Abstract
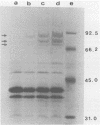
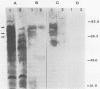
Iron starvation conditions limited the growth of Salmonella typhi, as evidenced by an increase in the lag phase of a culture and a decrease in the number of bacteria reached in the stationary phase. The analysis of the outer membrane of bacteria grown under these conditions identified new protein components with apparent molecular weights of 83,000, 78,000, and 69,000. The extent of induction of these proteins was regulated by increased iron deprivation. Immunoblot analysis showed that the serum of patients with typhoid fever exhibited an immunoglobulin G response to these iron-deprivation-induced proteins. The results of bioassays and DNA-DNA hybridization experiments indicated that pathogenic strains of S. typhi produced enterochelin but not aerobactin. Immunodetection with an anti-FepA antiserum confirmed that one of the induced proteins is the S. typhi analog of the Escherichia coli fepA gene product. These studies suggest a role for iron uptake in the pathogenesis of typhoid fever and confirm the immunogenicity of some of the outer membrane proteins of this pathogen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agüero M. E., Cabello F. C. Relative contribution of ColV plasmid and K1 antigen to the pathogenicity of Escherichia coli. Infect Immun. 1983 Apr;40(1):359–368. doi: 10.1128/iai.40.1.359-368.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K., Parr T. R., Jr, Parker C. D., Hancock R. E. Bordetella pertussis major outer membrane porin protein forms small, anion-selective channels in lipid bilayer membranes. J Bacteriol. 1986 Apr;166(1):212–216. doi: 10.1128/jb.166.1.212-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985 Nov;50(2):392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Cloning of the aerobactin-mediated iron assimilation system of plasmid ColV. J Bacteriol. 1983 Feb;153(2):1111–1113. doi: 10.1128/jb.153.2.1111-1113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón I., Lobos S. R., Rojas H. A., Palomino C., Rodríguez L. H., Mora G. C. Antibodies to porin antigens of Salmonella typhi induced during typhoid infection in humans. Infect Immun. 1986 Apr;52(1):209–212. doi: 10.1128/iai.52.1.209-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna B., Nicoletti M., Visca P., Casalino M., Valenti P., Maimone F. Composite IS1 elements encoding hydroxamate-mediated iron uptake in FIme plasmids from epidemic Salmonella spp. J Bacteriol. 1985 Apr;162(1):307–316. doi: 10.1128/jb.162.1.307-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss E. H., Hollifield W. C., Jr, Neilands J. B. Absence of ferric enterobactin receptor modification activity in mutants of Escherichia coli K-12 lacking protein a. Biochem Biophys Res Commun. 1979 Nov 14;91(1):29–34. doi: 10.1016/0006-291x(79)90578-3. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Nahlik M. S., Neilands J. B., McIntosh M. A. Physical and genetic characterization of cloned enterobactin genomic sequences from Escherichia coli K-12. Gene. 1985;34(1):47–54. doi: 10.1016/0378-1119(85)90293-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976 Sep;127(3):1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. 2. N Engl J Med. 1970 Oct 1;283(14):739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986 Aug 15;261(23):10797–10801. [PubMed] [Google Scholar]
- Moody E. E., Hayes W. Chromosome transfer by autonomous transmissible plasmids: the role of the bacterial recombination (rec) system. J Bacteriol. 1972 Jul;111(1):80–85. doi: 10.1128/jb.111.1.80-85.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlik M. S., Fleming T. P., McIntosh M. A. Cluster of genes controlling synthesis and activation of 2,3-dihydroxybenzoic acid in production of enterobactin in Escherichia coli. J Bacteriol. 1987 Sep;169(9):4163–4170. doi: 10.1128/jb.169.9.4163-4170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982 Dec;38(3):948–952. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. R., Neilands J. B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970 Mar 12;38(5):989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain of Escherichia coli K1. Infect Immun. 1984 Oct;46(1):159–167. doi: 10.1128/iai.46.1.159-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]