharyngeal carcinoma | Malignant soft tissue tumor | 47.9 | AO | M |
| Papillary thyroid cancer, meningioma | Endometrial adenocarcinoma | 73.5 | AO | F |
AO, adult onset; CO, childhood onset; F, female; GHD, GH deficiency; M, male.
During HypoCCS participation.
Potentially recurrent cancers, but second malignancy could not be discounted.
The SIRs for all primary invasive cancers in HypoCCS are shown by country when expected case count exceeds five and total in Table 3. The overall estimated SIR for evident cases (main analysis) was 0.88 (95% CI 0.74–1.04). The SIR for cancers in GH-treated patients from the USA was 0.94 (95% CI 0.73–1.18).
Table 3.
Primary invasive cancer incidence by country for GH-treated adult hypopituitary patients in HypoCCS. SIRs were calculated for reference population cancer rates from SEER program (24) for the USA, and from GLOBOCAN (23) for all other countries.
| Country | n | Observed cases | Expected cases | SIR (95% CI) |
|---|---|---|---|---|
| Denmark | 151 | 7 | 5.48 | 1.28 (0.51–2.63) |
| Germany | 435 | 5 | 9.85 | 0.51 (0.16–1.18) |
| Italy | 833 | 5 | 11.52 | 0.43 (0.14–1.01) |
| Sweden | 348 | 15 | 18.87 | 0.79 (0.44–1.31) |
| The Netherlands | 439 | 16 | 16.12 | 0.99 (0.57–1.61) |
| UK | 462 | 10 | 11.27 | 0.89 (0.43–1.63) |
| USA | 3165 | 71 | 75.79 | 0.94 (0.73–1.18) |
| Other countriesa | 1007 | 13 | 12.72 | 1.02 (0.54–1.75) |
| Total | 6840 | 142 | 161.63 | 0.88 (0.74–1.04) |
CI, confidence interval; SIR, standardized incidence ratio.
Sum of countries where expected case count was <5: Austria, Belgium, Canada, Czech Republic, France, Hungary, Iceland, Norway, and Spain.
The only country with sufficient patients for calculation of SIR in non GH-treated patients was the USA (Table 4). Overlapping 95% CIs for SIR were observed between the GH-treated and non GH-treated groups, with the SIR for non GH-treated patients from the USA being similar to the GH-treated value but with wider 95% CI (SIR 1.16, 95% CI 0.76–1.69). In addition, for the USA cohort, when age at entry into HypoCCS for GH-treated patients was analyzed by age quartiles (<35.0, 35.0 to <47.4, 47.4 to <57.5, and ≥57.5 years), the SIR was 3.79 (95% CI 1.39–8.26) for the lowest age group (SIR was not elevated in any other age quartile). However, this was based on six observed and 1.58 expected cases; four of the six patients had CO GHD, and for five of the six patients, the cancer was a second neoplasm following histories of leukemia (two cases), pineal dysgerminoma, craniopharyngioma, and pituitary adenoma. Also, when considering GHD onset in patients from the USA, the SIR was 2.74 (1.18–5.41) for CO patients (eight cases) versus 0.86 (0.66–1.11) for AO patients (63 cases). Other subgroup analyses for all countries at the main analysis level did not detect potential predictive factors in any of the following parameters: previous history of cancer (yes versus no), gender (male versus female), GHD onset (AO versus CO), GH therapy prior to HypoCCS (yes versus no).
Table 4.
Primary invasive cancer incidence in GH-treated and non GH-treated patients from the USA. SIRs were calculated using SEER data (24).
| Treatment group | Observed cases | Expected cases | SIR (95% CI) |
|---|---|---|---|
| GH-treated (n=3165) | 71 | 75.79 | 0.94 (0.73–1.18) |
| Non GH-treated (n=631) | 27 | 23.28 | 1.16 (0.76–1.69) |
CI, confidence interval; SIR, standardized incidence ratio.
