Abstract
Objectives.
Chronological age is the most frequently employed predictor in life-span developmental research, despite repeated assertions that it is best conceived as a proxy for true mechanistic changes that influence cognition across time. The present investigation explores the potential that selected functional biomarkers may contribute to the more effective conceptual and operational definitions of developmental time.
Methods.
We used data from the Victoria Longitudinal Study to explore both static and dynamic biological or physiological markers that arguably influence process-specific mechanisms underlying cognitive changes in late life. Multilevel models were fit to test the dynamic coupling between change in theoretically relevant biomarkers (e.g., grip strength, pulmonary function) and change in select cognitive measures (e.g., executive function, episodic and semantic memory).
Results.
Results showed that, independent of the passage of developmental time (indexed as years in study), significant time-varying covariation was observed linking corresponding declines for select cognitive outcomes and biological markers.
Discussion.
Our findings support the interpretation that cognitive decline is not due to chronological aging per se but rather reflects multiple causal factors from a broad range of biological and physical health domains that operate along the age continuum.
Keywords: Biological age, Chronological age, Cognitive change, Longitudinal, Physiological status
IN recent decades, despite many advances in developmental designs (e.g., Nesselroade & Baltes, 1979) and statistical procedures (e.g., McArdle, 2009), one challenging constant remains—the all but ubiquitous use of chronological age as the developmental time metric for charting performance differences and changes. Both earlier (e.g., Birren, 1959; Wohlwill, 1973) and more recently (Anstey, 2008; Birren, 1999; Dixon, 2011), the strengths and weaknesses of chronological age as a developmental index have been well documented. Although heuristically useful for indexing age-related cognitive and functional change, chronological age is but an indirect reflection of accumulated biological and environmental influences. Consequently, chronological age is not a causal mechanism underlying cognitive and functional decline, at least in the sense that merely knowing that chronological age is associated with cognitive decline says nothing about specific or general mechanisms underlying age-related cognitive impairment. Instead, age is a temporal dimension along which biological, environmental, health, and neurological causal factors operate (Baltes & Willis, 1977; MacDonald, Dixon, Cohen, & Hazlitt, 2004). For example, Peto and Doll (1997) noted that while increasing age is associated with increasing morbidity, age per se does not cause disease (or health). Rather, as natural selection buffers more strongly against death during early adulthood and mid-life as compared with late life, the increasing incidence of disease and impairment observed in late life is a function of this common set of evolutionary pressures. From this perspective, chronological age appears best conceived as a proxy for true mechanistic changes that influence functional capacity and adaptivity (including, but not limited to, cognition) across the lifetime.
Most longitudinal cognitive aging research parameterizes developmental time using three time basis structures (see Morrell, Brant, & Ferrucci, 2009): chronological age (e.g., years since birth), measurement occasion (e.g., 0, 1, 2 … ), and time in study (years from baseline assessment). Although convenient, plotting within-person cognitive change as a function of chronological age may fail to capture important underlying sources of heterogeneity. For example, variability due to underlying health conditions (e.g., cardiovascular disease [CVD]) may be misattributed to age (Spiro & Brady, 2008). Moreover, the exclusive use of chronological age assumes that within-person change in cognition is adequately described by average population age trends, an assumption that has long been qualified (Baltes & Willis, 1977; Thorvaldsson, Hofer, Hassing, & Johansson, 2008). Ultimately, a comprehensive understanding of age-related decline in cognition requires focusing on development as a function of change in critical functions (e.g., biological, psychosocial) that are expressed over time (Li & Schmiedek, 2002; Sliwinski & Mogle, 2008). Among recent promising avenues are time-to-event parameterizations, which can be employed to index change in cognitive functioning in relation to the onset of key biological or health events believed to underlie within-person change (e.g., years pre/postmenopause; Thilers, MacDonald, Nilsson, & Herlitz, 2010). Arguably, advances in our theoretical understanding of mechanisms underlying age-related functional change will ultimately require supplementation, integration, or perhaps supplantation of chronological age by biological and health variables that both indirectly or directly index theoretically significant causes of psychological aging (Baltes & Willis, 1977; Birren, 1999; Dixon, 2011).
Effective implementation of biological metrics of maturation requires theorizing as to how physiological processes may influence (a) structural and functional changes in the brain and (b) levels and trajectories of cognitive decline and impairment. Of particular interest are potential causal markers of developmental change that reflect underlying biological and health domains. Known as “functional biomarkers” (see Anstey, 2008), these indicators index maturation in terms of biological functions or processes that (a) index functional capacity, (b) decline with age, (c) can be objectively measured, (d) operationalize fitness and health conditions, and (e) share a systematic association with relatively specific outcomes (e.g., age-related increases in cognitive or functional impairment, disease). Ideally, a primary objective of biomarker research is to develop a biological index that represents a “surrogate end point” or proxy for a behavioral or clinical observation (Biomarkers Definitions Working Group, 2001). In the field of cognitive aging, biomarkers of cognitive and functional change and decline span a diverse continuum of biological and neurological processes and indicators (Raz et al., 2005). These include (but are not limited to) histopathological counts of plaques and tangles (e.g., Bennett, Schneider, Wilson, Bienias, & Arnold, 2004), oxidative stress markers derived from biochemical blood assays (e.g., Franceschi et al., 2007), genetic markers such as ApoE4 (Small, Rosnick, Fratiglioni, & Bäckman, 2004), white matter hyperintensities (Raz, Rodrigue, Kennedy, & Acker, 2007), whole-brain atrophy (Spulber et al., 2010), dopamine receptor binding (Bäckman, Lindenberger, Li, & Nyberg, 2010), and amyloid burden (Nordberg, 2010). As indicated in our own earlier work (MacDonald et al., 2004; Wahlin, MacDonald, de Frias, Nilsson, & Dixon, 2006), they also include health and physiological markers demonstrating more indirect neural and cognitive associations, including sensory functioning such as visual acuity or olfaction, grip strength, or forced expiratory volume (e.g., Anstey, 2008; Djordjevic, Jones-Gotman, De Sousa, & Chertkow, 2008; Raz et al., 2005). Literally, hundreds of variables have been used as putative biomarkers (Anstey, 2008; Spiro & Brady, 2008). In this study, we focus on functional biomarkers, extending a program of research examining theoretical and empirical associations, both static and longitudinal, among biological and cognitive functions in aging.
