Abstract
Objectives.
Upward trends in IQ, education, and mental work suggest that cognitive function among seniors should be rising strongly across cohorts. There is little sign of such improvement in recent decades, and some analyses find poorer function in the newer cohorts. This essay explores possible explanations of the anomaly.
Methods.
Major long-term trends that might increase cognitive impairment are reviewed, and their implications are considered.
Results.
Physical activity is declining, food is increasingly manufactured, body fat is increasing, diabetes and metabolic syndrome are on the rise, the number of prescription drugs per person is increasing, and the proportion of the population either old or obese is growing.
Discussion.
Technological and economic development may lower the cognitive function needed for survival. They also lower physical activity in daily life. Sedentary work, transportation, and leisure undermine the aerobic and metabolic fitness required for the brain to perform well. Some prescription drugs impair cognitive function, and others do so when taken for many years or in combination with others. The growing fraction of the population that is either old or obese may further lower physical activity norms and requirements and substitute medical intervention for health, accelerating a trend toward cognitive impairment.
Keywords: Bio-accumulators, BMI, Cognitive function, Metabolic syndrome, Pharm-accumulation, Population aging
THE upward 20th century trends in IQ, education, and mental work suggest that age-specific levels of cognitive function among seniors should be rising strongly across cohorts. There is little sign of such a trend in recent decades, and some analyses suggest the opposite, showing stagnant or poorer cognitive function in the newer cohorts (Alwin, McCammon, & Hofer, 2006; Alwin, McCammon, Wray, & Rodgers, 2008; Schaie, 2008). Demographic interpretations of this anomaly often refer to survival effects. Technological and economic development may lower the degree of cognitive function needed for survival. If so, individuals with poor cognitive function may survive longer in newer cohorts, diminishing the average levels of old-age function (Alwin et al., 2006, 2008). However, other forces also may be suppressing or countervailing the gains from higher IQs, longer schooling, and more engaging work. Notably, the same technological trends that may lower the degree of cognitive function needed to survive also lower the physical activity required in daily life. Increasingly, sedentary forms of work, transportation, and leisure undermine the aerobic and metabolic fitness required for the brain to perform well (Brownson & Boehmer, 2004; Brownson, Boehmer, & Luke, 2005; Duany, Plater-Zyberk, & Speck, 2000; Ewing, Schmid, Killingsworth, Zlot, & Raudenbush, 2003; Kumari, Brunner, & Fuhrer, 2000; Lakdawalla & Philipson, 2002; Transportation Research Board, 2005; U.S. Department of Health and Human Services, 1996; Wyatt & Hecker, 2006).
THE BRAIN’S METABOLIC NEEDS
Cognitive function is, ultimately, brain function. An adult human brain weighs about 3 pounds (1.4 kg; Clarke & Sokoloff, 1999). That is about 2% of total body mass. The brain’s rate of oxygen consumption, its metabolic rate, is about 49 ml/min, that is, about 20% of the body’s total basal energy use. The neurons operate using electrochemical potentials produced by ionic gradients, which in turn are created by sodium–potassium pumps. Those pumps need energy to work, in the form of adenosine triphosphate (ATP). The synthesis of ATP requires oxygen and glucose delivered to the brain’s dense vascular system and from there through the cell walls to the interiors of the active neurons. Normal blood flow to the brain is about 750 ml/minute, which is about 15% of the body’s total flow. The brain works even when the person rests. It works harder when the person thinks, perceives, speaks, reads, plans, acts, intends, or remembers. All that work depends on the body’s ability to deliver oxygen and glucose to the cerebrovascular system and on that system’s ability to route blood to the active neurons. The brain’s functioning depends heavily on cardiovascular, respiratory, and metabolic fitness (Cotman, Berchtold, & Christie, 2007; Hillman, Erickson, & Kramer, 2008; Kramer & Erickson, 2007; Kumari et al., 2000; Spiro & Brady, 2008). The progressive substitution of mechanical power for human physical activity in jobs, household chores, transportation, and leisure is undermining the physical fitness needed for cognitive function.
