Abstract
A 63-year-old female presented with a 12-week history of worsening proximal pain and stiffness. She was diagnosed with polymyalgia rheumatica and started on corticosteroids. The authors were unable to wean-off her steroid treatment, despite trying various steroid sparing agents on different occasions with no benefit. In August 2010, she was diagnosed with giant cell arteritis with a temporal artery biopsy and ultrasound of the temporal and axillary arteries. An fluorine-18-deoxyglucose positron emission tomography CT showed increased uptake in the aorta and major vessels, in keeping with widespread large vessel involvement. Due to the disease severity, the failure of previous disease-modifying agents and the development of steroid related sideeffects, the authors decided to treat her with intravenous tocilizumab (TCZ;an interleukin 6 blocker). After her first infusion, the patient reported excellent response with normalisation of her inflammatory markers. Prednisolone reduced from 20 mg to 3.5 mg /day after five infusions of TCZ (8 mg/kg).
Background
Giant cell arteritis (GCA) is the commonest vasculitis mainly involving the large-sized and medium-sized arteries.1 Aortic and large vessel involvement is increasingly recognised during long-term follow-up.2 GCA of the aorta can remain asymptomatic for many years and leads to an increased risk of aneurysms and dissections, particularly of the thoracic aorta.3 4 Evolving vascular imaging techniques such as duplex ultrasound,5 CT, MRI and fluorine-18-deoxyglucose positron emission tomography (18F-FDG-PET) have greatly increased the ability to detect arterial changes in large vessel vasculitis.6
Polymyalgia rheumatica (PMR) is an associated inflammatory rheumatic disease presenting with pain and stiffness in the shoulder and pelvic girdle, muscle tenderness of the arms and legs, constitutional symptoms such as fever, weight loss and fatigue.1 Several disorders mimic PMR and it is now felt that PMR is a heterogeneous disease that covers a spectrum of patients, who may have a seronegative inflammatory arthritis to patients with large vessel vasculitis.7 8 PMR is also associated with cranial GCA (temporal arteritis) in 10% of the cases and up to 50% of the cases of GCA may have polymyalgic symptoms at presentation.
Corticosteroids (CS) constitute the preferred treatment for both GCA and PMR. However, there is an unmet need for therapy when disease is refractory to steroids, treatment is prolonged or complicated by chronic side effects. A meta-analysis of 3 methotrexate trials has shown at best, a modest therapeutic effect9 and there is no conclusive evidence about other immunosuppressive agents such as azathioprine10 and biologic agents such as tumour necrosis factor-α (TNF–α) inhibitors including etanercept and infliximab.11 12
Elevation of interleukin 6 (IL-6) in both PMR and GCA was originally reported in 199013 and subsequent reports have shown association of circulating IL-6 in patients with active disease.14 15 Studies have shown significant decrease of IL-6 levels with remission of clinical symptoms. However, CS-induced suppression of circulating IL-6 levels is short-lived and continuous CS therapy is required for the IL-6 suppressive effect.16
IL-6 inhibition with tocilizumab (TCZ;humanised anti-IL-6 receptor monoclonal antibody) appears to be a logical option for treating gonococcus (GC)-resistant disease. It is the first recombinant humanised antihuman monoclonal antibody of the immunoglobulin G1 subclass directed against the IL-6R17 and shown to be efficacious and safe, in treatment of rheumatoid arthritis.18 Clinical efficacy and safety studies with TCZ are ongoing in other disease areas such as systemic-onset juvenile idiopathic arthritis. We describe the successful use of TCZ in a case of polymyalgic onset, temporal artery biopsy (TAB)-positive GCA with large vessel involvement confirmed by FDG-PET and duplex ultrasound scans.
Case presentation
A 63-year-old female, diagnosed with PMR and treated with oral steroids since August 2003, presented with 12-week history of worsening proximal pain and stiffness. She had tried steroid sparing agents including methotrexate (2004), leflunomide (2007) and azathioprine (2009), with lack of efficacy or tolerability and was unable to wean-off her steroids.
Her symptoms worsened in August 2010 accompanied by new onset of temporal headache, fatigue, loss of appetite, loss of weight, transient visual loss and C reactive protein (CRP) of 78 mg/l. Her steroids increased to 60 mg/day. Urgent investigations confirm GCA.