Considered together, a theoretically motivated selection of functional biomarkers may be used eventually to operationalize biological aging or bioage. As such, bioage may reflect the functioning of critical physiological systems and processes and consequently may contribute to accurately indexing an individual’s functional age relative to his or her own life span. Consider two individuals who are 70 years of age: Despite having the same chronological age, a 70-year-old individual with type 2 diabetes and other comorbidities (e.g., vascular dysfunction) may be “biologically older” (or at least more vulnerable) than a 70-year-old individual who is otherwise healthy (McFall, Geall, Dolcos, Fischer, & Dixon, 2010). An obvious question concerns how two individuals can be the same chronoage yet exhibit such disparate underlying biological ages and associated cognitive phenotypes. The answer, in part, reflects the fact that different body and brain systems age at different rates both between individuals, as well as within the same individual, with the onset and the rate of biological aging a function of both biological make-up and environmental exposures (e.g., Fozard, Metter, & Brant, 1990; Murray, 1951; Spiro & Brady, 2008; Wohlwill, 1973). As a consequence of this differential biological aging and the modest to moderate association between chronological age and bioage (Goffaux et al., 2005; MacDonald et al., 2004), the use of chronological age to index heterogeneity in cognitive function attributable to biological processes will be imprecise at best and may result in chronological age being inappropriately employed for making decisions regarding an individual’s functional capacity in numerous domains (e.g., ability to drive, employment).
Recent investigations have operationalized biological aging as an index of various key biomarkers, some of which include both functional and neural markers. For example, Goffaux and colleagues (2005) created a summary index of biological age based on measures of endurance, strength, flexibility, balance, cognition, depression, comorbidity, and exercise. Similar biological age indices have been employed in other studies (e.g., Anstey & Smith, 1999; MacDonald et al., 2004; Nakamura & Miyao, 2007; Wahlin et al., 2006). However, to be sure, investigations of biological markers of cognitive aging have been criticized on several fronts, including (a) spurious associations between biomarkers and cognition due to their association with chronological age (Lindenberger & Pötter, 1998), (b) the lack of a strong theoretical foundation for the selection of biomarkers used for the operationalization of bioage (Anstey, 2008, but see Anstey, in press for progress on this front), (c) the fact that chronological age is easily and validly measured, and (d) within a developmental epidemiological perspective, chronological age falls among the continua of risk factors underlying individual differences in biological age (Dixon, 2011) and may be usefully employed in multivariate representations of bioage (Klemara & Doubal, 2006).
In the present investigation, we test the potential utility of biological aging processes as important markers of cognitive change focusing on two key strengths: (a) the selection of biomarkers based on a priori theoretical hypotheses regarding links to cognitive aging and (b) the use of appropriate statistical models for the analysis of actual changes over a six-year longitudinal period. Regarding the first strength, when targeting plausible biological indicators of aging, we selected individual biomarkers that index critical body systems (respiratory, circulatory, and muscular) with potential mechanistic links to cognition. For example, with increasing age, respiratory and circulatory functions are among the best indicators of physiological health (Spiro & Brady, 2008). Reduced peak expiratory flow, an indicator of pulmonary function, has been linked to compromised cognitive performance, risk for CVD, diminished physiological reserve, and mortality (e.g., Cook et al., 1991; Deary, Whalley, Batty, & Starr, 2006; Richards, Strachan, Hardy, Kuh, & Wadsworth, 2005; Weiss, Segal, Sparrow, & Wager, 1995). Among the potential mechanisms, cognitive impairment for individuals with impaired pulmonary function may be due to factors such as increased white matter hyperintensities and brain atrophy (Sachdev et al., 2006). In effect, functional biomarkers like pulmonary function may exert a direct influence on brain structure and function, which in turn affects cognition. Closely related to the respiratory system, vascular indices of the circulatory system also share known associations with cognitive impairment and dementia risk, including systematic changes in systolic and diastolic blood pressure, hypertension, high cholesterol, and stroke (Brady, Spiro, & Gaziano, 2005; Elias, D’Agostino, Elias, & Wolf, 1995; Hertzog, Schaie, & Gribbin, 1978; Qiu, Winblad, & Fratiglioni, 2005; Stephan, Matthews, Khaw, Dufouil, & Brayne, 2009). Physiological indicators such as markers of grip strength, balance, and body composition, indexing the sudden loss of body mass index (BMI) or the degenerative loss of skeletal muscle mass and strength (sarcopenia), also share well-documented associations with cognitive impairment, frailty, and dementia risk (Atti et al., 2008; Jensen, 2008; Rosano, Brach, Studenski, Longstreth, & Newman, 2007; Schaap, Pluijm, Deeg, & Visser, 2006). Such functional biomarkers may be proxies for physiological resilience, exerting their influence on cognitive function through pathways such as increased physical activity levels that share known associations with increased expression of certain genes, brain plasticity, and improved cognitive function (e.g., Cotman & Berchtold, 2002). Similarly, basic functional markers of gait and balance serve as indirect indicators of brain structure, including white matter hyperintensities linked to vascular disease and impairment in cognitive function (e.g., Rosano et al., 2007). Irrespective of mechanism, associations between the pertinent functional biomarkers and cognition may reflect a biological continuum along which human development can be indexed in terms of relative biological age and probability of biological decline and mortality (Anstey, 2008; Nakamura & Miyao, 2003).
The second strength of the present investigation concerns our use of longitudinal data to provide theoretically meaningful within-person tests and confirmation of previous promising cross-sectional associations. Specifically, using six-year data from three older adult samples of the Victoria Longitudinal Study (VLS), we explored two key research objectives. First, we formally assessed whether each of the cognitive and biological markers exhibited significant longitudinal change across the six-year follow-up. Second, for those measures that demonstrated significant change, we further evaluated evidence for the time-varying covariation between change in theoretically relevant biomarkers (e.g., BMI, grip strength) and change in cognitive performance (e.g., executive functions, episodic and semantic memory). In contrast to previous studies that have created composite markers of bioage (Anstey, 2008; MacDonald et al., 2004; Wahlin et al., 2006), the focus of the present investigation was to examine the influence of key individual candidate biomarkers (e.g., pulmonary function) from the VLS measurement battery based on potential mechanistic relevance to cognitive decline. Following the logic of process-based approaches for indexing developmental time (Sliwinski & Mogle, 2008), we used linear mixed models to specifically test for evidence of the dynamic across-time coupling between change for various cognitive measures on the one hand and change in select biological processes on the other. Thus, our focus concerns the within-person dynamics of biological and cognitive aging, rather than the between-person statics of biological–cognitive differences. Our expectation is that some degree of cognitive decline reflects multiple causal biological factors (e.g., changes in vascular health) that operate along the chronological age continuum.
METHODS
Participants
At intake, VLS participants are noninstitutionalized (community-dwelling) adults between 55 and 85 years of age with no serious cognition-related health conditions. Specifically, initial exclusionary criteria included previous diagnoses of neurocognitive diseases (Alzheimer’s), psychiatric conditions or medications, preexisting serious cardiovascular and cerebrovascular conditions, corrected eyesight insufficient for normal reading, and corrected hearing insufficient for understanding spoken directions (cf. Dixon & de Frias, 2004; Hultsch, Hertzog, Dixon, & Small, 1998).