Previous exposure to GH before HypoCCS entry cannot be precisely determined for these analyses, either in terms of contribution to total number of cases or patient-years of exposure. However, for the USA, the SIR for all GH-treated patients was similar to the SIR for the GH-treated patients who were not previously exposed to GH before study entry. The SIR for GH-treated patients who were GH naive at study entry was 0.91 (0.69–1.18) based on 58 observed cases and an expected count of 63.68 versus the SIR of 0.94 (0.73–1.18) for all GH-treated patients.
A total of 16 cases of colorectal cancer were observed in the overall HypoCCS population (11 in the GH-treated group and five in the non GH-treated group), with 11 in the USA (six GH-treated and five non GH-treated). The estimated SIR for cases in GH-treated patients was 0.60 (0.30–1.08) versus 1.51 (0.49–3.52) for non GH-treated patients (Table 5). All 11 colorectal cancers in the GH-treated patients were in-patients with AO GHD, all of whom were considered naive to GH treatment at HypoCCS entry. Of the observed 11 cases, nine were male GH-treated patients; however, the 95% CI for the different genders showed substantial overlap (Table 5).
Table 5.
Primary colorectal cancer incidence in all GH-treated and non GH-treated patients and in GH-treated patients by gender. SIRs were calculated using SEER data (24) for cases from the USA (n=6, GH-treated and n=5, non GH-treated), and GLOBOCAN data (23) for patients from all other countries (n=5, GH-treated).
| Treatment group | Observed cases | Expected cases | SIR (95% CI) |
|---|---|---|---|
| GH-treated (n=6840) | 11 | 18.18 | 0.60 (0.30–1.08) |
| Non GH-treated (n=940) | 5 | 3.31 | 1.51 (0.49–3.52) |
| GH-treated males (n=3571) | 9 | 12.28 | 0.73 (0.34–1.39) |
| GH-treated females (n=3269) | 2 | 5.91 | 0.34 (0.04–1.22) |
CI, confidence interval; SIR, standardized incidence ratio.
Inclusion of cases where malignant status could not be clarified increased the number of observed cases from 142 to 144 (level 1 sensitivity, representing probable cases) and 176 (level 2 sensitivity, representing possible cases), giving SIRs of 0.89 (95% CI 0.75–1.05) and 1.09 (0.93–1.26) respectively, for all countries, and 0.96 (0.75–1.21) and 1.31 (1.06–1.59) respectively, for patients from the USA. The 34 cases added for the sensitivity analyses included event terms of breast biopsy/lump/mass/neoplasm (eight cases), elevated PSA/prostate nodule (six cases), lung lesion/neoplasm/nodule (five cases), abnormal pap smear (four cases), unspecified brain lesions (two cases), and renal mass (two cases).
Discussion
Replacement therapy with GH in adults with GHD was first approved in 1995. Studies in childhood cancer survivors indicated an approximate two- to three-fold increased risk of second neoplasms, and there is a concern that adult GH treatment could be associated with an increased cancer risk (17, 19, 20). In contrast to previous studies in GH-treated adults, this is the first reported prospective, large-scale, follow-up examining cancer occurrence. This study indicated no increased risk for primary cancers in GH-treated adult hypopituitary patients in HypoCCS compared with general population cancer rates, standardized by country, gender, and age.
Our analysis included 21 patients with a previous history of malignant disease prior to HypoCCS entry, including eight with history of intracranial tumors or leukemia. Analysis of cancer cases in the USA by age quartiles indicated an increased risk for the youngest group (<35 years), based on 1.58 expected cases and six observed cases. The second neoplasms followed intracranial tumors or leukemia in five of the six cases, and four of these had CO GHD. Similarly, analysis by GHD onset in patients from the USA, indicated an elevated SIR for CO patients, based on eight cases. The 95% CIs in these subgroups were wide, with lower confidence limits close to 1, and it is reasonable to suggest that such patients may have had a higher risk of second neoplasms, consistent with previous reports demonstrating an increased risk associated with survival of childhood neoplastic disease (13, 14). Previous radiotherapy for childhood cancer may be an important influence on the increased cancer incidence in this group. However, there are insufficient data, regarding history, type, and quantity of radiotherapy in the HypoCCS database, to conduct a specific analysis to assess the risk for radiation-associated cancer morbidity.
