Abstract
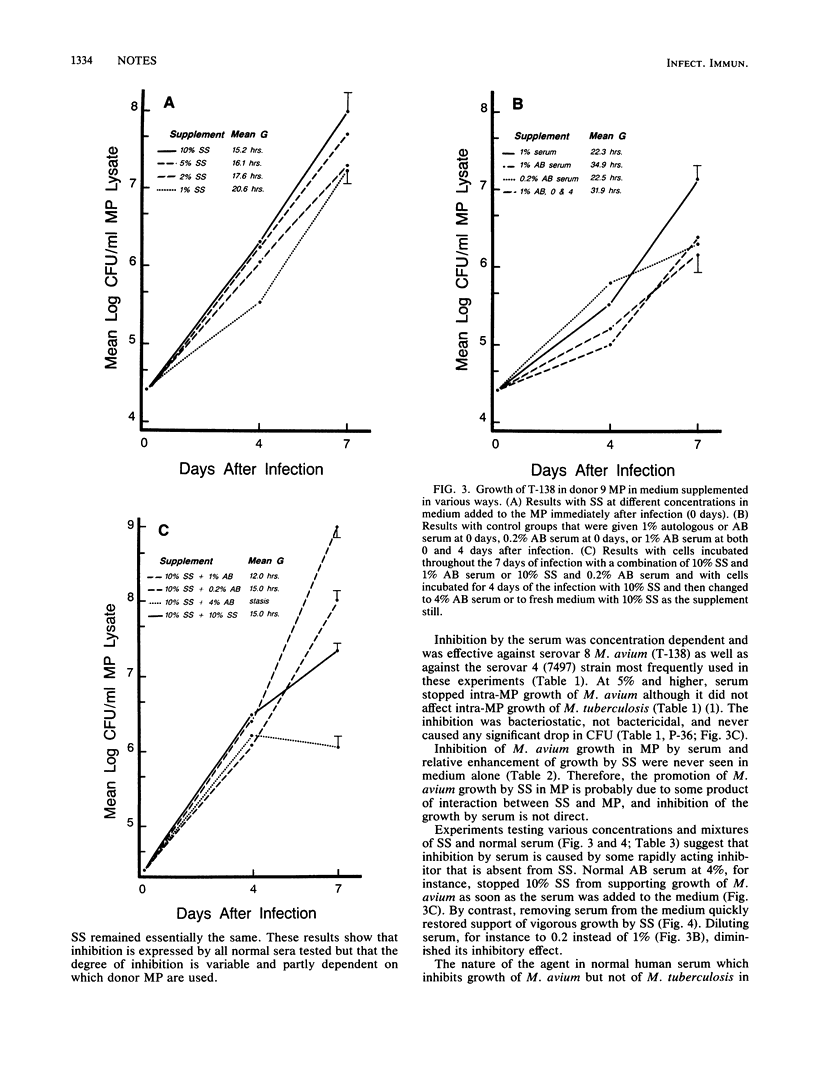
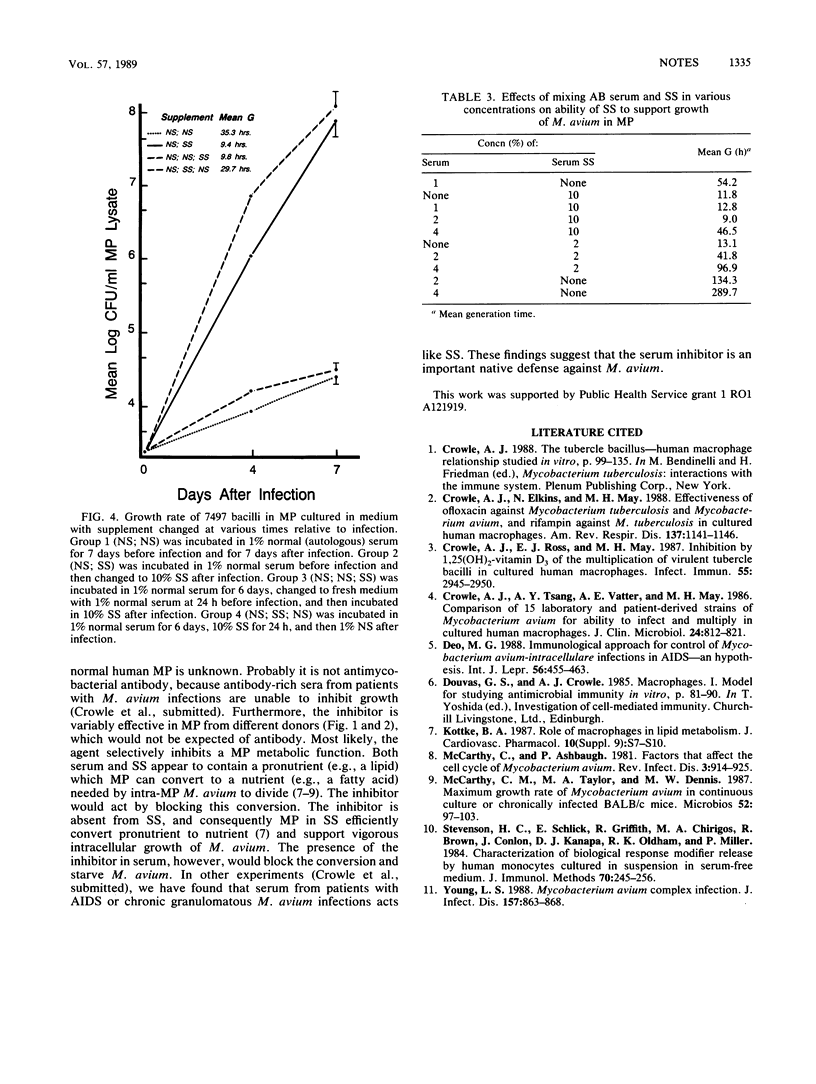
Normal human serum used as a protein supplement in RPMI 1640 medium inhibited growth in blood-derived human macrophages (MP) of virulent Mycobacterium avium serovars 4 and 8, derived from patients with acquired immunodeficiency syndrome, but not virulent Mycobacterium tuberculosis. A defined serum substitute (SS) promoted the intramacrophage growth of M. avium but not of M. tuberculosis. The effects of serum or SS were measured by counting viable bacteria in lysates of the MP at 0, 4, and 7 days after their infection by the bacteria. Neither serum nor SS inhibited or enhanced M. avium growth in the absence of MP. The results suggest that a nutrient essential for intracellular replication of M. avium is made by MP from a pronutrient present in both SS and serum and that something in serum inhibits MP conversion of the pronutrient to nutrient. This inhibition may be an important mechanism of native resistance against M. avium in normal people.