Participants are followed at roughly three-year retest intervals or waves (see Dixon & de Frias, 2004 for further design characteristics). Analyses in the present study are based upon data from select waves of all three VLS samples that contain key biological and cognitive measures of interest: Sample 1 Waves 5–7 (n = 130, Mage = 78.93 years); Sample 2 Waves 3–5 (n = 336, Mage = 73.50 years); and Sample 3 Waves 1–2 (n = 577, Mage = 68.30 years). Although the cognitive outcomes were administered across all samples and waves of the VLS, the biological markers were first assessed at Wave 5 for Sample 1, at Wave 3 for Sample 2, and at Wave 1 for Sample 3. Data were assembled to form a three-wave (six-year) longitudinal design, thereby facilitating examination of how change for select biological measures influences change in cognitive functioning.
VLS participants exhibit the typical selectivity of longitudinal samples relative to the general population. The final sample used for this investigation included 1,043 participants (n = 684 females and 359 males). At baseline, these participants had completed an average of 15.05 (SD = 3.03) years of education, achieved an average score of 28.57 (SD = 1.49) on the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975), exhibited respective systolic and diastolic mean blood pressure readings of 128.46 mmHg (SD = 15.32) and 75.35 mmHg (SD = 9.44), reported self-rated health estimates relative to perfect of 1.85 (SD = 0.77) and 1.64 (SD = 0.73) relative to same-aged peers on a scale from 1 (very good) to 5 (very poor), and reported an average Center for Epidemiologic Studies–Depression Scale (Radloff, 1977) score of 7.55 (SD = 5.65). Among select physician-diagnosed chronic conditions, 3.9% of participants reported moderate to serious atherosclerosis, 11.4% reported moderate to serious hypertension, 10.1% reported moderate to serious heart conditions, 3.6% reported moderate to serious type 2 diabetes, 9.3% reported experiencing moderate to serious cancer, with 1.2% having experienced a moderate to serious stroke. Taken together, these background characteristics indicate that these VLS participants are in very good health and that any observed estimates of cognitive change or cognition–biomarker associations likely underestimate the true effects.
Cognitive Measures
Indicators of five cognitive constructs (i.e., fluid reasoning, working memory, episodic memory, semantic memory, and crystallized ability) sensitive to cognitive health and aging were selected from previous VLS confirmatory factor analyses (see Hertzog, Dixon, Hultsch, & MacDonald, 2003). These indicators span a continuum from processes (process based, executively demanding, fluid) to products (knowledge based, more automatized, crystallized) of cognition. For further description of the tasks and associated psychometric properties, see VLS source citations (Dixon & de Frias, 2004; Hultsch et al., 1998).
Fluid reasoning.—
Fluid reasoning ability was indexed by the letter series task. This task assessed how well participants could deduce the pattern of a series of letters. Participants were asked to place the last letter in a series of letters that would continue the established pattern (e.g., A B D A B D A B … ?). Participants received a score out of a total possible of 20.
Working memory.—
The computation span task was used as an indicator of working memory requiring storage and simultaneous processing of information (Salthouse & Babcock, 1991). Participants were asked to solve a series of arithmetic problems while holding the final digit from each problem in memory for later recall. The number of problems in a series increased from one to seven, with three trials at each series length. The highest span correctly recalled for two of the three trials was the measure used.
Episodic memory.—
The word recall test was based on six categorized lists of common English nouns from the Howard (1980) and Battig and Montague (1969) norms. Two word lists were used, with each list consisting of six words from five taxonomic categories (e.g., birds) typed on a single page in unblocked order. Participants were given 2 min to study each list and 5 min to write their free recall. The average number of correctly recalled words was the outcome measure. Equivalent form lists were employed such that participants did not see the same word list across the six-year period in the present study.
Semantic memory.—
The fact recall test assesses acculturated knowledge and is based on norms developed by Nelson and Narens (1980). Participants wrote their answers under self-paced conditions to two 40-item recall tests of world facts from multiple domains (science, history, art, sports, and geography). The outcome measure was the average number of correct items out of 40.
Crystallized ability.—
English vocabulary was indexed by a 54-item recognition vocabulary measure, adapted from the Kit of Factor Referenced Cognitive Tests (Ekstrom, French, Harman, & Derman, 1976). Participants were given 15 min to complete the test, with the outcome measure reflecting the total number of correct responses.
Biological Measures
Key biomarkers of interest with well-established links to cognitive decline in normal aging included (a) grip strength, (b) peak expiratory flow (pulmonary function), (c) systolic and diastolic blood pressure (vascular health), and (d) BMI.
Grip strength.—
Grip strength was measured using a Smedley hand dynamometer. The strength of each hand was measured in kilograms of force while the participant was in a seated position. For each hand, participants were given two attempts. The best score of the two attempts for the dominant hand was used.
Peak expiratory flow.—
Peak expiratory flow was measured using a MiniWright Peak Flow meter. Participants were asked to take as deep a breath as possible and place the mouthpiece into their mouth. They were told to execute a quick hard blow while in a standing position. A final score was calculated in liters per minute based on the largest maximum volume expired out of three attempts.
Body mass index.—
Anthropometric measures of height and weight were used to derive each individual’s BMI. Height was measured in centimeters and weight was measured in kilograms. BMI reflects the height/weight ratio calculated by taking the weight in kilograms divided by the square of one’s height in meters.
Blood pressure.—
Blood pressure was taken from the left arm using an Omron Automatic Oscillometric Digital Blood Pressure monitor (model HEM-713C) while the participant was seated. Indices of systolic and diastolic blood pressure measured in millimeters of mercury were obtained for four readings on each of two sessions approximately one week apart. Two blood pressure readings were taken at the beginning and end of each of the two testing sessions. The average of the eight readings was used.
