Abstract
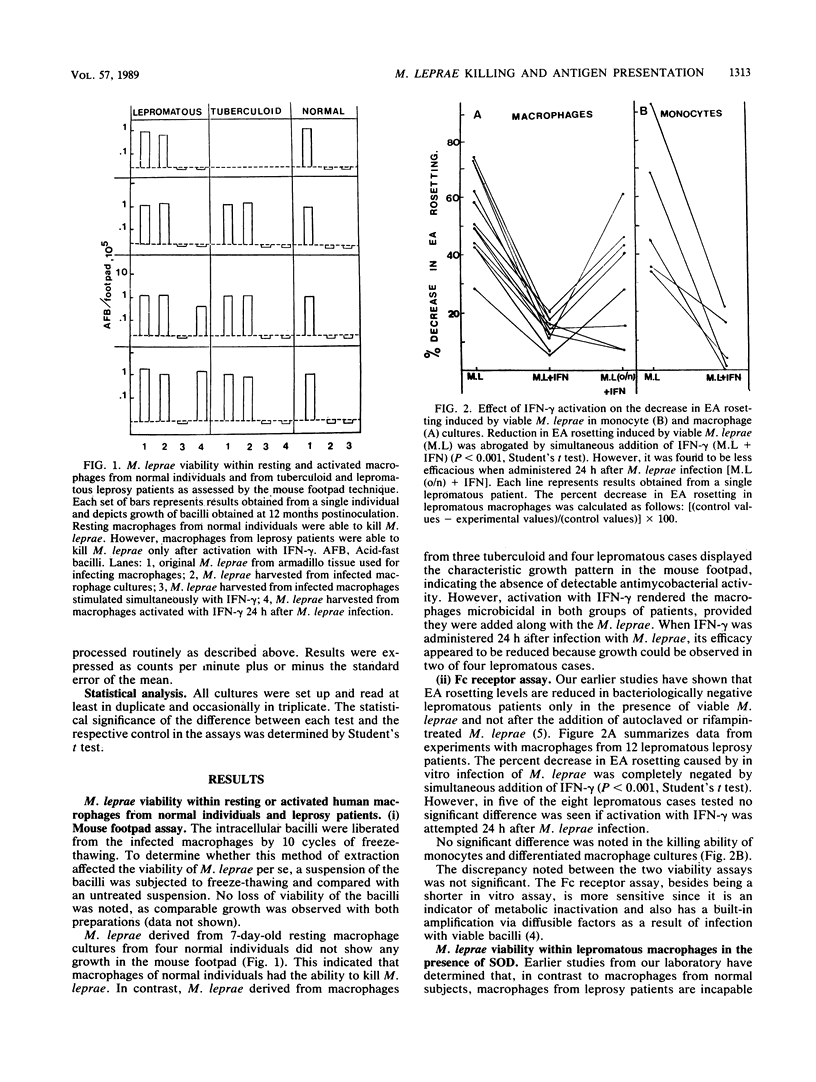
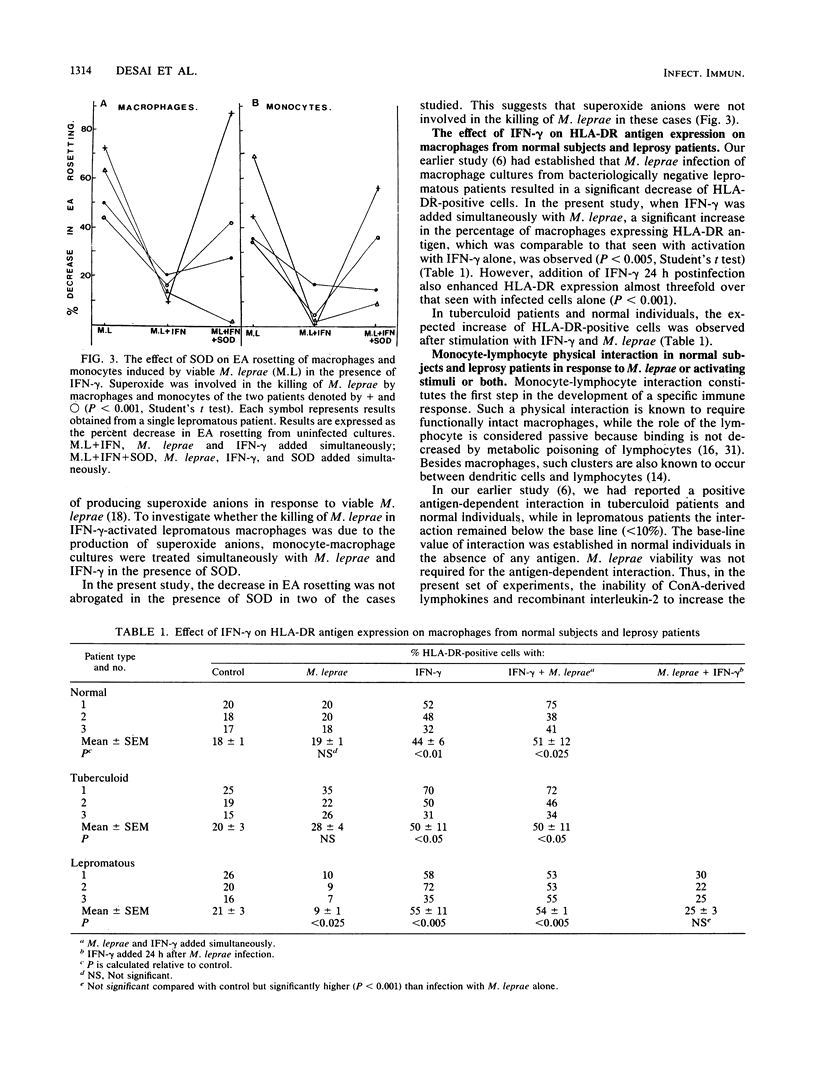
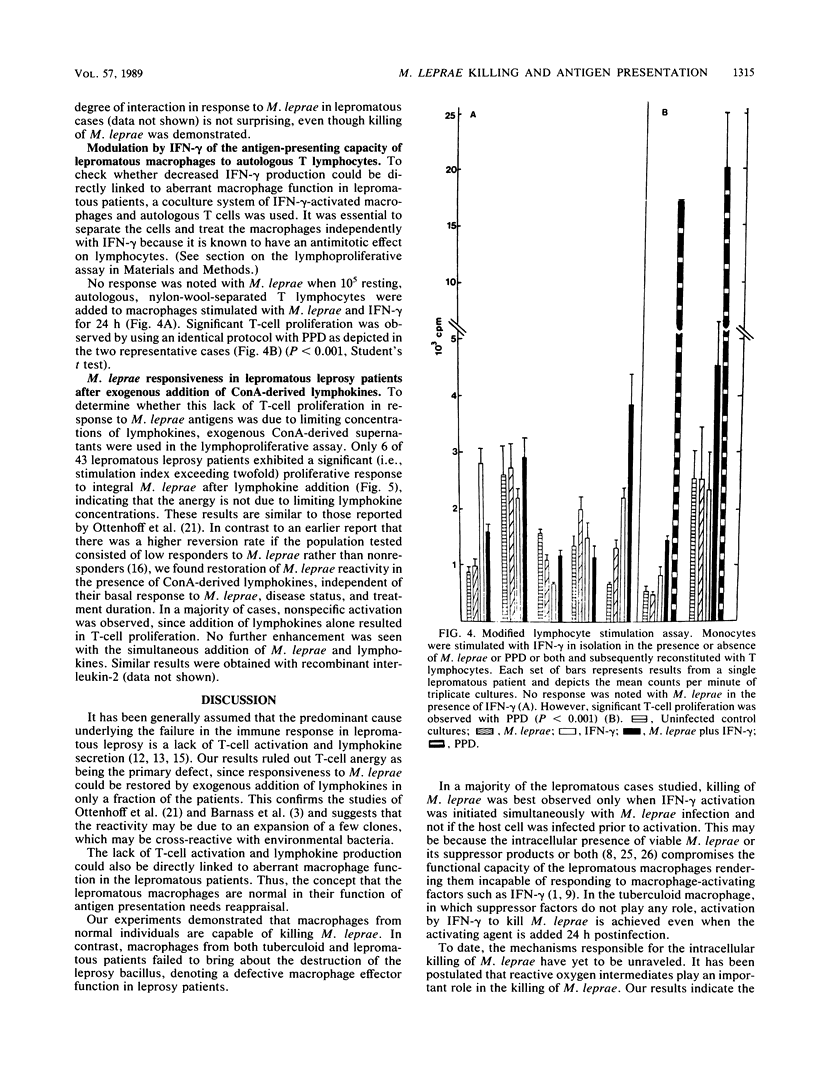
The killing of Mycobacterium leprae by resting and gamma interferon (IFN-gamma)-activated macrophages in normal subjects and leprosy patients was assessed. Resting macrophages from normal individuals demonstrated the ability to kill M. leprae. For macrophages from tuberculoid patients, killing of M. leprae was only achieved in the presence of IFN-gamma, suggesting that initial T-cell activation occurs prior to the killing of M. leprae. In contrast, though activation with IFN-gamma rendered the lepromatous macrophages microbicidal, it failed to induce lymphocyte proliferation, suggesting a defect at either the antigen-presenting cell or the lymphocyte level or both. The concept that T-cell anergy is primarily due to lack of lymphokine generation was ruled out by our results, since responsiveness was restored in only a small proportion of lepromatous patients after exogenous lymphokine addition. In conclusion, this study demonstrated that killing and antigen presentation are two independent events. It appears that the ability of the macrophages per se to kill M. leprae may be of greater importance than lymphocyte-mediated activation for protection against M. leprae infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Vemuri N., Mahadevan P. R. Macrophage membrane alterations in leprosy as determined by change in sialic acid level. J Clin Lab Immunol. 1986 Mar;19(3):119–122. [PubMed] [Google Scholar]
- Barnass S., Mace J., Steele J., Torres P., Gervasoni B., Ravioli R., Terencio J., Rook G. A., Waters M. F. Prevalence and specificity of the enhancing effect of three types of interleukin 2 on T cell responsiveness in 97 lepromatous leprosy patients of mixed ethnic origin. Clin Exp Immunol. 1986 Apr;64(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- Birdi T. J., Mistry N. F., Mahadevan P. R., Antia N. H. Alterations in the membrane of macrophages from leprosy patients. Infect Immun. 