Full text
PDF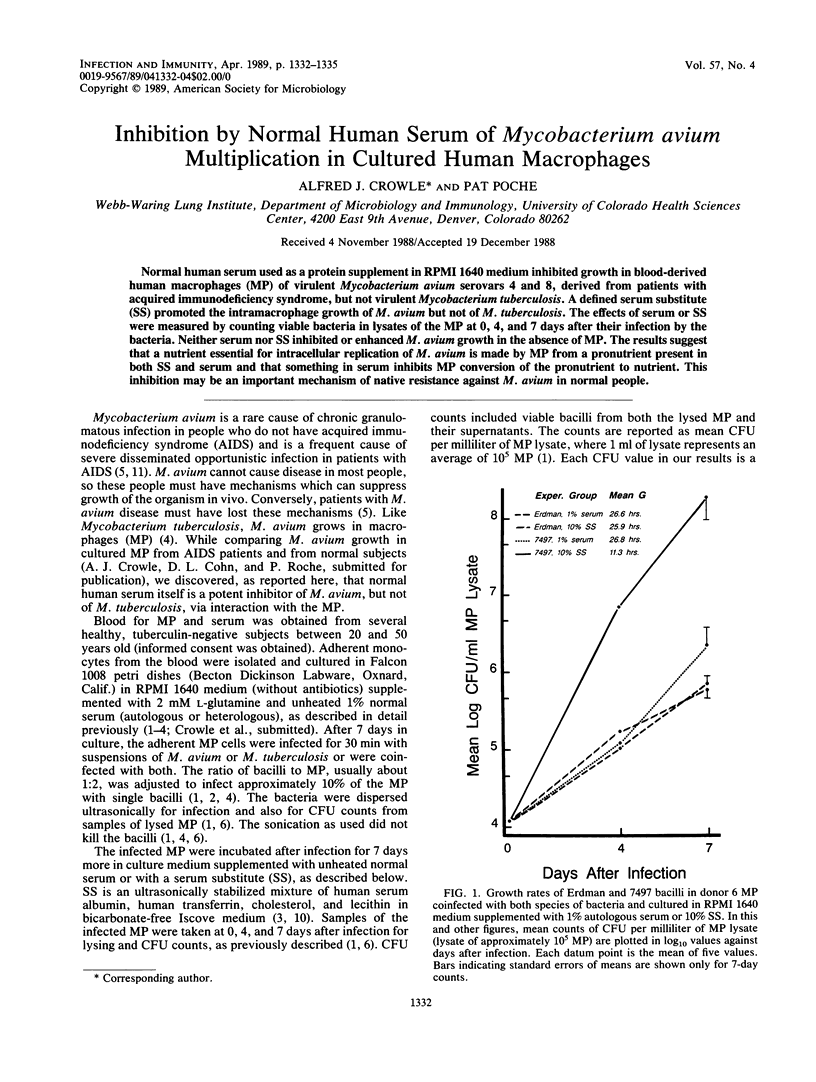
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J., Elkins N., May M. H. Effectiveness of ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium, and rifampin against M. tuberculosis in cultured human macrophages. Am Rev Respir Dis. 1988 May;137(5):1141–1146. doi: 10.1164/ajrccm/137.5.1141. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Ross E. J., May M. H. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987 Dec;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo M. G. Immunological approach for control of Mycobacterium avium-intracellulare infections in AIDS-an hypothesis. Int J Lepr Other Mycobact Dis. 1988 Sep;56(3):455–463. [PubMed] [Google Scholar]
- Kottke B. A. Role of macrophages in lipid metabolism. J Cardiovasc Pharmacol. 1987;10 (Suppl 9):S7–10. [PubMed] [Google Scholar]
- McCarthy C. M., Taylor M. A., Dennis M. W. Maximum growth rate of Mycobacterium avium in continuous culture or chronically infected BALB/c mice. Microbios. 1987;52(211):97–103. [PubMed] [Google Scholar]
- McCarthy C., Ashbaugh P. Factors that affect the cell cycle of Mycobacterium avium. Rev Infect Dis. 1981 Sep-Oct;3(5):914–925. doi: 10.1093/clinids/3.5.914. [DOI] [PubMed] [Google Scholar]
- Stevenson H. C., Schlick E., Griffith R., Chirigos M. A., Brown R., Conlon J., Kanapa D. J., Oldham R. K., Miller P. Characterization of biological response modifier release by human monocytes cultured in suspension in serum-free medium. J Immunol Methods. 1984 May 25;70(2):245–255. doi: 10.1016/0022-1759(84)90189-3. [DOI] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]