Statistical Procedure
Consistent with a process-based approach to indexing developmental time (cf. Sliwinski & Mogle, 2008), we used HLM 6.06 (Raudenbush, Bryk, & Congdon, 2004) to fit linear mixed models to test two main research questions. First, we tested whether each of the cognitive and biological markers displayed significant longitudinal changes. Initial within-person models of change were fit to examine linear change as a function of time in study (see equations 1a and 1b).
| (1a) |
| (1b) |
Second, for those measures that exhibited significant change, we further examined how within-person change in cognition and biomarkers traveled together across time. To identify intra-individual covariates of cognitive change, we constructed “time-varying covariation models” (see equation 2) by including an index of developmental time (time in study) as well as various indices of biological time (e.g., change in grip strength). This Level 1 model
| (2) |
assumes that cognitive functioning at any given time depends upon the amount of time since baseline, change in biological functioning, as well as person-specific residuals. The β1i slope parameter in this model reflects rate of linear change in cognition across time independent of biomarker function, whereas the β2i slope parameter assesses whether higher (or lower) biomarker function for a given occasion is linked to higher (or lower) cognitive function independent of linear cognitive change over time. Time in study was modeled as years since baseline (centered at 0), with the slopes for each biomarker grand mean centered to reflect cognitive functioning for the population average. Time-varying covariation between Level 1 variables indicates that scores on these variables “travel together” over time. As time in study shares a close association with variable coding approaches used to address practice effects, detrending for developmental time also adjusts for practice in our change estimates. To control for the potential confound of sex differences in physiology, the influence of sex was partialled from both grip strength and peak expiratory flow prior to further analysis (cf. Anstey, Lord, & Smith, 1996; Christensen et al., 2000). Chronological age at baseline was entered as a covariate in all models (cf. Morrell et al., 2009). Parameters were estimated using full information maximum likelihood. Where variance terms (random effects) were not significant, they were trimmed from the model (Snijders & Bosker, 1999).
RESULTS
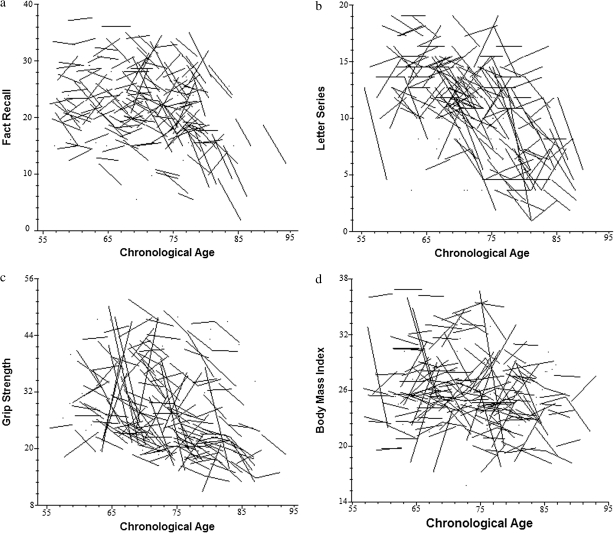
To address our first research question, we conducted analyses confirming the presence of longitudinal change separately for each of the cognitive outcomes (letter series, computation span, word recall, fact recall, and vocabulary) and biological processes (grip strength, peak expiratory flow, BMI, and systolic and diastolic blood pressure) under consideration. Table 1 summarizes the parameters from each linear mixed model, modeling change as a function of time in study, covarying for age at the initial time of testing (cf. Morrell et al., 2009). Consistent with expectations, significant declines were observed for all cognitive outcomes and biomarkers of interest. Each additional year increase in chronological age above the baseline grand mean was associated with significantly poorer functioning for all cognitive outcomes (p < .001) except vocabulary, as well as all biomarkers (p < .05) except for BMI. For heuristic purposes, the panels of Figure 1 characterize change for select cognitive outcomes and biomarkers as a function of chronological age. These panels clearly demonstrate both population average change as well as individual change.
Table 1.
Change in Cognitive Performance and Biological Capacity as a Function of Time in Study
| Variables | Intercept,γ00 | Slope,γ10/γ20 | SE | p |
| Cognitive | ||||
| Letter series | 11.14 | −0.954 | 0.094 | <.001 |
| −0.048 | 0.012 | <.001 | ||
| Computation span | 3.12 | −0.323 | 0.037 | <.001 |
| −0.025 | 0.005 | <.001 | ||
| Word recall | 17.44 | −1.042 | 0.113 | <.001 |
| −0.094 | 0.014 | <.001 | ||
| Fact recall | 20.38 | −0.866 | 0.130 | <.001 |
| −0.105 | 0.016 | <.001 | ||
| Vocabulary | 43.05 | −1.281 | 0.109 | <.001 |
| −0.004 | 0.015 | .776 | ||
| Biomarker | ||||
| Grip strength | 29.17 | −2.197 | 0.275 | <.001 |
| −0.084 | 0.033 | <.05 | ||
| Peak exp. flow | 387.77 | −7.425 | 3.435 | <.05 |
| −1.851 | 0.427 | <.001 | ||
| BMI | 26.75 | −0.240 | 0.114 | <.05 |
| −0.009 | 0.014 | .513 | ||
| Blood pressure | ||||
| Systolic | 128.28 | −0.412 | 0.376 | .273 |
| −0.130 | 0.051 | <.05 | ||
| Diastolic | 75.28 | −3.111 | 0.223 | <.001 |
| −0.082 | 0.031 | <.01 | ||
Notes: γ00 = Average performance at wave = 0 for the grand mean of baseline sample age; γ10 = average rate of linear change per additional year in study for an individual of average sample age; γ20 = average difference in rate of linear change per additional year of age above the grand mean; BMI = body mass index. Cognitive coefficients reflect values out of 20 for letter series, values on a 0 to 6 scale for computation span, and values out of 30, 40, and 54, respectively, for word recall, fact recall, and vocabulary. Biomarker coefficients reflect kilograms of force for grip strength, liters per minute for peak expiratory flow, kilograms divided by the square of one’s height in meters for BMI, and millimeters of mercury for systolic and diastolic blood pressure. All intercept values were significantly different from 0, as were corresponding variance components. Age at baseline, centered at the grand mean (71.25 years, SD = 8.54), was entered as a covariate for all models. For the cognitive models, df ranged from 1729 for letter series to 1959 for vocabulary, with biomarker values ranging from 1004 for peak expiratory flow to 1785 for BMI.
Figure 1.
Raw data trajectories of cognitive and biological change as a function of chronological age. Individual trajectories are plotted for a 25% random sample of all possible cases. (a) Fact recall, (b) letter series, (c) grip strength, and (d) body mass index.
Observing significant linear change in both the cognitive and biomarker measures as a function of time in study represents a necessary first step. A more stringent test of whether change in cognition is systematically associated with change in key biological functions requires the fitting of dynamic time-varying covariation models (see equation 2). Specifically, independent of population average change, we evaluated whether cognitive functioning was higher (or lower) on occasions when functioning for each biological indicator was also higher (or lower). In effect, these statistical models provide an inferential test that is akin to, for example, superimposing developmental change in fact recall (see Figure 1a) on change in grip strength (see Figure 1c).