1983 Jul;41(1):121–127. doi: 10.1128/iai.41.1.121-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdi T. J., Mistry N. F., Mahadevan P. R., Antia N. H. Antigen specific macrophage-lymphocyte interaction in lepromatous leprosy. J Clin Lab Immunol. 1984 Apr;13(4):189–194. [PubMed] [Google Scholar]
- Birdi T. J., Salgame P. R., Antia N. H. The role of macrophages in leprosy as studied by protein synthesis of macrophages from resistant and susceptible hosts--a mouse and human study. Lepr India. 1979 Jan;51(1):23–42. [PubMed] [Google Scholar]
- Churchill W. H., Wong C. Mediator-induced macrophage activation, as shown by enhanced cytotoxicity for tumor, requires macrophage surface fucose and sialic acid. Cell Immunol. 1980 Oct;55(2):490–498. doi: 10.1016/0008-8749(80)90180-x. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Attempts to characterize the mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages. Infect Immun. 1988 Jun;56(6):1464–1469. doi: 10.1128/iai.56.6.1464-1469.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Longley J., Bjune G., Mustafa A. S., Godal T. The role of interleukin-2 (IL-2) in the specific unresponsiveness of lepromatous leprosy to Mycobacterium leprae: studies in vitro and in vivo. Immunol Lett. 1985;11(3-4):249–252. doi: 10.1016/0165-2478(85)90175-0. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Accessory cell-T lymphocyte interactions. Antigen-dependent and -independent clustering. J Exp Med. 1986 Feb 1;163(2):247–261. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Cohn Z. A. Cellular immunity in lepromatous and tuberculoid leprosy. Immunol Lett. 1985;11(3-4):205–209. doi: 10.1016/0165-2478(85)90169-5. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975 Jan 1;141(1):138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Jagannathan R., Bhagaria A., Vejare S., Agarwal S. Host-pathogen interaction--new in vitro drug test systems against Mycobacterium leprae--possibilities and limitations. Lepr Rev. 1986;57 (Suppl 3):182–200. doi: 10.5935/0305-7518.19860110. [DOI] [PubMed] [Google Scholar]