An increased risk for colorectal cancer (SIR=7.9, 95% CI 1.0–28.7) was reported in adults from the UK who received pituitary GH between 1959 and 1985 during childhood or early adulthood (19). This was based on two cases observed versus a very small expected count. Although the authors acknowledged that their findings were based on a small observed case count and that data collected on pituitary GH dosing regimens may not relate directly to modern biosynthetic GH regimens, further investigation of colorectal cancer in current cohorts was justified. Specific analysis for colorectal cancer in HypoCCS yielded 16 cases in total from all countries. The SIR was 0.60 (95% CI 0.3–1.08) for the 11 cases in the GH-treated group and 1.51 (0.49–3.52) for the five cases in the non GH-treated group. All 11 colorectal cancers in the GH-treated group were inpatients naive to GH treatment at HypoCCS entry and, in contrast to the population studied previously (19), all had AO GHD.
Our study included sensitivity analyses that incorporated cases of uncertain malignant status. Although the analyses were deliberately liberal, no overall SIR was significantly elevated upon inclusion of these additional cases. The SIR for patients from the USA was only elevated for the level 2 sensitivity analysis (possible cases). Because such unconfirmed cases will not be recorded in the comparator registries and the SIR increase was small and only for patients from the USA, we believe that this result is not clinically significant and unlikely to indicate a true increased risk of primary malignancy in the HypoCCS USA cohort.
There were a number of limitations of this analysis, particularly the time of follow-up in HypoCCS (mean 3.7 years), which is relatively short but, at least in part, limited by the time since approval of the adult GHD indication. Similar analysis with longer follow-up is needed to confirm assurance of no increase in cancer risk during GH therapy in adults. In addition, the general population may not be an ideal comparison group due to differences in the health profile versus the GH-deficient population. However, our results are reassuring since no increase relative to the general population was found, and GHD patients are unlikely to be healthier than the general population. Our study covered the period 1996–2008 but did not take into account changes in clinical practice during that period. The etiologies of GHD in the overall cohort changed during the course of HypoCCS, with a relative decrease in the proportion of intracranial tumors and pituitary dysfunction diagnoses (22). In comparison of SIRs for GH-treated and non GH-treated patients, those treated with GH were relatively younger and had a higher rate of idiopathic GHD diagnosis (Table 1), which may influence the risk for primary cancers; additionally, the size of the non GH-treated group was much smaller than the GH-treated group. Cancer cases were included in the analysis without considering induction time; cases diagnosed soon after GH initiation for GH naive patients in HypoCCS were unlikely due to GH treatment, leading to overestimation of observed cases. In addition, follow-up time was only during HypoCCS, and a patient treated with GH prior to HypoCCS would have had extra time not added to the patient-years calculation. However, we attempted to address this potential bias by calculating SIR for patients who were believed to be GH naive at HypoCCS entry, which was found to be similar to the overall SIR.
In conclusion, the incidence of primary cancer in patients with GHD enrolled in HypoCCS who were treated with GH as adults appears similar to that of the general population. However, such surveillance should be continued to provide a thorough assessment of cancer risk during longer follow-up, for specific tumors and patients in specific risk groups.
Declaration of interest
C J Child, A G Zimmermann, J J Li and H Jung are employees and stockholders of Eli Lilly and Company (Indianapolis, IN, USA). E M Erfurth, L L Robison and W W Woodmansee are members of Medical Research advisory boards and have received consulting fees from Eli Lilly and Company. W W Woodmansee is also a former employee and stockholder of Eli Lilly and Company. D M Green had no competing interest or financial disclosures to report.
Funding
HypoCCS is sponsored by Eli Lilly and Company (Indianapolis, IN, USA).
Acknowledgements
The authors gratefully thank all participating investigators and patients. Additionally, the authors would like to thank Dr Peter Bates (Cambridge Medical Writing Services, UK) for his editorial review of the manuscript, and Qi Rong (inVentiv Clinical Solutions, Indianapolis, USA) for assistance with statistical programing and analysis.