Table 2 summarizes the findings from the time-varying covariation models. Independent of the passage of developmental time (indexed as years in this study), the multilevel findings indicate select links between corresponding declines for select cognitive outcomes and biological markers. Beginning with cognitive processes, decline in fluid reasoning (letter series) shared a significant time-varying association with declines in grip strength. Upon entering grip strength in the model, the developmental time effect was reduced from −0.954 letter series units of decline per year to −0.882 units of decline, representing an attenuation of 7.5% ([−0.954 to −0.882]/−0.954 × 100 = 7.5%) of the total developmental effect. Decline in systolic blood pressure was also uniquely associated with fluid reasoning decline but did not attenuate the developmental time effect. Similarly, decline in working memory (computation span) was significantly linked to declines in grip strength and peak expiratory flow, with the developmental time effect attenuated by 0.3% for grip strength but not at all for peak flow. Decline in semantic memory (fact recall) shared unique links to corresponding declines in muscle strength, body mass, pulmonary capacity, as well as systolic blood pressure: The developmental time effect was attenuated by 19%, 3%, 8%, and 7%, respectively, by partialling the effect of these biomarkers. Finally, declines in vocabulary and corresponding declines in peak expiratory flow as well as systolic and diastolic blood pressure were linked, but these unique associations were entirely independent of the developmental time effect.
Table 2.
Time-Varying Covariation Models: Cognitive Performance as a Function of Time in Study and Biological Function
| Variables | Intercept,γ00 | Slope,γ10/γ20 | SE | p |
| Letter series | ||||
| Peak exp. flow | 11.21 | −0.922 | 0.096 | <.001 |
| 0.001 | 0.0008 | .135 | ||
| Grip strength | 11.18 | −0.882 | 0.098 | <.001 |
| 0.016 | 0.009 | .098* | ||
| BMI | 11.14 | −0.970 | 0.094 | <.001 |
| 0.011 | 0.021 | .602 | ||
| Blood pressure | ||||
| SBP | 11.19 | 0.003 | 0.006 | .616 |
| −0.002 | 0.001 | <.05 | ||
| DBP | 11.11 | 0.013 | 0.010 | .190 |
| −0.001 | 0.001 | .223 | ||
| Computation span | ||||
| Peak exp. flow | 3.13 | −0.347 | 0.037 | <.001 |
| 0.001 | 0.001 | <.005 | ||
| Grip strength | 3.13 | −0.322 | 0.038 | <.001 |
| 0.010 | 0.003 | <.005 | ||
| BMI | 3.13 | −0.355 | 0.036 | <.001 |
| −0.011 | 0.007 | .12 | ||
| Blood pressure | ||||
| SBP | 3.13 | 0.003 | 0.002 | .160 |
| −0.001 | 0.001 | .273 | ||
| DBP | 3.11 | 0.007 | 0.003 | <.05 |
| −0.001 | 0.001 | .748 | ||
| Word recall | ||||
| Peak exp. flow | 17.53 | −0.899 | 0.122 | <.001 |
| 0.001 | 0.001 | .595 | ||
| Grip strength | 17.52 | −0.895 | 0.120 | <.001 |
| −0.005 | 0.011 | .673 | ||
| BMI | 17.46 | −1.017 | 0.114 | <.001 |
| −0.006 | 0.023 | .798 | ||
| Blood pressure | ||||
| SBP | 17.45 | 0.010 | 0.007 | .130 |
| 0.001 | 0.001 | .178 | ||
| DBP | 17.49 | 0.007 | 0.011 | .534 |
| 0.002 | 0.001 | <.05 | ||
| Fact recall | ||||
| Peak exp. flow | 20.40 | −0.717 | 0.137 | <.001 |
| 0.004 | 0.001 | <.01 | ||
| Grip strength | 20.41 | −0.628 | 0.141 | <.001 |
| 0.064 | 0.014 | <.001 | ||
| BMI | 20.39 | −0.859 | 0.131 | <.001 |
| 0.085 | 0.029 | <.01 | ||
| Blood pressure | ||||
| SBP | 20.47 | 0.037 | 0.009 | <.001 |
| −0.003 | 0.001 | <.01 | ||
| DBP | 20.27 | 0.048 | 0.014 | <.01 |
| −0.002 | 0.002 | .308 | ||
| Vocabulary | ||||
| Peak exp. flow | 43.37 | −1.57 | 0.116 | <.001 |
| 0.004 | 0.001 | <.01 | ||
| Grip strength | 43.15 | −1.56 | 0.117 | <.001 |
| 0.017 | 0.013 | .212 | ||
| BMI | 43.18 | −1.67 | 0.114 | <.001 |
| 0.001 | 0.028 | .988 | ||
| Blood pressure | ||||
| SBP | 43.24 | −0.009 | 0.008 | .237 |
| −0.002 | 0.001 | <.05 | ||
| DBP | 43.15 | −0.010 | 0.012 | .394 |
| −0.005 | 0.001 | <.01 | ||
Notes: γ00 = Average performance at wave = 0 for the grand mean of a given biomarker and baseline sample age; γ10 = average rate of linear change per additional year in study for an individual of average sample age, independent of a given biomarker’s influence; γ20 = estimated change in cognitive function per unit change in a given biomarker for an individual of average sample age, independent of the average rate of linear change per additional year in study; BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure. All intercept values were significantly different from 0, as were corresponding variance components. Age at baseline, centered at the grand mean (71.25 years, SD = 8.54), was entered as a covariate for all models.
*p < .05, one tailed.
DISCUSSION
Despite the status of chronological age as the most frequently employed developmental index in the field of cognitive aging, several arguments qualifying this practice have been raised for at least the past 5 decades (e.g., Birren, 1965; Peto & Doll, 1997; Spiro & Brady, 2008; Wohlwill, 1973). Identified problems are both statistical (e.g., spurious associations may arise from age-related mean trends; Hofer & Sliwinski, 2001) and conceptual (e.g., chronological age does not inform specific or general mechanisms underlying age-related cognitive impairment; MacDonald et al., 2004) in nature. To what extent should chronological age be conceived as a proxy for true mechanistic changes that influence cognition across time? The present study sought to build on recent calls for more effective conceptual and operational definitions of developmental time (Baltes & Willis, 1977; Sliwinski & Mogle, 2008). Specifically, we explored models of late-life cognitive change that considered the predictive utility of (a) a conventional index of developmental change (time in study) as well as (b) targeted functional biological markers that arguably could reflect more process-specific mechanisms underlying cognitive decline (Anstey, in press).