Investigations
GCA was confirmed with a TAB showing giant cells and intimal hyperplasia, and a temporal artery ultrasound showing the typical ‘halo’ sign in both temporal as well as axillary arteries (figure 1). An FDG-PET-CT showed increased uptake in the entire aorta up to its bifurcation, axillary and subclavian arteries in keeping with widespread large vessel involvement (figure 2).
Figure 1.
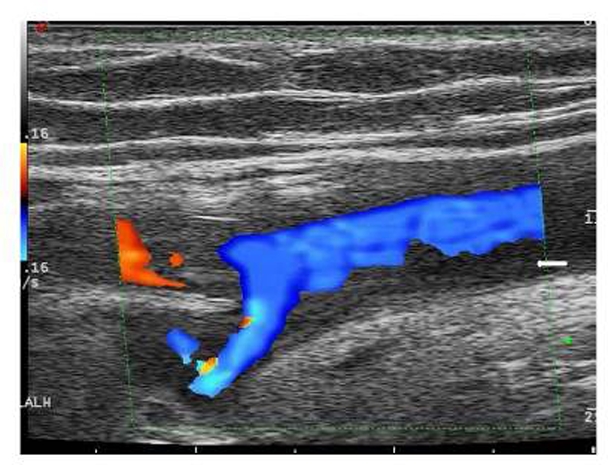
Duplex ultrasound scan of the right axillary artery showing the typical ‘halo’ sign (see arrow).
Figure 2.
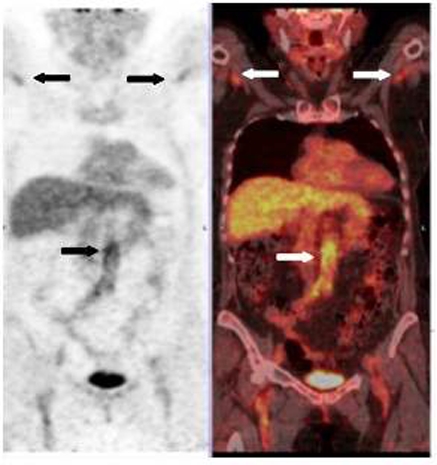
Fluorine-18-deoxyglucose positron emission tomography scan showing uptake in the abdominal wall of the aorta and subclavian axillary arteries.
Treatment
In the context of new onset of TAB-positive GCA with FDG-PET and ultrasound showing aortic and large vessel involvement, and a background of steroid and disease-modifying antirheumatic drug refractory PMR–she was treated with TCZ 8 mg/kg monthly intravenous infusions, after obtaining written informed consent.
Outcome and follow-up
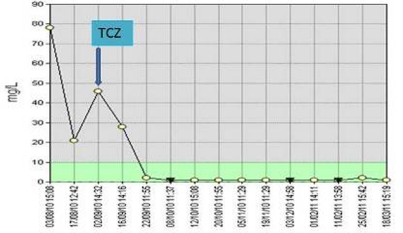
Within 24 h of the first infusion, the patient reported excellent response with resolution of pain in her shoulders and pelvic girdle (100% improvement in global visual analogue scale), morning stiffness and constitutional features. Her quality of life (SF-36 physical and mental component scores) also registered significant improvement. Her CRP decreased from 78 mg/l to 1 mg/l in a week and has remained normal thereafter (figure 3).
Figure 3.
C reactive protein levels after initiation of tocilizumab.
Serum IL-6 levels were increased with pre-TCZ baseline of 10.21 pg/ml (range 0.22–4.62). IL-6 levels registered further marked elevation prior to second infusion (248 pg/ml) a month later. The pre-infusion IL-6 levels thereafter showed decrease with pre-6th infusion value of 23.37 pg/ml. A repeat FDG-PET scan after three TCZ infusions showed good partial metabolic response along with significant reduction in uptake in the wall of the abdominal aorta and uptake in both subclavian and axillary arteries showing similar activity to the previous scan. Standardised uptake values (SUVmax) from 5.8 pretreatment was reduced to less than 3.