THE DEFAULT AMERICAN LIFESTYLE
Physical activity in work, transportation, and recreation has been trending down for a century or more (Brownson & Boehmer, 2004; Brownson et al., 2005; Lakdawalla & Philipson, 2002; Mirowsky & Ross, 2010; Wyatt & Hecker, 2006). A concurrent decline in the cost of food and a shift from home-made to manufactured and restaurant meals magnifies the impact of declining physical activity on the metabolic and vascular systems (Cook & Daponte, 2008; Cutler, Glaeser, & Shapiro, 2003; Hill, 2009; Kessler, 2009; Pollan, 2006; Rashad, Grossman, & Chou, 2005; Schlosser, 2002). An increasing fraction of successive birth cohorts spend an increasing number of years before old age with increasingly severe problems controlling blood pressure, cholesterol, and blood sugar or delivering oxygen on demand (Bray & Bellanger, 2006; Centers for Disease Control and Prevention, 2010; Duncan, Li, & Zhou, 2004; Ford, Giles, & Mokdad, 2004; Mensah et al., 2004). On a population level, medical interventions have not, and probably cannot, eliminate the physiological consequences of too much food and too little physical activity throughout life. Those physiological consequences eventually degrade cognitive function (e.g., Cournot et al., 2006; Grodstein, Wilson, Chen, & Manson, 2001; Morley, 2004; Sabia, Kivimaki, Shipley, Marmot, & Singh-Manous, 2009).
The default American lifestyle is unhealthy (Mirowsky & Ross, 2010). It is not a default in the old sense of a failure to perform a task or fulfill an obligation. It is a default in the newer sense of an option, assigned automatically by an operating system, that remains in effect unless canceled or overridden by the operator. The automatic routines of 21st century affluent society are set by the economic system and physical infrastructure. They developed from two related centuries-old trends: the progressive increase in per-capita productivity and wealth and the progressive substitution of mechanical energy and work for human labor (Brownson & Boehmer, 2004; Brownson et al., 2005). These have nearly eliminated the threats to health and survival common in 1900, increasing life expectancy, but leaving as residue the diseases of affluence.
Abundance has its risks. The industrial production of food products provides an excess of cheap calories always ready at hand (Chou, Grossman, & Saffer, 2002; Cook & Daponte, 2008; Cutler et al., 2003; Hill, 2009; Rashad et al., 2005). The food is engineered for production, transportation, marketing, and convenience as much or more than for nutrition (Kessler, 2009; Pollan, 2006; Schlosser, 2002). Gas engines, electric motors, and electronic communication make travel, work, play, and commerce increasingly sedentary (Cutler et al., 2003; Lakdawalla & Philipson, 2002). The locales of daily activity are separated by distances and obstacles that are forbidding and dangerous to anyone not in a motorized vehicle (Duany et al., 2000; Ewing et al., 2003;Transportation Research Board, 2005). So, people eat lots of packaged and prepared foods, drive from place to place, and sit while working, playing, and socializing. Their physiological systems, evolved for physical effort in contexts of scarcity, drift further and further from balance. Their muscles become atrophied, their joints inflamed and calcified, their bones brittle and misaligned, their hearts weak, and their arteries clogged and hardened. They take medicines to control blood pressure, cholesterol, and glucose; to regulate bowel movements, urination, stomach acidity, and reflux; and to stifle anxiety, depression, and pain (Critser, 2005; Petersen, 2008). This is the unhealthy lifestyle that remains in effect unless canceled or overridden by the operator.
BODY MASS TRAJECTORIES AND TRENDS
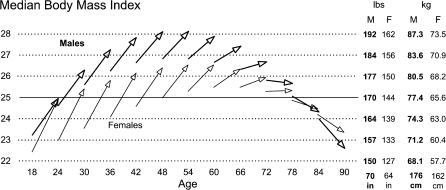
The epidemic of excess body weight provides the most obvious sign of trouble to come. Figure 1 shows age–vectors of the body mass index (BMI in kilograms per square meter) for a representative household sample of English-speaking U.S. adults interviewed in 1995, 1998, and 2001. The arrows illustrate the results of a six-year linear latent growth model with the origins and slopes regressed on polynomial functions of age (Mirowsky & Kim, 2007). Each arrow represents the predicted median BMI score at the beginning of the study and the change over the six-year follow-up. For visual clarity, the figure only shows the arrows for every sixth one-year birth cohort (persons born in the same calendar year). It also shows separate vector sets for men and women.
