Abstract
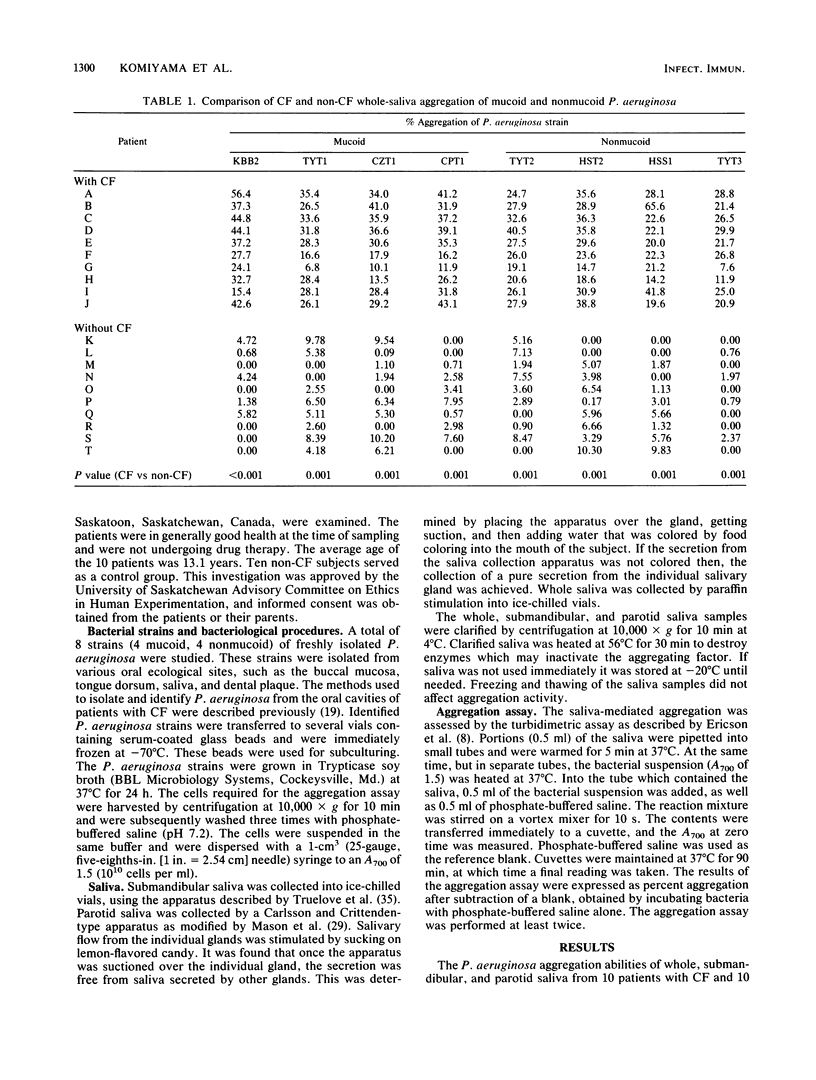
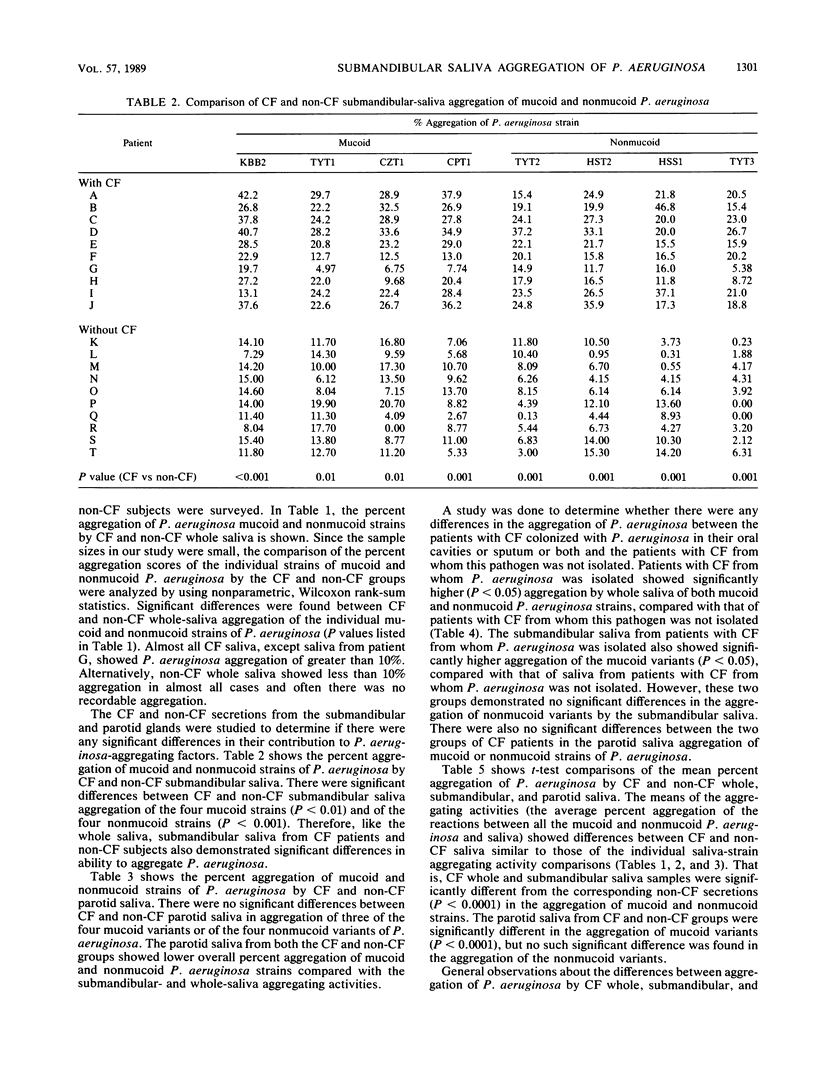
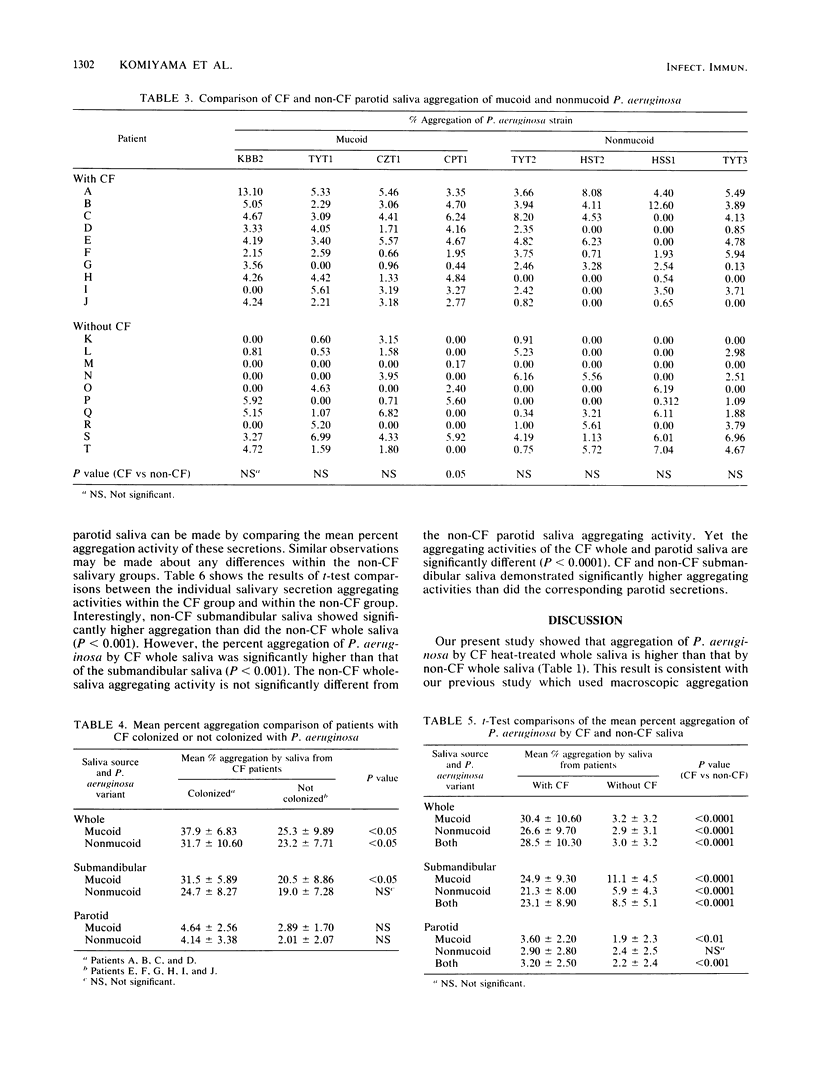
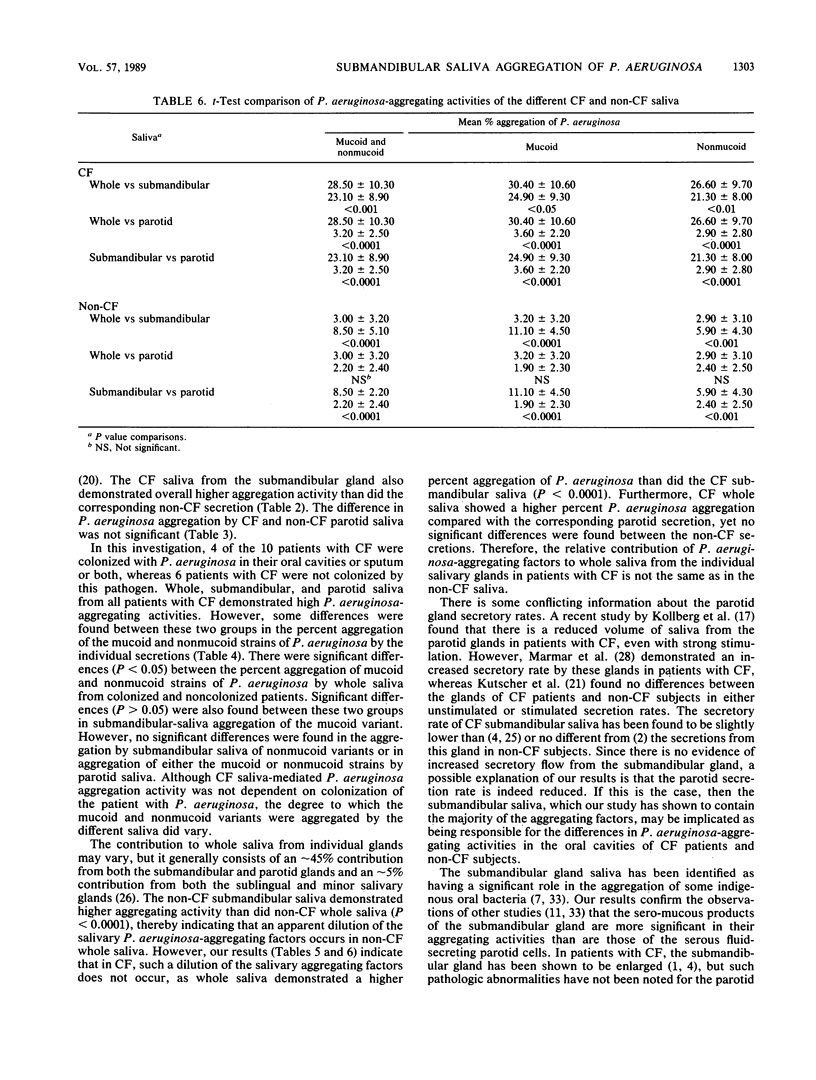
The aggregation of mucoid and nonmucoid Pseudomonas aeruginosa by submandibular, parotid, and whole saliva from patients with cystic fibrosis (CF) and non-CF subjects was investigated. There were significant differences (P less than 0.01) in aggregation of mucoid and nonmucoid variants of P. aeruginosa by submandibular and whole saliva from CF patients and non-CF subjects. However, the differences in the parotid secretion were not as pronounced. Patients with CF who were colonized with P. aeruginosa demonstrated a significantly higher (P less than 0.05) percent aggregation of the mucoid variants by the submandibular secretion and of both mucoid and nonmucoid variants by whole saliva, compared with corresponding secretions from patients with CF not colonized with this pathogen. The parotid saliva aggregation activity was not markedly different for the two groups with CF. From patients with CF, whole saliva demonstrated a higher percent P. aeruginosa aggregation than did the submandibular saliva. In non-CF subjects, however, the percent aggregation of P. aeruginosa by submandibular saliva was higher than that by whole saliva. Our results indicate that the sero-mucous products of the submandibular gland have a more significant role in P. aeruginosa aggregation than the serous secreting parotid cells and that the submandibular secretion is possibly responsible for the differences in oral colonization by this pathogen in subjects with and without CF.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBERO G. J., SIBINGA M. S. Enlargement of the submaxillary salivary glands in cystic fibrosis. Pediatrics. 1962 May;29:788–793. [PubMed] [Google Scholar]
