Abstract
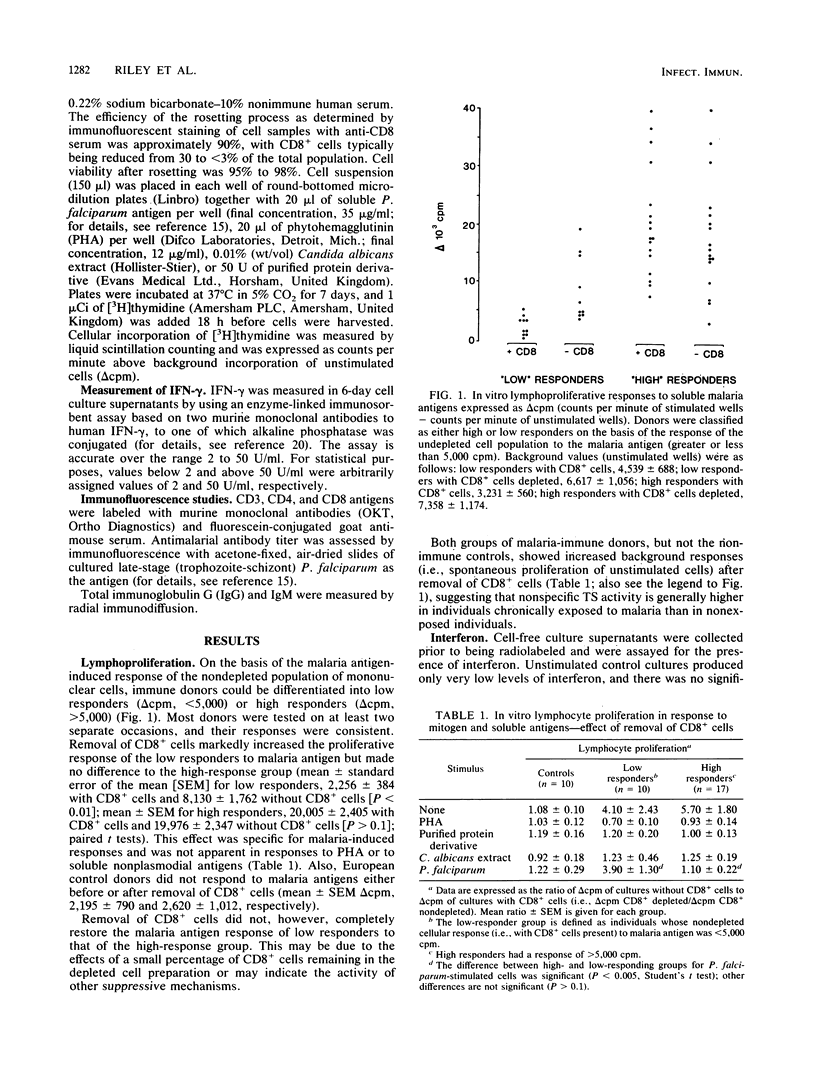
Infection with Plasmodium falciparum induces marked disturbances in normal immunoregulatory functions. Antigen-specific immunosuppression is a feature of acute malaria and has been linked to activation of CD8+ T suppressor cells. Among immune adults, cell-mediated immune responses to malaria antigens are extremely variable when measured in vitro, and there is no obvious relation between responsiveness and resistance to clinical disease. In this study, when CD8+ cells were removed from peripheral blood mononuclear cell preparations obtained from individuals who responded poorly to a soluble malaria antigen preparation, both lymphoproliferation and gamma interferon production were significantly enhanced, but responses to other soluble antigens and mitogen were unaffected. No effect of CD8+ cell depletion was seen in individuals whose undepleted mononuclear cells gave a high response to the malaria antigen. This suggests that for some malaria-exposed individuals, CD8+ cells activated in vitro by exposure to malaria antigens suppress other cellular responses and may obscure the presence of potentially protective immune mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brasseur P., Agrapart M., Ballet J. J., Druilhe P., Warrell M. J., Tharavanij S. Impaired cell-mediated immunity in Plasmodium falciparum-infected patients with high-parasitemia and cerebral malaria. Clin Immunol Immunopathol. 1983 Apr;27(1):38–50. doi: 10.1016/0090-1229(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Brown I. N., Watson S. R., Sljivić V. S. Antibody response in vitro of spleen cells from Plasmodium yoelii-infected mice. Infect Immun. 1977 May;16(2):456–460. doi: 10.1128/iai.16.2.456-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druilhe P., Rhodes-Feuillette A., Canivet M., Gentilini M., Periês J. Circulating interferon in patients with Plasmodium falciparum, P. ovale and P. vivax malaria. Trans R Soc Trop Med Hyg. 1982;76(3):422–423. doi: 10.1016/0035-9203(82)90207-3. [DOI] [PubMed] [Google Scholar]
- Gilbreath M. J., MacDermott R. P., Pavanand K., Phisphumvithi P., Kongchareon S., Wimonwattrawatee T. Deficiency of Con A-induced suppressor cell activity in peripheral blood mononuclear cells from Thai adults naturally infected with Plasmodium falciparum and Plasmodium vivax. Parasite Immunol. 1983 Sep;5(5):431–440. doi: 10.1111/j.1365-3024.1983.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K., Looareesuwan S., Supanaranond W., Phillips R. E., Chanthavanich P., Warrell D. A. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986 Apr;153(4):763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Piessens W. F., Ratiwayanto S., Hussein P. R., Kurniawan L., Piessens P. W., Campbell J. R., Marwoto H. A. Reduction of suppressor T lymphocytes in the tropical splenomegaly syndrome. N Engl J Med. 1984 Feb 9;310(6):337–341. doi: 10.1056/NEJM198402093100601. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Sprott V. M., Playfair J. H. Differential involvement of non-specific suppressor T cells in two lethal murine malaria infections. Clin Exp Immunol. 1981 Aug;45(2):433–438. [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Micklem H. S., Ure J. M. Immunosuppression in murine malaria. I. Response to type III pneumococcal polysaccharide. Immunology. 1977 May;32(5):635–644. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Riley E. M., Andersson G., Otoo L. N., Jepsen S., Greenwood B. M. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin Exp Immunol. 1988 Jul;73(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- Riley E. M., Jepsen S., Andersson G., Otoo L. N., Greenwood B. M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988 Mar;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Hamilton A. J., Nicholas J., Sinden R. E. The fate of the circumsporozoite antigens during the exoerythrocytic stage of Plasmodium berghei. Eur J Cell Biol. 1988 Apr;46(1):25–30. [PubMed] [Google Scholar]
- Theander T. G., Bygbjerg I. C., Jepsen S., Svenson M., Kharazmi A., Larsen P. B., Bendtzen K. Proliferation induced by Plasmodium falciparum antigen and interleukin-2 production by lymphocytes isolated from malaria-immune individuals. Infect Immun. 1986 Jul;53(1):221–225. doi: 10.1128/iai.53.1.221-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Andersson G., Stoczkowska M., Shabo R., Romero P., Patarroyo M. E., Wigzell H., Perlmann P. Production of IL 2 and IFN-gamma by T cells from malaria patients in response to Plasmodium falciparum or erythrocyte antigens in vitro. J Immunol. 1985 Nov;135(5):3498–3504. [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann H., Patarroyo M. E., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983 Aug;53(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Romero P., Patarroyo M. E., Björkman A., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. III. Proliferative response to antigen in vitro and subset composition of T cells from patients with acute infection or from immune donors. Clin Exp Immunol. 1984 Nov;58(2):380–387. [PMC free article] [PubMed] [Google Scholar]
- Weidanz W. P. Malaria and alterations in immune reactivity. Br Med Bull. 1982 May;38(2):167–172. doi: 10.1093/oxfordjournals.bmb.a071754. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Weintraub J., Nkrumah F. K., Evans C. B., Tigelaar R. E., Rosenberg Y. J. Immunity to Plasmodium berghei yoelii in mice. II. Specific and nonspecific cellular and humoral responses during the course of infection. J Immunol. 1978 Aug;121(2):629–636. [PubMed] [Google Scholar]
- Zlotnik A., Shimonkevitz R. P., Gefter M. L., Kappler J., Marrack P. Characterization of the gamma-interferon-mediated induction of antigen-presenting ability in P388D1 cells. J Immunol. 1983 Dec;131(6):2814–2820. [PubMed] [Google Scholar]



