Abstract
Acute stroke could be the presentation of unrecognised cardiomyopathy postanabolic androgenic steroid (AAS) abuse. A 39-year-old male patient displayed signs of acute stroke, which were associated with AAS abuse over the last 3 years. Despite the absence of symptoms and signs of congestive heart failure at presentation, AAS-induced cardiomyopathy with a thrombus in the left ventricle was discovered to be the aetiology of his stroke and peripheral vascular disease. Awareness of the complications of AAS led to the prompt treatment of the initially unrecognised dilated cardiomyopathy, and stroke.
Background
Acute presentation of stroke in a young patient with prolonged anabolic steroid use should alert us to underlying cardiomyopathy with thromboembolic events and peripheral vascular disease.
Case presentation
A 39-year-old male body builder presented with dizziness and expressive aphasia for the last 6 h.
Three months earlier, the patient presented to medical emergency with a transient ischaemic attack in the form of sudden loss of vision in left eye associated with weakness and numbness in the left upper and lower limbs lasting less than 1 h. The patient had intermittent claudication in the left lower limb. Neurological examination at the time, followed by CT of the head was completely normal. The patient refused admission to the hospital and was discharged on aspirin and follow-up which he did not pursue.
The patient had no medical issues, 3 weeks prior to admission at the hospital. However, the patient admitted to have been abusing anabolic androgenic steroids (AAS) for the last 3 years, which were administered as intramuscular injections of nandrolone twice weekly.
On physical examination, he was alert and conscious with motor aphasia. Heart rate was 100/min, and blood pressure 140/100 mm Hg. Chest, heart and abdomen were normal. Jugular venous pressure was not elevated, and no peripheral oedema was noted. Peripheral pulsations were present on right side and absent dorsalis pedis pulsation on left side. Pupils were normal to exam. His fundi were normal with no visual field defects and no nystagmus. Right facial palsy, upper motor neuron lesion. No motor weakness was detected. Deep reflexes were normal in upper and lower limbs. Plantar reflexes were normal.
Investigations
Complete blood picture, erythrocyte sedimentation rate and C reactive protein were within normal range. Fasting blood sugar, liver function test, kidney profile and serum electrolytes were normal. Troponin, and coagulation profile were normal and his creatine kinase was 500 U/l (normal range 5–130 U/l).
Serum triglycerides 1.8 mmol/l (normal <2.20 mmol/l), total serum cholesterol 5.4 mmol/l (normal <5.2 mmol/l), high density lipoprotein-C 0.85 mmol/l (normal >0.9 mmol/l), low density lipoprotein-C (LDL-C) 4.19 mmol/l (normal <3.37 mmol/l), apolipoprotein B 1.29 mg/dl (normal range 0.60–1.33 mg/dl). Full thrombophilia screen, antiphospholipid antibodies, virology screen and immunology screen were negative. Urinalysis and microscopy was normal. Ankle brachial index: right side=1.2, 1eft side=0.69. Chest x-ray showed cardiomegaly. ECG showed sinus rhythm. Q waves were present in leads II, III and AVF. Poor R waves were observed in V1–V3. CT and MRI of brain showed left frontal infarction (figure 1). Echocardiography showed dilated left ventricle (LV) with global hypokinaesia. Left ventricular cavity size was enlarged, end diastolic diameter was 6.9 cm and end systolic diameter was 5.7 cm. Left ventricular ejection fraction was 35% and there was an apical thrombus (figure 2). The left apical thrombus was mobile, measuring 1.6×1.5 cm. Left atrium diameter was 4.1cm. Carotid Doppler ultrasound showed no significant stenosis. Dipyridamol stress test of heart ruled out myocardial ischaemia. Magnetic resonance angiogram of left lower limb showed that there was an abrupt cut-off at the left superficial femoral artery at the beginning of the left popliteal artery, with total occlusion of left popliteal artery (figure 3).
Figure 1.
