Abstract
Air embolism (AE) is a potential complication during transthoracic needle biopsy (TNB). The authors report on venous and systemic AE during CT-guided TNB under general anaesthesia. During the intervention, the radiologist observed accumulation of air bubbles in the left heart chambers, in the right subclavian vein, the superior vena cava and the right atrium. This was presumably due to pressure infusion of contrast medium (CM) air entrained via a stop-cock improperly fixed to the venous cannula or via the injection valve of the cannula by Venturi forces. Prevention of AE related to CM infusion is a subject for institutional risk management. Stop-cocks and injection valves should not be used in intravenous lines supplied by pressure infusions. Adverse outcome may be avoided by placing the patient head down, increasing FiO2 to 1.0, administering antithrombotic therapy and immobilizing the patient on the intervention table until CT has proved complete remission of AE.
Background
CT-guided transthoracic needle biopsy (TNB) is performed by interventional radiologists in order to evaluate pulmonary, hilar, mediastinal and pleural nodules or masses.1 Core biopsies with a cutting needle are used to obtain a cylinder of tissue for histological analysis. Beside pneumothorax, hemoptysis, parenchymal haemorrhage and subcutaneous emphysema, air embolism (AE) is a potential complication of TNB.2 The incidence of AE during TNB is more frequent than previously reported.3 4
Case presentation
A 27-year-old man, with weight 58 kg, height 168 cm and ASA physical status II was scheduled for CT-guided TNB at the Department of Radiology. The patient with testicular neoplasm was suspected to have disseminated metastases in the lungs. Physical examination and preoperative routine laboratory results were not remarkable, in particular, clotting parameters and thrombocyte count were within normal limits. Spirometry showed a minor restrictive ventilation disorder (FVC: 4.75 l, FEV1: 4.18 l, FEF (50%): 5.37 l/s, FEF (25%): 8.3 l/s). CT scan prior to the intervention revealed a small area in the right upper lobe (figure 1A) that was sensitive to tracer accumulation in positron emission tomography. Prior to the investigation, baseline blood pressure was 110/60 mm Hg, heart rate was 70 beats/min and ECG revealed sinus rhythm. Anaesthesia was induced with 200 mg propofol and 0.2 mg fentanyl. After administration of 40 mg rocuronium, the airway was secured by tracheal tube. Anaesthesia was maintained with a volatile anaesthetic (0.8 vol% isoflurane) and remifentanil (0.5 mg/h). After placing the patient in the prone position, puncture of the right upper lobe was uneventful. Ventilation was performed with FiO2 0.4; the time interval between inspiration and expiration was kept at 1:2. The ventilator was not disconnected during puncture. Approximately, 100 ml of sodium solution and contrast medium (CM) were infused by motor pump at a rate of 4 ml/s via a peripheral venous cannula.
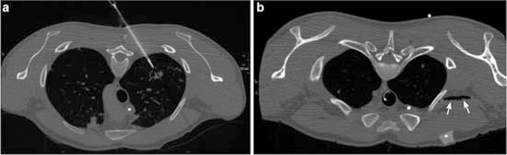
Figure 1.
(A) Biopsy of a suspect, thick-walled cavern in the left upper lobe of the lung, with the patient in the prone position; 18 G core biopsy needle. (B) Air (arrows) in the right subclavian vein. CT slices with a slice thickness of 1.25 mm.
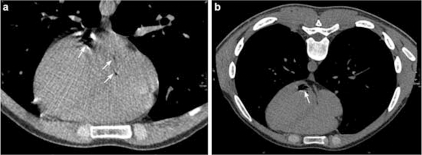
About 30 min after onset of CM administration, the radiologist detected air bubbles on the CT cross-section in the right subclavian vein and superior vena cava (figure 1B). Subsequent CT scan of the chest revealed additional air bubbles in the right and left atrium and in the left ventricle indicating the presence of a patent oval foramen (figure 2A,B). The patient’s circulatory and respiratory parameters remained stable (blood pressure: 100/60 mmHg, heart rate: 65 beats/min, partial oxygen saturation: 99%, endtidal CO2: 30 mmHg).
Figure 2.
(A) Air embolism (arrows) in the left, and in the right atrium of the heart. The left atrium is located dorsally. (B) Air (arrow) in the left ventricle. CT slices with a slice thickness of 1.25 mm.
Treatment
Initially, the entrance spot of AE could not be identified. The patient was kept in prone and head-down position, general anaesthesia was maintained and FiO2 was increased to 1.0. An indwelling urinary catheter was placed. Antithrombotic treatment with unfractionated heparin was started with a 5000 IU bolus and maintained at a rate of 400 IU/h. Arterial blood gas examination revealed pH: 7.47, BE: 2.4, HCO3: 25.7, pO2: 369.9 mmHg and pCO2: 35.6 mmHg.