- Blomfield J., Rush A. R., Allars H. M., Brown J. M. Parotid gland function in children with cystic fibrosis and child control subjects. Pediatr Res. 1976 Jun;10(6):574–578. doi: 10.1203/00006450-197606000-00004. [DOI] [PubMed] [Google Scholar]
- Bratthall D., Carlén A. Salivary agglutinin and secretory IgA reactions with oral streptococci. Scand J Dent Res. 1978 Dec;86(6):430–443. doi: 10.1111/j.1600-0722.1978.tb00650.x. [DOI] [PubMed] [Google Scholar]
- CHERNICK W. S., BARBERO G. J., PARKINS F. M. Studies on submaxillary saliva in cystic fibrosis. J Pediatr. 1961 Dec;59:890–898. doi: 10.1016/s0022-3476(61)80319-3. [DOI] [PubMed] [Google Scholar]
- Davis P. B. Pathophysiology of cystic fibrosis with emphasis on salivary gland involvement. J Dent Res. 1987 Feb;66(Spec No):667–671. doi: 10.1177/00220345870660S210. [DOI] [PubMed] [Google Scholar]
- Diaz E., Mosovich L. L., Neter E. Serogroups of Pseudomonas aeruginosa and the immune response of patients with cystic fibrosis. J Infect Dis. 1970 Mar;121(3):269–274. doi: 10.1093/infdis/121.3.269. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Bratthall D., Borgström M., Howley T. P. Actinomyces viscosus and Actinomyces naeslundii agglutinins in human saliva. Scand J Dent Res. 1983 Aug;91(4):263–273. doi: 10.1111/j.1600-0722.1983.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Gahnberg L., Olsson J., Krasse B., Carlén A. Interference of Salivary immunoglobulin A antibodies and other salivary fractions with adherence of Streptococcus mutans to hydroxyapatite. Infect Immun. 1982 Aug;37(2):401–406. doi: 10.1128/iai.37.2.401-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. E., Thaler M., Davis C., Malamud D. Bacterial aggregating activity in human saliva: simultaneous determination of free and bound cells. Infect Immun. 