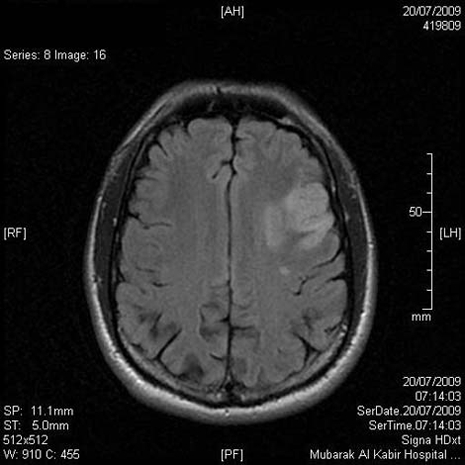
MRI of brain and neck showed left frontal infarction.
Figure 2.
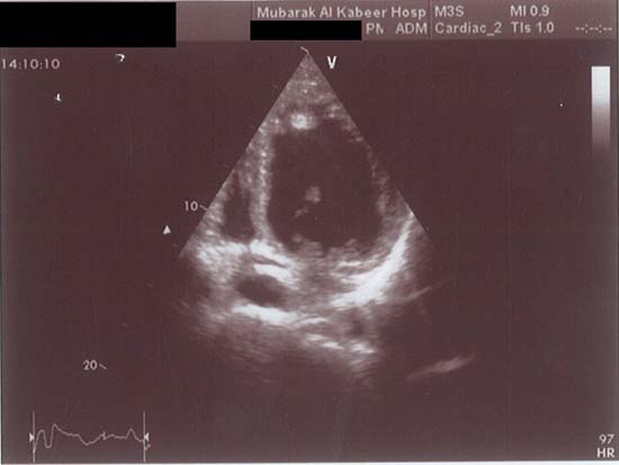
Echocardiography showed severely dilated left ventricle (LV) with epical thrombus.
Figure 3.
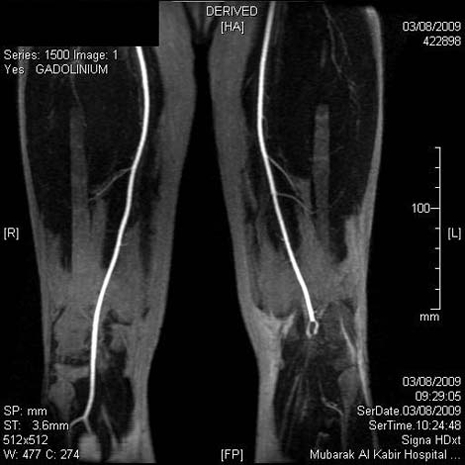
Magnetic resonance angiogram of left lower limb showed occlusion of left superficial femoral artery.
Outcome and follow-up
Patient was managed with intravenous unfractionated heparin infusion, statins, angiotensin converting enzyme inhibitors and β-blockers. Repeat CT showed no evidence of haemorrhagic transformation with progressive improvement of motor aphasia. In addition to the previously mentioned medications, the patient was discharged on aspirin and warfarin as well.
Upon follow-up after 3 months, review echo showed resolution of thrombus with partial improvement of ejection fraction (40–45%).
Upon follow-up after 6 months, ankle brachial index was improved, right side=1.20 and left side=0.82. The patient’s symptoms improved and he was able to resume work.
Discussion
Some athletes whether in competitive or non-competitive sports, abuse AAS to improve their performance or even their appearance as body builders.1
Abusers typically use 5–15 times the recommended medical doses of AAS. Athletes abusing AAS for years have high potential for arterial hypertension, cardiovascular, cerebrovascular disease and lipid metabolism disorder.2
We reported a 39-year-old man, who developed dilated cardiomyopathy, embolic stroke and peripheral vascular disease after self-administration of AAS for 3 years.