Mill-wheel murmur was not detected on heart auscultation. Anaesthesia was maintained until repeated CT controls displayed decline of AE first in the heart and then delayed in the superior vena cava. Five hours after the incident, no more air bubbles were detected on the CT scan and the patient could be safely weaned from the ventilator.
Outcome
Observation overnight at the intermediate care unit was unremarkable and 18 h after onset of AE, the patient was moved to the general ward. Neurological examination revealed normal tone, reflexes, strength and sensation in all extremities. Control chest X-ray remained without pathological findings.
Discussion
We report the occurrence of AE in a 27-year-old patient who underwent TNB under general anaesthesia. The diagnosis was based on radiological findings. No changes in vital signs were noted during the intervention and there was no evidence of hypoxemia and hypercapnia on arterial blood gas examination.
TNB of pulmonary or mediastinal lesions is a routine diagnostic performance at our hospital. Needle biopsy yields an overall diagnostic accuracy exceeding 95% in patients with cancer; Westcott and colleagues reported an overall sensitivity of 93% and specificity of 100% in pulmonary nodules of less than 15 mm diameter.5 In order to avoid involuntary movements of the patients during the procedure, TNB is frequently performed under general anaesthesia.
There are numerous ways as to how AE may occur during CT-guided TNB. First, air can enter the pulmonary circulation when the needle tip is inserted into the pulmonary vein and the stylet is removed in spontaneous breathing patients. Second, air can enter through a bronchovenous fistula created by the needle passing through the lung parenchyma during elevated intra-alveolar pressure under positive pressure ventilation.6 Third, venous AE can arise from pressure infusion of CM,7 most likely the cause in our patient. When maintaining infusion systems, air can be infused by gravity or more likely as assumed in our case, air might have been delivered under pressure into the venous system by an infusion pump. There is the probability that a bolus of air was unintentionally administered by the infusion pump without detection of air in the line due to erroneous replacement of the perfusion line after pump adjustment. Furthermore, air could have entered via a stop-cock improperly fixed to the venous cannula or via the injection valve of the venous cannula by Venturi forces. This is especially risky when intravenous fluids and CM are propelled through the venous line by motor pump. Finally, there is the potential of pump failure including leakage of the filter. Usually, the infusion pump would have given an alarm when air bubbles accumulate in the line but air can be detected only in a small central part of the infusion system. Presumably, this safety feature was bypassed when an unfilled line was plugged accidentally. The volume of air that fills the peripheral tubing to the patient amounts to 21 ml, an amount that comes up to what the radiologist had estimated in the superior vena cava and the heart.
Additional factors that facilitate air entrainment in AE include cachexia, hypovolemia, high-negative intrathoracic pressure, particularly when seated upright, bulbous lung disease, inflammation or vascular lesions, all of which were absent in our patient.8
AE may affect the respiratory, cardiovascular and central nervous system. The morbidity and mortality of AE are related to the volume of air influx, rate of entrainment, the patient’s underlying cardiorespiratory status and the patient’s position.9 In human beings, the actual volume of gas that can be tolerated is unknown; mortality has been reported with injection of as little as 100–300 ml. Large volumes can be fatal by causing right ventricular outflow obstruction but already small volumes of no more than 10–20 ml can be lethal when ascending to the brain either retrograde through the venous system or when entering the arterial circulation of the left heart.10 However, small volumes of air in the pulmonary perfusion are rapidly reabsorbed or excreted by the lungs.
There are several reports of venous AE in the literature, but current treatment guidelines do not consider the potential of retrograde ascending embolism.11 General recommendations include 100% oxygen to maximise the patient’s oxygenation as well as to reduce embolus volume by eliminating nitrogen.12 Hyperbaric oxygen therapy is beneficial in severe cases as it causes compression of existing air bubbles by establishing a high diffusion gradient to speed dissolution of bubbles and by improving oxygenation in the ischemic tissues.13 In case of cardiovascular compromise, inotropic support is indicated.14 In cases of venous AE, the patient should be positioned left side down in order to gather intracardiac air in the apex of the right ventricle and to prevent outflow to the lungs or to the left ventricle in case of patent ovale foramen.15 Although many groups recommend application of right lateral decubitus and Trendelenburg position if air bubble is detected in the left ventricle (before it embolizes to brain),16 some investigators are of the opinion that buoyancy of gas bubbles is not sufficient to counteract the blood flow and they recommend the use of flat supine position to avoid aggravation of cerebral oedema that could develop in these patients.17 Due to the good outcome of our patient who was kept in head-down position, we prefer this position in the immediate management of AE. As intravascular air may cause thrombus formation, antithrombotic therapy should be provided with unfractionated heparin.18 In operation theatres with air quality III or less (> 500 particles/m3), administration of antibiotics is worth considering.