Figure 1.
Arrows representing the predicted level and change in median body mass index (BMI) by age and sex, for every sixth one-year birth cohort, U.S. 1995–2001. One unit of BMI is 6.8 pounds (3.1 kg) for average height men (5′ 10″: 176 cm), and 5.8 pounds (2.6 kg) for average height women (5′ 4″: 162 cm).
Several things stand out about the body mass vectors. To begin with, median BMI increased considerably within the young and middle-aged cohorts as they aged six years. Expected median BMI increased in every birth cohort up to the ones in their early 70s at the beginning of the study. The largest within-cohort increases were on the order of 12.6 pounds (5.7 kg) over the six-year period. The figure shows that the majority of the men and women between the ages of 30 and 78 were above the BMI cutoff of 25 usually considered overweight. Taking the integral of the age-specific slopes suggests that, by the time the youngest cohorts in the survey reach age 40 (around the year 2020), the majority will be above the BMI cutoff of 30 usually considered obese.
The arrows in Figure 1 show considerable between-cohort trends in age-specific weight. The trends show up in the figure as vertical gaps between adjacent within-sex arrows. The model shows upward BMI trends at all ages for both sexes. The largest gap, implying the fastest age-specific trend, was 2.0 BMI units for the female cohorts aged 24 in 1995 and 2001. In other words, the weight of 24-year-old women was increasing at a rate of nearly 2 pounds (0.9 kg) per calendar year. For men, the fastest upward trend was around age 40 at about 1.3 BMI units or nearly 1.5 pounds (0.68 kg) per calendar year. Although the trend in body mass varies by age and sex, it was upward at all ages for both sexes. Across a broad range of ages, median age-specific body weight was increasing at a rate at or above a pound (0.45 kg) per year.
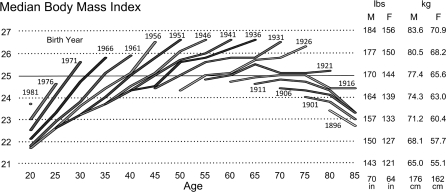
Demographic traditionalists might be skeptical of results from a polynomial regression on age of the parameters in a six-year linear latent-growth model. Figure 2 illustrates data from an age–period–cohort (APC) analysis based on twenty-five years of serial cross-sectional data (1976–2001) from the National Health Interview Survey (NHIS; Cook & Daponte, 2008, table 4). The study used five-year intervals and combined men and women. The last interval for each cohort covers roughly the same period as in the previous graph: 1996–2001. The largest within-cohort five-year increases are on the order of 1.8 BMI units (12.2 pounds [5.6 kg] for an average height men and 10.4 pounds [4.7 kg] for an average height women). As in the latent-growth model of Figure 1, the APC model of Figure 2 shows no indication that median BMIs stop rising before the cohorts reach their 70s.
Figure 2.
Median body mass index by cohort and age, based on twenty-five years of serial cross-sectional data (1976–2001) from the U.S. National Health Interview Survey (Cook & Daponte, 2008, table 4). Birth years and ages are in five-year groupings (±2 years of the values shown).
The NHIS data, too, show a substantial trend between cohorts toward higher age-specific body mass. The increases in age-specific BMI between 1995 and 2001 range from 0.3 to 1.2 U, with a mode around 0.8 U. This again implies that median age-specific body weight was increasing at a rate at or above a pound (0.45 kg) per year across a broad range of ages.