Our initial analyses focused on demonstrating significant change, as a function of time in study, for each biomarker and cognitive outcome. We observed six-year decline for each of our five cognitive outcomes (letter series, computation span, word recall, fact recall, and vocabulary). Similarly, six-year declines were also observed for targeted indicators of the various biological processes (grip strength, peak expiratory flow, BMI and, systolic and diastolic blood pressure). Having demonstrated significant declines for both the cognitive and biological markers, we proceeded to explore the dynamic relationship between them. Consistent with our expectations, we observed significant time-varying covariation between theoretically relevant functional biomarkers of cognition and corresponding decline for a number of markers of cognition. Building on previous findings examining cross-sectional differences in biological status (e.g., Anstey, 2008; MacDonald et al., 2004; Spiro & Brady, 2008; Wahlin et al., 2006), significant change in key functional-health biomarkers including muscle strength, cardiovascular health, pulmonary function, and body composition were all linked to cognitive impairment. Diminished grip strength, increasing systolic but lower diastolic blood pressure, reduced lung volume based on peak expiratory flow estimates, and declining body mass showed the following dynamic patterns: (a) They were all selectively linked to cognitive decline and (b) all linkages were in the expected direction based on previous research (e.g., Deary et al., 2006; Qiu et al., 2005; Schaap et al., 2006).
Of note, the most robust predictive effects for the biological indicators were observed for more crystallized (semantic memory) than fluid (reasoning) or episodic memory abilities. Whereas declines in muscle strength, body mass, pulmonary capacity, and blood pressure shared only selective links with the fluid or episodic memory measures, they were all significantly associated with declines in semantic memory. Beyond sheer number of significant biomarker predictors, the largest attenuation of the developmental time effect was also observed for semantic memory (19% attenuation of the time effect by grip strength). One plausible reason why the select biomarkers examined in this study were differentially associated with crystallized abilities may reflect the likelihood that normative aging processes differentially account for decline for more fluid measures of cognitive function due to mid-life structural, functional, and neuromodulatory brain changes. For example, changes in dopamine modulation, as well as changes in prefrontal and medial temporal cortex as early as age 30, may indicate a more fundamental and uniform developmental influence—one that is well described by chronological age—on cognitive outcomes such as working or episodic memory subserved by these neurotransmitters and brain regions (e.g., Bäckman et al., 2010; Raz et al., 2005). Whereas such normative developmental changes influence every individual to varying degrees and may thus be adequately indexed by chronological age, non-normative aging due to select pathology (e.g., CVD) does not affect all individuals and may exhibit considerable heterogeneity in terms of age of onset (cf., Bäckman & MacDonald, 2006; Spiro & Brady, 2008). Thus, the relative predictivity of any biomarker will, in part, depend on the extent to which the bodily system indexed by the biomarker (e.g., cardiovascular) is relevant to a given cognitive or functional outcome and the disease processes that affect such outcomes. As research evolves in this area, maximizing variance accounted for will require the identification of biomarkers that are sensitive to both normative and pathological aging. With regard to the present study, although the proportion of the total variance in developmental cognitive change accounted for by any individual biomarker is relatively modest (cf. Hofer, Berg, & Era, 2003; Lindenberger & Ghisletta, 2009), the unique predictivity of cognitive decline by the select biomarkers, independent of developmental time, suggests that potential causal factors that operate along the age continuum can indeed be identified.
Integrating biological and chronological markers for indexing cognitive decline will not occur without methodological effort (e.g., Klemara & Doubal, 2006; Nakamura & Miyao, 2007). In addition, to date, the selection of individual biological predictors or the operationalization of biological age in the study of cognitive aging has often lacked a strong theoretical foundation (e.g. Anstey, 2008; Baltes & Willis, 1977). However, improved theorizing and methods will permit us to (a) identify biomarkers that share potential mechanistic links with cognition, (b) better articulate the promising concept of biological age, and (c) improve our understanding of late-life cognitive change. In particular, developing core criteria for the selection of biomarkers from a variety of physiological and neurological systems, and establishing corresponding psychometric properties, represents an important next step for continuing research in this area (Ingram, Nakamura, Smucny, Roth, & Lane, 2001; Nakamura, Lane, Roth, & Ingram, 1998). By successfully identifying biomarkers that distinguish normal cognitive decline from accelerated decline or impairment due to pathology, we may be better able to focus on early detection and intervention, as opposed to post-diagnosis treatment, remedial operations, or palliative care.
To the extent that biomarkers index important aspects of biological functions, especially as they affect downstream neurological structure and function, they may be more theoretically linked to the underlying mechanisms of cognitive change than is the simple passage of time (chronological years). A key challenge in future research concerns which mechanistic pathways of aging, and associated indicators, we should target. Notably, research in a number of fields, including genetics, is providing great assistance in refining our focus. For example, a recent review by Cluett and Melzer (2009) reported that of over 300 published genome-wide association studies, a total of 50 genetic variants have been directly linked to four key age-related diseases: CVD, type 2 diabetes, osteoporosis, and prostate cancer. The primary mechanistic pathways believed to underlie these four age-related diseases include potential cognitively related biomarkers such as cell senescence, oxidative stress, endocrine signaling, and inflammation. Accordingly, future research may target such mechanisms and associated biomarkers of cognitive decline. Two of the mechanistic pathways in particular, inflammation and oxidative stress, have been directly linked to changes in functional biomarkers examined in the present study (e.g., Wang et al., 2009) and show considerable promise for indexing cognitive decline, functional impairment, and associated disease processes (e.g., Ershler, 2007; Giunta et al., 2008; Yaffe et al., 2004).
Several limitations of the present investigation should be noted. First, the biomarkers employed may be classified as indirect functional markers of the underlying pathways that, through their influence on brain health, are associated with cognitive functioning (Anstey, 2008). In future research, a combination of theoretically selected indirect and direct measures of biological function believed to share such indirect (and perhaps direct) association with cognitive functions should be employed. For example, recent advances in the use of diffusion tensor imaging have revealed clear links between white matter degradation and cognitive impairment (Davis et al., 2009). A second limitation concerns the number of available longitudinal assessments. Although the time-varying covariation models employed were well suited to our research questions, such models are computationally intensive. As additional waves of measurement become available in the VLS, it will be possible to fit these models without having to constrain parameters (e.g., the random slope effect for biomarkers). A third limitation concerns the univariate modeling approach adopted in the present study, which was designed to investigate each biomarker individually in a time-intensive design. Although this approach is essential for building the case with longitudinal data, it can be elaborated methodologically in future prospective studies. Given that a diversity of biomarker predictors have been shown to exert differential influences on cognitive performance, as well as the many specific relations documented between cognition and brain structure and function, the search for single causal mechanisms of age-related declines in cognition appears less tenable than multicausal interactive biological explanations ranging from the direct neurobiological to the indirect physiological. Ultimately, adopting a multivariate approach to this challenge (e.g., identifying latent factors of biological aging in a structural equation modeling [SEM] framework and relating them to latent cognitive outcomes) will be advantageous for multiple reasons (e.g., SEM attenuates for measurement error). Progress is being made in this regard, as in recent years, theoretical models and implementations of biological age have been appearing more frequently (cf. Goffaux et al., 2005; Nakamura & Miyao, 2007). A fourth limitation concerns the possibility that practice effects on the cognitive outcomes may have obscured the reported estimates of cognitive change in the present study. Given the initially select nature of the VLS sample relative to the general population, the present estimates of change likely underestimate the timing, extent, explanations, and magnitude of typical cognitive decline (cf. Salthouse, 2009, but see also Schaie, 2009; Dixon, Small, MacDonald, & McArdle, in press).