Several studies show that high doses of AAS such as nandrolone, may lead to growth-promoting effects on cardiac tissue, as seen in hypertrophic cardiomyopathy, followed by apoptotic cell death which is mediated by membrane-receptor second messenger cascades that increase intracellular Ca2+ influx and mobilisation, leading to the release of apoptogenic factors.3 4
AAS abuse associated with sudden cardiac death, myocardial infarction, ventricular remodelling and cardiomyopathy is related to apoptosis.5 This relation may explain the clinical observations that AAS can lead to myocardial death without coronary thrombosis or atherosclerosis.6 7
Several studies in isolated human myocytes have shown that AAS bind to androgen receptors and may directly cause hypertrophy, via tissue upregulation of the renin-angiotensin system.8
AAS abuse causes decrease in high density lipoprotein cholesterol by 20% and increase in LDL cholesterol by 20% due to lipolytic degradation of lipoproteins and their removal by receptors through modification of apolipoprotein A-I and B synthesis.9 Apolipoprotein B has been experimentally linked to the development of atherosclerosis, mediating the interaction between LDL-C and the arterial wall.1
These lipoprotein abnormalities increase the risk for coronary artery disease by three to sixfold and may occur within 9 weeks of AAS self-administration.8 10 Fortunately, lipid effects seem to be reversible after discontinuation.1
AAS enhance platelet aggregation and thrombus formation by increasing platelet production of thromboxane A2, decreasing production of prostacyclin and increasing fibrinogen levels.1
Ischaemic stroke can occur as a result of atherothrombosis or embolisation either in the carotids or the heart as AAS has been associated with changes in vascular reactivity, lipid profile, haemostasis and platelet aggregation.1 Accordingly, peripheral vascular disease can occur through the same mechanism.1
Our case is unique as our patient developed an embolic stroke and peripheral vascular disease in the absence of any risk factors for either of the diseases, but rather as a complication of dilated cardiomyopathy with LV thrombus formation. These complications were deduced to be the result of AAS abuse after ruling out other aetiological factors.
Learning points.
-
▶
AAS abuse can lead to complications in young patients
-
▶
We present a case report of a young patient who developed acute embolic stroke, cardiomyopathy and peripheral vascular disease as a result of AAS abuse
-
▶
Physicians should be aware of the complications and side effects of AAS abuse in young patients.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Santamarina RD, Besocke AG, Romano LM, et al. Ischemic stroke related to anabolic abuse. Clin Neuropharmacol 2008;31:80–5 [DOI] [PubMed] [Google Scholar]
- 2.Lane HA, Grace F, Smith JC, et al. Impaired vasoreactivity in bodybuilders using androgenic anabolic steroids. Eur J Clin Invest 2006;36:483–8 [DOI] [PubMed] [Google Scholar]
- 3.D’Ascenzo S, Millimaggi D, Di Massimo C, et al. Detrimental effects of anabolic steroids on human endothelial cells. Toxicol Lett 2007;169:129–36 [DOI] [PubMed] [Google Scholar]
- 4.Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol 2010;106:893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaugg M, Jamali NZ, Lucchinetti E, et al. Anabolic-androgenic steroids induce apoptotic cell death in adult rat ventricular myocytes. J Cell Physiol 2001;187:90–5 [DOI] [PubMed] [Google Scholar]
- 6.Fineschi V, Baroldi G, Monciotti F, et al. Anabolic steroid abuse and cardiac sudden death: a pathologic study. Arch Pathol Lab Med 2001;125:253–5 [DOI] [PubMed] [Google Scholar]
- 7.Wysoczanski M, Rachko M, Bergmann SR. Acute myocardial infarction in a young man using anabolic steroids. Angiology 2008;59:376–8 [DOI] [PubMed] [Google Scholar]
- 8.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 2003;24:313–40 [DOI] [PubMed] [Google Scholar]
- 9.Hartgens F, Rietjens G, Keizer HA, et al. Effects of androgenic-anabolic steroids on apolipoproteins and lipoprotein (a). Br J Sports Med 2004;38:253–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maravelias C, Dona A, Stefanidou M, et al. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol Lett 2005;158:167–75 [DOI] [PubMed] [Google Scholar]