BMI AND THREATS TO COGNITIVE FUNCTION
The body mass trends provide the most visible and readily measured sign of hidden physiological trends that threaten the cognitive function of America’s future seniors. Body mass in itself may or may not degrade mental capacities. Among the elderly, a low or declining BMI generally indicates an unhealthy loss of muscle and bone as well as fat, resulting from inactivity, poor nutrition, and chronic conditions (e.g., Dziura, de Leon, Kasl, & DiPietro, 2004; Kahng, Dunkle, & Jackson, 2004). Among seniors who have long been overweight or obese, remaining so may be a relatively good sign for cognitive function (Kuo et al., 2006). For middle-aged adults, though, higher BMI is associated with lower cognitive function and faster subsequent declines in it (Cournot et al., 2006). Likewise, obesity in early and middle adulthood or rapid increases in BMI during adulthood are associated with lower cognitive function near the end of middle age (around age 61; Sabia et al., 2009).
BMI values are correlated with many measures of physiological dysfunctions that may directly or indirectly undermine cognitive function. There are some reasons to suspect that body fat may contribute directly to cognitive dysfunction. Fat cells secrete inflammatory cytokines (Abraham, Brunner, Eriksson, & Robertson, 2007), and the fat they store is triglyceride, which when circulating increases blood viscosity (Kumari et al., 2000). However, most explanations refer to BMI’s relationship to several factors that may undermine cognitive function. They include high blood pressure, high ratios of low-density to high-density lipoprotein, type 2 diabetes, insulin resistance and poor blood sugar control, oxidative stress, heart disease, stroke, deep vein thrombosis, and signs of the inflammatory processes that contribute to the formation of atherosclerotic plaques and embolisms (Abraham et al., 2007; Bray & Bellanger, 2006; Duncan et al., 2004; Ford, Giles, & Dietz, 2002; Ford et al., 2004; Haffner, 2006; Hillman et al., 2008; Kopelman, 2000; Laaksonen et al., 2002; Mensah et al., 2004; Ogden, Yanovski, Carroll, & Flegal, 2007; Vincent, Innes, & Vincent, 2007). The collection of biomarkers related to BMI is often called the metabolic syndrome. Poor cognitive function is related to type 2 diabetes (Arvanitakis, Wilson, Bienias, Evans, & Bennett, 2004; Grodstein et al., 2001) and the metabolic syndrome (Kumari et al., 2000; Morley, 2004), particularly in the presence of inflammation (Yaffe et al., 2004). In addition, the low physical activity associated with higher BMI has a direct association with cognitive dysfunction (Angevaren, Aufdemkampe, Verhaar, Aleman, & Vanhees, 2008; Kramer & Erickson, 2007), partly because of low aerobic capacity.
Many researchers criticize BMI as an indicator of underlying problems and trouble to come (Folsom et al., 2000; Gallagher et al., 2000; Prentice & Jebb, 2001; Snijder, van Dam, Visser, & Seidell, 2006). They note that BMI measures muscle mass and bone density (signs of good health) as well as body fat; that measures of abdominal or visceral fat may be better indicators of underlying health problems; or that BMI’s relationship to health problems differs by age, sex, and race/ethnicity. Some researchers also debate whether body fat causes problems, such as high blood pressure and insulin resistance, or simply acts as an easy-to-measure correlate of the things that cause the metabolic syndrome (e.g., Campos, Saguy, Ernsberger, Oliver, & Gaesser, 2005). Whatever view one takes on those issues, the BMI trends suggest that an avalanche of health problems is on its way. Higher prevalence of old-age cognitive impairment seems likely, given its relationship to low physical activity, high BMI, the metabolic syndrome, and type 2 diabetes. In addition, just as type 2 diabetes is no longer solely found among old people, age-related dementia may begin to appear in ever younger segments of the population.
BIO-ACCUMULATION AND PHARM-ACCUMULATION
Exercise and eating habits, body mass, blood pressure, blood triglyceride and cholesterol levels, insulin resistance, and arteriosclerosis are all accumulators (Mirowsky & Ross, 2003). By definition, accumulators have two properties: they gather many small effects into one large one and once present they tend to stay present. Many known bio-accumulators influence the body’s ability to meet the brain’s metabolic needs, such as resting metabolic rate, cardiac output, vital capacity, and oxidative damage to mitochondrial DNA, in addition to the ones mentioned above (Mirowsky & Ross, 2003; and see earlier references regarding the metabolic syndrome and exercise).