The patterns of significance for each of the biomarkers examined in the present investigation imply specific physiological mechanisms of age-related cognitive impairment. As noted, developing core criteria for the selection of biomarkers from a variety of physiological systems and establishing corresponding psychometric properties represents an important next step for continuing research in this area. The relative benefit of functional biomarkers concerns the more objective indexing of underlying disease processes and progression of cognitive and functional impairment—conceptualizing, operationalizing, and indexing bioage should be a primary goal of a wide range of aging research. Ultimately, our focus concerning the mechanisms of cognitive decline should shift from careless referral to aging as an “undefined physical concept” (Peto & Doll, 1997) to the targeted search for specific mechanistic pathways (e.g., oxidative stress, inflammation) of age-related performance declines.
FUNDING
This research was supported by a grant from the National Institutes of Health/National Institute on Aging (R37 AG008235-20) to R. A. Dixon, who also acknowledges support from the Canada Research Chairs program. S. W. S. MacDonald was supported by a Career Investigator Scholar Award from the Michael Smith Foundation for Health Research.
Acknowledgments
We thank the volunteer participants of the VLS for their time and effort and VLS staff members for their assistance in data collection and preparation. Portions of this study were based on an invited presentation at the Penn State Conference on Cognition, Health & Aging: Integrating Cross-Disciplinary Perspectives (October 30–31, 2009, State College, PA). For further information about the VLS, contact R. A. Dixon (rdixon@ualberta.ca) or visit http://www.ualberta.ca/∼vlslab/.
References
- Anstey KJ. Cognitive aging and functional biomarkers: What do we know, and where to from here? In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. pp. 327–339. [Google Scholar]
- Anstey KJ. Biomarkers and memory aging: A lifecourse perspective. In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. New York, NY: Psychology Press; in press. [Google Scholar]
- Anstey KJ, Lord SR, Smith G. Measuring human functional age: A review of empirical findings. Experimental Aging Research. 1996;22:245–266. doi: 10.1080/03610739608254010. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychology and Aging. 1999;14:605–618. doi: 10.1037//0882-7974.14.4.605. [DOI] [PubMed] [Google Scholar]
- Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: Nine-year follow-up data from the Kungsholmen Project. Journal of the American Geriatric Society. 2008;56:111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li S-C, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neuroscience and Biobehavioral Reviews. 2010;34:670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Bäckman L, MacDonald SWS. Death and cognition: Synthesis and outlook. European Psychologist. 2006;11:224–235. [Google Scholar]
- Baltes PB, Willis SL. Toward psychological theories of aging and development. In: Birren JE, Schaie KW, editors. Handbook on psychology of aging. New York, NY: Reinhold-Van Nostrand; 1977. [Google Scholar]
- Battig WF, Montague WE. Category norms of verbal items in 56 categories: A replication and extension of the Connecticut category norms. Journal of Experimental Psychology Monographs. 1969;80:1–46. [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Archives of Neurology. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Birren JE. Principles of research on aging. In: Birren JE, editor. Handbook of aging and the individual: Psychological and biological aspects. Chicago: University of Chicago Press; 1959. pp. 42–3. [Google Scholar]
- Birren JE. Age changes in speed of behavior: Its central nature and physiological correlates. In: Welford AT, Birren JE, editors. Behavior, Aging, and the Nervous System. Springfield: Thomas; 1965. pp. 216–191. [Google Scholar]
- Birren JE. Theories of aging: A personal perspective. In: Bengtson VL, Schaie KW, editors. Handbook of theories of aging. New York, NY: Springer; 1999. [Google Scholar]
- Brady CB, Spiro A, Gaziano JM. Effects of age and hypertension status on cognition: The Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19:770–777. doi: 10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Christensen H, Korten AE, Mackinnon AJ, Jorm AF, Henderson AS, Rodgers B. Are changes in sensory disability, reaction time and grip strength associated with changes in memory and crystallized intelligence? A longitudinal analysis in an elderly community sample. Gerontology. 2000;46:276–292. doi: 10.1159/000022172. [DOI] [PubMed] [Google Scholar]
- Cluett C, Melzer D. Human genetic variations: Beacons on the pathways to successful aging. Mechanisms of Ageing and Development. 2009;130:553–563. doi: 10.1016/j.mad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Cook NR, Evans DA, Scherr PA, Speizer FE, Taylor JO, Hennekens CH. Peak expiratory flow rate and 5-year mortality in an elderly population. American Journal of Epidemiology. 1991;133:784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioural intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;45:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Batty GD, Starr JM. Physical fitness and lifetime cognitive change. Neurology. 2006;67:1195–1200. doi: 10.1212/01.wnl.0000238520.06958.6a. [DOI] [PubMed] [Google Scholar]
- Dixon RA. Enduring theoretical themes in psychological aging: Derivation, functions, perspectives, and opportunities. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. San Diego, CA: Elsevier; 2011. pp. 3–23. 7th ed. [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. [Google Scholar]
- Dixon RA, Small BJ, MacDonald SWS, McArdle JJ. Yes, memory declines with aging—But when, how, and why? In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. New York, NY: Psychology Press; in press. [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Derman D. Manual for kit of factor referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Elias PK, D’Agostino RB, Elias MF, Wolf PA. Blood pressure, hypertension, and age as risk factors for poor cognitive performance. Experimental Aging Research. 1995;21:393–417. doi: 10.1080/03610739508253992. [DOI] [PubMed] [Google Scholar]
- Ershler WB. A gripping reality: Oxidative stress, inflammation, and the pathway to frailty. Journal of Applied Physiology. 2007;103:3–5. doi: 10.1152/japplphysiol.00375.2007. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fozard JL, Metter J, Brant LJ. Next steps in describing aging and disease in longitudinal studies. Journal of Gerontology. 1990;45:116–127. doi: 10.1093/geronj/45.4.p116. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Salvioli S. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J. Inflammaging as a prodrome to Alzheimer's disease. Journal of Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. doi:10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffaux J, Friesinger GC, Lambert W, Shroyer LW, Moritz TE, McCarthy M, Hammermeister KE. Biological age—A concept whose time has come: A preliminary study. Southern Medical Journal. 2005;98:985–993. doi: 10.1097/01.smj.0000182178.22607.47. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, MacDonald SWS. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? Psychology and Aging. 2003;18:755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Schaie KW, Gribbin K. Cardiovascular disease and changes in intellectual functioning from middle to old age. Journal of Gerontology. 1978;33:872–883. doi: 10.1093/geronj/33.6.872. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Berg S, Era P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychology and Aging. 2003;18:285–305. doi: 10.1037/0882-7974.18.2.285. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding Ageing: An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47:341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Howard DV. Category norms: A comparison of the Battig and Montague (1969) norms with the responses of adults between the ages of 20 and 80. Journal of Gerontology. 1980;35:884–890. doi: 10.1093/geronj/35.2.225. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Experimental Gerontology. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- Jensen GL. Inflammation: Role in aging and sarcopenia. Journal of Parenteral and Enteral Nutrition. 2008;32:656–659. doi: 10.1177/0148607108324585. [DOI] [PubMed] [Google Scholar]