As a consequence of this complication, hospital administration and the manufacturer agreed on the recommendation that infusion of CM by pump is no longer permitted through venous cannula with integrated valves for injection and with stop-cocks fixed to the cannula. Both, the radiologist and anaesthetist teams were instructed to always inspect the peripheral perfusion line as to whether it is adequately filled with saline before its connection to the venous catheter.
We did not perform transthoracal or transoesophageal echocardiography19 nor did we apply precordial Doppler and end-tidal nitrogen monitoring to demonstrate intracardiac air.20 Furthermore, aspiration of the venous AE, for example, via a central venous catheter was not attempted in our patient.
Learning points.
-
▶
Prevention of AE due to unintentional administration of air during CM infusion is a subject for institutional risk management.
-
▶
Stop-cocks and injection valves should not be used in intravenous lines supplied by pressure infusion devices.
-
▶
After early recognition of AE during CT-guided TNB, adverse outcome may be avoided by keeping the patient in a head-down position, commencing antithrombotic therapy, as well as immobilizing and maintaining the patient on the CT intervention table until AE has been demonstrated to have subsided.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.de Gregorio Ariza MA, Alfonso Aguirán ER, Villavieja Atance JL, et al. Transthoracic aspiration biopsy of pulmonary and mediastinal lesions. Eur J Radiol 1991;12:98–103 [DOI] [PubMed] [Google Scholar]
- 2.Li H, Boiselle PM, Shepard JO, et al. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 1996;167:105–9 [DOI] [PubMed] [Google Scholar]
- 3.Hiraki T, Fujiwara H, Sakurai J, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest 2007;132:684–90 [DOI] [PubMed] [Google Scholar]
- 4.Ibukuro K, Tanaka R, Takeguchi T, et al. Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: single-institution experience. AJR Am J Roentgenol 2009;193:W430–6 [DOI] [PubMed] [Google Scholar]
- 5.Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology 1997;202:97–103 [DOI] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Ansaarie I, Bader M, et al. Coronary artery air embolism complicating a CT-guided transthoracic needle biopsy of the lung. Chest 2002;121:993–6 [DOI] [PubMed] [Google Scholar]
- 7.Woodring JH, Fried AM. Nonfatal venous air embolism after contrast-enhanced CT. Radiology 1988;167:405–7 [DOI] [PubMed] [Google Scholar]
- 8.Ho AM, Ling E. Systemic air embolism after lung trauma. Anesthesiology 1999;90:564–75 [DOI] [PubMed] [Google Scholar]
- 9.Toung TJ, Rossberg MI, Hutchins GM. Volume of air in a lethal venous air embolism. Anesthesiology 2001;94:360–1 [DOI] [PubMed] [Google Scholar]
- 10.Holcomb BW, Loyd JE, Byrd BF, 3rd, et al. Iatrogenic paradoxical air embolism in pulmonary hypertension. Chest 2001;119:1602–5 [DOI] [PubMed] [Google Scholar]
- 11.Schlimp CJ, Loimer T, Rieger M, et al. The potential of venous air embolism ascending retrograde to the brain. J Forensic Sci 2005;50:906–9 [PubMed] [Google Scholar]
- 12.Sibai AN, Baraka A, Moudawar A. Hazards of nitrous oxide administration in presence of venous air embolism. Middle East J Anesthesiol 1996;13:565–71 [PubMed] [Google Scholar]
- 13.Blanc P, Boussuges A, Henriette K, et al. Iatrogenic cerebral air embolism: importance of an early hyperbaric oxygenation. Intensive Care Med 2002;28:559–63 [DOI] [PubMed] [Google Scholar]
- 14.Shaikh N, Ummunisa F. Acute management of vascular air embolism. J Emerg Trauma Shock 2009;2:180–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong J, Gadalla F, Druzin M. Venous emboli occurring caesarean section: the effect of patient position. Can J Anaesth 1991;38:191–5 [DOI] [PubMed] [Google Scholar]
- 16.Arnold BW, Zwiebel WJ. Percutaneous transthoracic needle biopsy complicated by air embolism. AJR Am J Roentgenol 2002;178:1400–2 [DOI] [PubMed] [Google Scholar]
- 17.Muth CM, Shank ES. Gas embolism. N Engl J Med 2000;342:476–82 [DOI] [PubMed] [Google Scholar]
- 18.Ryu KH, Hindman BJ, Reasoner DK, et al. Heparin reduces neurological impairment after cerebral arterial air embolism in the rabbit. Stroke 1996;27:303–9; discussion 310. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe RA, Siegel LC, Schnittger I, et al. Epidural air injection assessed by transesophageal echocardiography. Reg Anesth 1995;20:152–5 [PubMed] [Google Scholar]
- 20.Souders JE. Pulmonary air embolism. J Clin Monit Comput 2000;16:375–83 [DOI] [PubMed] [Google Scholar]