Because bio-accumulators represent the sum of many small consequences over time, they provide little or no feedback for protective behavioral conditioning. Body fat serves as a good example of bio-accumulators in general (Mirowsky & Ross, 2003). A man who weighs 150 pounds at age 24 will weigh more than 300 pounds at age 54 simply by eating 50 kcal/day more than he burns. An extra 50 kcal/day of food or of activity is not self-evident. It is the number of calories in one Oreo cookie or half a Fig Newton and the number burned in 10 min of gardening, bicycling, brisk walking, or going up and down stairs. The body mass accumulation cannot be seen on a daily, weekly, or even monthly basis. With careful measurement, it can be seen on a quarterly basis. Over a year, the extra 5 pounds may be visible in a mirror or in the fit of clothing, but the link to behavior will remain invisible. This absence of perceptible feedback means that personally controlling the accumulation requires information, planning, and self-direction (Mirowsky & Ross, 2010). Avoiding the weight gain takes relatively little will or effort compared with losing 150 pounds after having gained it. Just skip the cookies and build some physical activity into daily life. Simple as that seems, fewer and fewer Americans manage it (Khan et al., 2009; Nestle & Jacobson, 2000).
Much of contemporary medical science searches for bio-accumulators that pose long-run risk of death, impairment, or suffering. That provides the information individuals can use to manage their own bio-accumulations. However, medical science and practice and the medical industry lack the ability and incentive to change the way people live and work. Many doctors, and perhaps most, tell patients the facts and urge them to choose the healthy way. Even so, few people go to the doctor to be told how to live. They go to get treated, typically after a bio-accumulation has become a problem. The medical industry looks for drugs, devices, or surgeries that promise to manage the accumulators or soften their consequences. The products generally do not eliminate the underlying dysfunction. A stent, for example, reopens an artery or vein without altering the pathophysiology that clogged it. In time, the patient needs another and then another. Stop taking medication for hypertension, low-density cholesterol, or insulin resistance and the accumulator returns to its pre-medication state (or worse).
The strategy of “secondary prevention” inadvertently creates what I call “pharm-accumulation”: the overlaying of medications, protraction of their use, and accrual of their side effects and interactions. Pharm-accumulation creates well-known problems for seniors (Beers & Jones, 2004; Fick et al., 2003). They have had the longest time to accumulate the problems for which the drugs are taken, and the longest time to have taken the drugs. In addition, the extent and variety of their accumulated pathologies makes them both more exposed to complications and interactions and more vulnerable to them.
Pharm-accumulation seriously threatens the cognitive function of seniors. A surprisingly large number of medications produce delirium or dementia, particularly in seniors (Lisi, 2000; Rogers, Wiese, & Rabheru, 2008). Not surprisingly, the list includes antidepressants, benzodiazepines, and opioids. It also includes antihistamines, antispasmodics, antiarrhythmics, diuretics, antiparkinsonians, antibiotics, bladder stabilizers, H2 receptor antagonists (taken for peptic ulcer, gastroesophageal reflux, and dyspepsia), anti-inflammatories, antiemetics, anticonvulsants, alpha 1 blockers (taken for hypertension or benign prostatic hyperplasia), other types of antihypertensives, and bronchial dilation inhalers (taken for asthma attacks or chronic obstructive pulmonary disease; Lisi, 2000; Rogers et al., 2008). Many of the drugs that cause delirium or dementia in seniors are widely prescribed, such as diazepam, the beta-blockers, and cimetidine. Some sell over the counter, such as diphenhydramine and ibuprofen.