- Klemara P, Doubal S. A new approach to the concept of computation of biological age. Mechanisms of Ageing and Development. 2006;127:240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Li S.-C., Schmiedek F. Age is not necessarily aging: Another step towards understanding the ”clocks” that time aging. Gerontology. 2002;48:5–12. doi: 10.1159/000048917. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Ghisletta P. Cognitive and sensory deficits in old age: Gauging the evidence for a common cause. Psychology and Aging. 2009;24:1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Pötter U. The complex nature of unique and shared effects in hierarchical linear regression: Implications for developmental psychology. Psychological Methods. 1998;3:218–230. [Google Scholar]
- MacDonald SWS, Dixon RA, Cohen A.-L., Hazlitt JE. Biological age and 12-year cognitive change in older adults: Findings from the Victoria Longitudinal Study. Gerontology. 2004;50:64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McFall GP, Geall BP, Fischer AL, Dolcos S, Dixon RA. Testing covariates of Type 2 diabetes-cognition associations in older adults: Moderating or mediating effects? Neuropsychology. 2010;24:547–562. doi: 10.1037/a0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. The Journals of Gerontology, Series A: Medical Sciences and Biological Sciences. 2009;64:215–222. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IM. Assessment of physiological age by a combination of serial criteria: Vision, hearing, blood pressure, and muscle force. Journal of Gerontology. 1951;6:120–126. doi: 10.1093/geronj/6.2.120. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Ingram DK. A strategy for identifying biomarkers of aging: Further evaluation of hematology and blood chemistry data from a calorie restriction study in rhesus monkeys. Experimental Gerontology. 1998;33:421–443. doi: 10.1016/s0531-5565(97)00134-4. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. Further evaluation of the basic nature of the human biological aging process based on a factor analysis of age-related physiological variables. The Journals of Gerontology, Series A: Medical Sciences and Biological Sciences. 2003;58:196–204. doi: 10.1093/gerona/58.3.b196. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. The Journals of Gerontology, Series A: Medical Sciences and Biological Sciences. 2007;62:1096–1105. doi: 10.1093/gerona/62.10.1096. [DOI] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Norms of 300 general information questions: Accuracy of recall, latency of recall, and feeling-of-knowing ratings. Journal of Verbal Learning and Verbal Behavior. 1980;19:338–368. [Google Scholar]
- Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. New York, NY: Academic Press; 1979. [Google Scholar]
- Nordberg A. Amyloid imaging in early detection of Alzheimer’s disease. Neurodegenerative Diseases. 2010;7:136–138. doi: 10.1159/000289223. [DOI] [PubMed] [Google Scholar]
- Peto R, Doll R. There is no such thing as aging. British Medical Journal. 1997;315:1030–1032. doi: 10.1136/bmj.315.7115.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raudenbush SW, Bryk A, Cheong YF, Congdon RT. HLM 6: Hierarchical Linear and Nonlinear Modeling (version 6.06) [Computer Software] Chicago, IL: Scientific Software International; 2004. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Richards M, Strachan D, Hardy R, Kuh D, Wadsworth M. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosomatic Medicine. 2005;67:602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- Rosano C, Brach J, Studenski S, Longstreth WT, Jr., Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Anstey KJ, Parslow RA, Wen W, Maller J, Kumarc R, Jorm AF. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dementia and Geriatric Cognitive Disorders. 2006;21:300–308. doi: 10.1159/000091438. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. American Journal of Medicine. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Schaie KW. When does age-related cognitive decline begin? Salthouse again reifies the cross-sectional fallacy. Neurobiology of Aging. 2009;30:528–533. doi: 10.1016/j.neurobiolaging.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Mogle J. Time-based and process-based approaches to analysis of longitudinal data. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. pp. 477–491. [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- Spiro A, III, Brady CB. Integrating health into cognitive aging research and theory: Quo vadis? In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. pp. 260–282. [Google Scholar]
- Spulber G, Niskanen E, MacDonald SWS, Smilivici O, Chen K, Reiman EM, Soininen H. Whole brain atrophy rate predicts progression from MCI to Alzheimer’s disease. Neurobiology of Aging. 2010;31:1601–1605. doi: 10.1016/j.neurobiolaging.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Stephan BCM, Matthews FE, Khaw K.-T., Dufouil C, Brayne C. Beyond mild cognitive impairment: Vascular cognitive impairment, no dementia (VCIND) Alzheimer’s Research and Therapy. 2009;1:4. doi: 10.1186/alzrt4. doi:10.1186/alzrt4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilers PP, MacDonald SWS, Nilsson L.-G., Herlitz A. Accelerated postmenopausal cognitive decline is restricted to women with normal BMI: Longitudinal evidence from the Betula project. Psychoneuroendocrinology. 2010;35:516–524. doi: 10.1016/j.psyneuen.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Thorvaldsson V, Hofer SM, Hassing LB, Johansson B. Cognitive change as conditional on age heterogeneity in onset of mortality-related processes and repeated testing effects. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. New York, NY: Sage Publications; 2008. pp. 284–297. [Google Scholar]
- Wahlin Å., MacDonald SWS, de Frias CM, Nilsson L.-G., Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: Moderating, mediating, or both? Psychology and Aging. 2006;21:318–332. doi: 10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- Wang H, Li H, Hou Z, Pan L, Shen X, Li G. Role of oxidative stress in elevated blood pressure induced by high free fatty acids. Hypertension Research. 2009;32:152–158. doi: 10.1038/hr.2008.35. [DOI] [PubMed] [Google Scholar]
- Weiss ST, Segal MR, Sparrow D, Wager C. Relation of FEV1 and peripheral blood leukocyte count to total mortality: The Normative Aging Study. American Journal of Epidemiology. 1995;142:493–498. doi: 10.1093/oxfordjournals.aje.a117665. [DOI] [PubMed] [Google Scholar]
- Wohlwill JF. The study of behavioral development. New York, NY: Academic Press; 1973. [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Newman AB. The metabolic syndrome, inflammation and risk of cognitive decline. Journal of the American Medical Association. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]