The prednisolone dosage was reduced from pre-TCZ value of 20 mg daily to 3.5 mg/day after five infusions of TCZ (8 mg/kg) and the patient remains in clinical remission. The patient has had asymptomatic transient neutropaenia post TCZ (white cell count and the neutrophil count dropped to 2.8 × 109/l of 0.9 × 109/l, respectively) in view of the remission, the dose of TCZ has been reduced to 4 mg/kg after the fourth infusion.
Discussion
We report the successful use of TCZ in a case of resistant large vessel, TAB-positive GCA complicating PMR. The patient had an excellent clinical, laboratory and radiological response and remains well after six cycles of TCZ. The steroid dosage as well as TCZ dosage has reduced considerably. The striking increase in serum IL-6 levels provoked by initial TCZ infusions and decrease with further TCZ treatment has been reported in other diseases. This phenomenon can be interpreted as a proof of an IL-6 driven disease process, where IL-6R blockade in an IL-6 overproduction state may cause paradoxically heightened levels of IL-6 by blocking IL-6R uptake with TCZ and hence cytokine degradation.19 Our findings are in accordance with results from two other reports.20 In the Swiss series, five patients with GCA were successfully treated with TCZ with all patients achieving rapid, sustained and complete clinical response and normalisation of the acute phase proteins. Prednisone dosage reduced within 12 weeks to a mean of 2.5 mg/day. No relapse and no drug-related side effects were noted.
Learning points.
-
▶
There is an unmet need for better treatment of GCA and PMR due to frequent relapses and adverse events seen with steroid therapy.
-
▶
IL-6 blockage is promising target for treatment of refractory GCA with large vessel involvement.
-
▶
There is a need for randomised control trials (RCTs) for assessing the efficacy and safety of TCZ.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008;372:234–45 [DOI] [PubMed] [Google Scholar]
- 2.Brack A, Martinez-Taboada V, Stanson A, et al. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999;42:311–17 [DOI] [PubMed] [Google Scholar]
- 3.Hunder GG, Ward LE, Burbank MK. Giant-cell arteritis producing an aortic arch syndrome. Ann Intern Med 1967;66:578–82 [DOI] [PubMed] [Google Scholar]
- 4.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003;48:3522–31 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt WA, Kraft HE, Vorpahl K, et al. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336–42 [DOI] [PubMed] [Google Scholar]
- 6.Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006;55:131–7 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Gay MA, Garcia-Porrua C, Salvarani C, et al. The spectrum of conditions mimicking polymyalgia rheumatica in Northwestern Spain. J Rheumatol 2000;27:2179–84 [PubMed] [Google Scholar]
- 8.Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of polymyalgia rheumatica. Rheumatology (Oxford) 2010;49:186–90 [DOI] [PubMed] [Google Scholar]
- 9.Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007;56:2789–97 [DOI] [PubMed] [Google Scholar]
- 10.De Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis 1986;45:136–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Taboada VM, Rodríguez-Valverde V, Carreño L, et al. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann Rheum Dis 2008;67:625–30 [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GS, Cid MC, Rendt-Zagar KE, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007;146:621–30 [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta B, Panayi GS. Interleukin-6 in serum of patients with polymyalgia rheumatic and giant cell arteritis. British J of Rheumatology 1990;29:256–458 [DOI] [PubMed] [Google Scholar]
- 14.Roche NE, Fulbright JW, Wagner AD, et al. Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum 1993;36:1286–94 [DOI] [PubMed] [Google Scholar]
- 15.Corrigall VM, Dolan AL, Dasgupta B, et al. The sequential analysis of T lymphocyte subsets and interleukin-6 in polymyalgia rheumatica patients as predictors of disease remission and steroid withdrawal. Br J Rheumatol 1997;36:976–80 [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Taboada VM, Alvarez L, RuizSoto M, et al. Giant cell arteritis and polymyalgia rheumatica: role of cytokines in the pathogenesis and implications for treatment. Cytokine 2008;44:207–20 [DOI] [PubMed] [Google Scholar]
- 17.Nishimoto N, Miyasaka N, Yamamoto K, et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009;68:1580–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29 [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008;112:3959–64 [DOI] [PubMed] [Google Scholar]
- 20.Seitza M, Reichenbacha S, Bonelc HM, et al. Rapid induction of remission in large vessel vasculitis by IL-6 blockade: a case series. Swiss Med Wkly 2011;141:w13156. [DOI] [PubMed] [Google Scholar]