HypoCCS Advisory Board
Membership of the HypoCCS Advisory Board as of February 2011 was Andrea F Attanasio (Agliano Terme, Italy), Paolo Beck-Peccoz (Milan, Italy), Werner F Blum (Bad Homburg, Germany), Roger Bouillon (Leuven, Belgium), Philippe Chanson (Le Kremlin-Bicêtre, France), Kazuo Chihara (Kakogawa, Japan), David R Clemmons (Chapel Hill, USA), Eva Marie Erfurth (Lund, Sweden), Mark Hartman, (Indianapolis, USA), Ken Y Ho (Brisbane, Australia), Heike Jung (Bad Homburg, Germany), David L Kleinberg (New York, USA), Steven W J Lamberts (Rotterdam, Netherlands), Shlomo Melmed (Los Angeles, USA), Richard J Ross (Sheffield, United Kingdom), Les L Robison (Memphis, USA), Akira Shimatsu (Kyoto, Japan), Christian J Strasburger (Berlin, Germany), Susan M Webb (Barcelona, Spain), Whitney W Woodmansee (Boston, USA), and Alan G Zimmermann (Indianapolis, USA).
References
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocrine Reviews. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncology. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic V, Mattsson AF, Gaillard RC, Wilton P, Koltowska-Häggstöm M, Ranke MB. Serum insulin-like growth factor I (IGF-I), IGF-binding proteins 2 and 3, and the risk for development of malignancies in adults with growth hormone (GH) deficiency treated with GH: data from KIMS (Pfizer International Metabolic Database) Journal of Clinical Endocrinology and Metabolism. 2010;95:4449–4454. doi: 10.1210/jc.2010-0287. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- Attanasio AF, Bates PC, Ho KKY, Webb SM, Ross RJ, Strasburger CJ, Bouillon R, Crowe B, Selander K, Valle D, Lamberts SWJ, for the International HypoCCS Advisory Board Human growth hormone replacement in adult hypopituitary patients: long-term effects on body composition and lipid status – 3-year results from the HypoCCS database. Journal of Clinical Endocrinology and Metabolism. 2002;87:1600–1606. doi: 10.1210/jc.87.4.1600. [DOI] [PubMed] [Google Scholar]
- Abs R, Bengtsson BÅ, Hernberg-Stâhl E, Monson JP, Tauber JP, Wilton P, Wüster C, for the KIMS Study Group and the KIMS International Board GH replacement in 1034 growth hormone deficient hypopituitary adults: demographic and clinical characteristics, dosing and safety. Clinical Endocrinology. 1999;50:703–713. doi: 10.1046/j.1365-2265.1999.00695.x. [DOI] [PubMed] [Google Scholar]
- Chung TT, Drake WM, Evanson J, Walker D, Plowman PN, Chew SL, Grossman AB, Besser GM, Monson JP. Tumour surveillance imaging in patients with extrapituitary tumours receiving growth hormone replacement. Clinical Endocrinology. 2005;63:274–279. doi: 10.1111/j.1365-2265.2005.02338.x. [DOI] [PubMed] [Google Scholar]
- Jostel A, Mukherjee A, Hulse PA, Shalet SM. Adult growth hormone replacement therapy and neuroimaging surveillance in brain tumour survivors. Clinical Endocrinology. 2005;62:698–705. doi: 10.1111/j.1365-2265.2005.02282.x. [DOI] [PubMed] [Google Scholar]
- Murray RD, Darzy KH, Gleeson HK, Shalet SM. GH-deficient survivors of childhood cancer: GH replacement during adult life. Journal of Clinical Endocrinology and Metabolism. 2002;87:129–135. doi: 10.1210/jc.87.1.129. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Reddingius RE, Higgins CD, Spoudeas HA, Phipps K, Qiao Z, Ryder DJ, Brada M, Hayward RD, Brook CGD, Hindmarsh PC, Shalet SM. Growth hormone treatment of children with brain tumors and risk of tumor recurrence. Journal of Clinical Endocrinology and Metabolism. 2000;85:4444–4449. doi: 10.1210/jc.85.12.4444. [DOI] [PubMed] [Google Scholar]
- Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database) Journal of Pediatrics. 2010;157:265–270. doi: 10.1016/j.jpeds.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Sklar CA, Mertens AC, Mitby P, Occhiogrosso G, Qin J, Heller G, Yasui Y, Robison LL. Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the childhood cancer survivor study. Journal of Clinical Endocrinology and Metabolism. 2002;87:3136–3141. doi: 10.1210/jc.87.7.3136. [DOI] [PubMed] [Google Scholar]
- Ergun-Longmire B, Mertens AC, Mitby P, Qin J, Heller G, Shi W, Yasui Y, Robison LL, Sklar CA. Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. Journal of Clinical Endocrinology and Metabolism. 2006;91:3494–3498. doi: 10.1210/jc.2006-0656. [DOI] [PubMed] [Google Scholar]
- Popovic V, Damjanovic S, Micic D, Nesovic M, Djurovic M, Petakov M, Obradovic S, Zoric S, Simic M, Penezic Z, Marinkovic J. Increased incidence of neoplasia in patients with pituitary adenomas. The Pituitary Study Group. Clinical Endocrinology. 1998;49:441–445. doi: 10.1046/j.1365-2265.1998.00536.x. [DOI] [PubMed] [Google Scholar]
- Stochholm K, Gravholt CH, Laursen T, Laurberg P, Andersen M, Kristensen LØ, Feldt-Rasmussen U, Christiansen JS, Frydenberg M, Green A. Mortality and GH deficiency: a nationwide study. European Journal of Endocrinology. 2007;157:9–18. doi: 10.1530/EJE-07-0013. [DOI] [PubMed] [Google Scholar]
- Svensson J, Bengtsson BA, Rosén T, Odén A, Johannsson G. Malignant disease and cardiovascular morbidity in hypopituitary adults with or without growth hormone replacement therapy. Journal of Clinical Endocrinology and Metabolism. 2004;89:3306–3312. doi: 10.1210/jc.2003-031601. [DOI] [PubMed] [Google Scholar]
- Erfurth EM, Bülow B, Mikoczy Z, Hagmar L. Incidence of a second tumor in hypopituitary patients operated for pituitary tumors. Journal of Clinical Endocrinology and Metabolism. 2001;86:659–662. doi: 10.1210/jc.86.2.659. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Adlard P, Preece MA. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet. 2002;360:273–277. doi: 10.1016/S0140-6736(02)09519-3. [DOI] [PubMed] [Google Scholar]
- Mills JL, Schonberger LB, Wysowski DK, Brown P, Durako SJ, Cox C, Kong F, Fradkin JF. Long-term mortality in the United States cohort of pituitary-derived growth hormone recipients. Journal of Pediatrics. 2004;144:430–436. doi: 10.1016/j.jpeds.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Monson JP. Long-term experience with GH replacement therapy: efficacy and safety. European Journal of Endocrinology. 2003;148(Suppl 2):S9–S14. doi: 10.1530/eje.0.148S009. [DOI] [PubMed] [Google Scholar]
- Webb SM, Strasburger CJ, Mo D, Hartman ML, Melmed S, Jung H, Blum WF, Attanasio AF. Changing patterns of the adult growth hormone deficiency diagnosis documented in a decade-long global surveillance database. Journal of Clinical Endocrinology and Metabolism. 2009;94:392–399. doi: 10.1210/jc.2008-0713. [DOI] [PubMed] [Google Scholar]
- GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide (2002 estimates). International Agency for Research on Cancer (IARC). http://globocan.iarc.fr/ (accessed 26 November 2010).
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M & Edwards BK (eds). SEER Cancer Statistics Review, 1975–2004, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2004/ (accessed 26 November 2010).
- Breslow NE & Day NE (eds). Statistical Methods in Cancer Research, Volume II – The design and analysis of cohort studies. IARC Scientific Publications 1987 82 65–69. [PubMed]