- Mistry N. F., Birdi T. J., Antia N. H. M. leprae phagocytosis and its association with membrane changes in macrophages from leprosy patients. Parasite Immunol. 1986 Mar;8(2):129–138. doi: 10.1111/j.1365-3024.1986.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., de Vries R. R. Unresponsiveness to Mycobacterium leprae in lepromatous leprosy in vitro: reversible or not? Int J Lepr Other Mycobact Dis. 1984 Sep;52(3):419–424. [PubMed] [Google Scholar]
- Pierce S. K., Margoliash E. Antigen processing: an interim report. Trends Biochem Sci. 1988 Jan;13(1):27–29. doi: 10.1016/0968-0004(88)90015-1. [DOI] [PubMed] [Google Scholar]
- Prasad H. K., Nath I. Incorporation of 3H-thymidine in Mycobacterium leprae within differentiated human macrophages. J Med Microbiol. 1981 Aug;14(3):279–293. doi: 10.1099/00222615-14-3-279. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- SHEPARD C. C., MCRAE D. H. MYCOBACTERIUM LEPRAE IN MICE: MINIMAL INFECTIOUS DOSE, RELATIONSHIP BETWEEN STAINING QUALITY AND INFECTIVITY, AND EFFECT OF CORTISONE. J Bacteriol. 1965 Feb;89:365–372. doi: 10.1128/jb.89.2.365-372.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P. R., Mahadevan P. R., Antia N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect Immun. 1983 Jun;40(3):1119–1126. doi: 10.1128/iai.40.3.1119-1126.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Bhutani L. K., Sharma A. K., Nath I. Monocyte-derived soluble suppressor factor(s) in patients with lepromatous leprosy. Infect Immun. 1983 Dec;42(3):890–899. doi: 10.1128/iai.42.3.890-899.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P., Wallach D., Bach M. A. Interleukin-2-induced T-cell response to M. leprae in lepromatous leprosy: reversion of a suppressor mechanism or expansion of a small M. leprae-reactive T-cell pool? Int J Lepr Other Mycobact Dis. 1985 Dec;53(4):649–652. [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987 Feb;55(2):446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]