1979 Dec;26(3):1028–1034. doi: 10.1128/iai.26.3.1028-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):551–558. [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolberg H., Danielsson A., Glitterstam K., Henriksson R., Marklund S. Studies on parotid saliva in cystic fibrosis. Acta Paediatr Scand. 1982 Mar;71(2):321–322. doi: 10.1111/j.1651-2227.1982.tb09422.x. [DOI] [PubMed] [Google Scholar]
- Komiyama K., Gibbons R. J. Inhibition of lactose-reversible adherence between Actinomyces viscosus and oral streptococci by salivary components. Caries Res. 1984;18(3):193–200. doi: 10.1159/000260765. [DOI] [PubMed] [Google Scholar]
- Komiyama K., Habbick B. F., Martin T., Tumber S. K. Characterization by pyocine typing and serotyping of oral and sputum strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. Can J Microbiol. 1987 Mar;33(3):221–225. doi: 10.1139/m87-038. [DOI] [PubMed] [Google Scholar]
- Komiyama K., Habbick B. F., Tumber S. K. Role of sialic acid in saliva-mediated aggregation of Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1987 Oct;55(10):2364–2369. doi: 10.1128/iai.55.10.2364-2369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutscher A. H., Mandel I. D., Thompson R. H., Jr, Wotman S., Zegarelli E. V., Fahn B. S., Denning C. R., Goldstein J. A., Taubman M., Khotim S. Parotid saliva in cystic fibrosis. 1. Flow rate. Am J Dis Child. 1965 Dec;110(6):643–645. doi: 10.1001/archpedi.1965.02090030671009. [DOI] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D., Appelbaum B., Kline R., Golub E. E. Bacterial aggregating activity in human saliva: comparisons of bacterial species and strains. Infect Immun. 1981 Mar;31(3):1003–1006. doi: 10.1128/iai.31.3.1003-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D., Kutscher A., Denning C. R., Thompson R. H., Jr, Zegarelli E. V. Salivary studies in cystic fibrosis. Am J Dis Child. 1967 Apr;113(4):431–438. doi: 10.1001/archpedi.1967.02090190077005. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;(8):25–47. [PubMed] [Google Scholar]
- Marks M. I. The pathogenesis and treatment of pulmonary infections in patients with cystic fibrosis. J Pediatr. 1981 Feb;98(2):173–179. doi: 10.1016/s0022-3476(81)80631-2. [DOI] [PubMed] [Google Scholar]
- Marmar J., Barbero G. J., Sibinga M. S. The pattern of parotid gland secretion in cystic fibrosis of the pancreas. Gastroenterology. 1966 Apr;50(4):551–556. [PubMed] [Google Scholar]
- Mason D. K., Harden R. M., Rowan D., Alexander W. D. Recording the pattern of salivary flow. J Dent Res. 1966 Sep-Oct;45(5):1458–1463. doi: 10.1177/00220345660450053301. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Rosan B., Appelbaum B., Golub E., Malamud D., Mandel I. D. Enhanced saliva-mediated bacterial aggregation and decreased bacterial adhesion in caries-resistant versus caries-susceptible individuals. Infect Immun. 1982 Dec;38(3):1056–1059. doi: 10.1128/iai.38.3.1056-1059.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelove E. L., Bixler D., Merritt A. D. Simplified method for collection of pure submandibular saliva in large volumes. J Dent Res. 1967 Nov-Dec;46(6):1400–1403. doi: 10.1177/00220345670460064301. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Adherence of Streptococcus salivarius HB and HB-7 to oral surfaces and saliva-coated hydroxyapatite. Infect Immun. 1980 Oct;30(1):150–158. doi: 10.1128/iai.30.1.150-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]