Drug effects on cognitive function tend to add up or even multiply. For example, the odds of drug-induced dementia is nine times greater among seniors taking four or five medications than among those taking one or none (Lisi, 2000). A variety of drugs have anticholinergic effects that can impair memory, learning, concentration and lucidity (Jeffrey, 2008; Lisi, 2000; Rogers et al., 2008; Tsao et al., 2008). Anticholinergic effects add up across drugs prescribed for a variety of diseases and conditions. The effects are greater for older persons because as people age, the body produces less acetylcholine and has fewer sites where it binds. Many medical drugs produce enough anticholinergic effect to impair memory by themselves, including cimetidine, prednisolone, digoxin, lanoxin, theophylline, warfarin, furosemide, nifedipine, isosorbide, and ranitidine. Others can nudge the total anticholinergic effect above its critical level, including dyazide, dipyridamole, and codeine. Over-the-counter sleep aids such as diphenhydramine and dimenhydrinate also can impair memory by themselves and add to the total anticholinergic effect.
The problems with anticholinergic effects provide a warning. Similar problems with a variety of drugs that increase serotonin have begun to emerge, producing a syndrome that includes agitation, restlessness, disorientation, confusion, and unresponsiveness (Keck & Arnold, 2000). Drugs that contribute to serotonin toxicity include antidepressant selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, narcotic painkillers, over-the-counter cough, and cold remedies containing dextromethorphan, the anticonvulsant valproate, triptans used to treat and prevent migraines, the antibiotic Zyvox, antinausea drugs, the anti-Parkinson’s drug L-dopa, the weight-loss drug Meridia, and lithium; the dietary supplements tryptophan, St. John’s wort and ginseng; and several drugs of abuse, including ecstasy, lysergic acid diethylamide, amphetamines, and the hallucinogens foxy methoxy and Syrian rue (Brody, 2007). The future may reveal other classes of pharm-accumulation that degrade cognitive function.
Clinical experiments sometimes find that cognitive function in patients with a specific disease can be improved with precise medical control of a bio-accumulator, particularly hypertension and hyperglycemia (e.g., Amenta, Mignini, Rabbia, Tomassoni, & Veglio, 2002; Duron & Hanon, 2008). “Can be” is a significant part of that statement. Antihypertensive drugs can add to the total anticholinergic burden (Lisi, 2000; Rogers et al., 2008), and antihyperglycemic drugs can produce hypoglycemic events that damage the brain (Whitmer, Karter, Yaffe, Quesenberry, & Selby, 2009). Clinical experiments represent idealized medical practice that often bears little resemblance to actual practice. Experimenters generally select patients without multiple complicating diseases, follow precisely defined dosage protocols designed to maximize potential benefit while minimizing possible side effects, take objective measures of outcomes before and after treatment, and know that the symptoms, treatments, and outcomes are being recorded and reviewed. Real practice is often less coherent (Grimshaw & Russell, 1994; Kohn, Corrigan, & Donaldson, 2000; Thomas et al., 2000; Wagner & Groves, 2002; Wennberg, 2002), with a variety of drugs prescribed by different specialists for a variety of disorders at different times, over-the-counter or herbal medication mixed in, and little or no follow-up short of another crisis, which often leads to another specialist who adds new prescriptions but, like the others, bears no responsibility for the full set and does not feel free to remove drugs prescribed by others.
A case study illustrates the cognitive effects of pharm-accumulation. A 65-year-old man was admitted to a geriatric memory clinic with a Mini-Mental State Examination (MMSE) score of 13 of 30 (Iwuagwu, Steiner, & Raji, 2008). (Scores less than 27 indicate cognitive impairment, and those less than 11 indicate severe impairment.) A year earlier the man had undergone a coronary artery bypass graft surgery. During the postoperative hospitalization, he became delirious and agitated. He was discharged with prescriptions for lithium, doxepin (a tricyclic antidepressant), clonazepam (an anxiolytic), and rivastigmine (a cholinergic agent used to treat dementia). At home, he relied entirely on his wife for most activities of daily living and required assistance getting in and out of bed, dressing, bathing, and using the toilet. Eventually, he started using a wheelchair because of fatigue and near falls. The physicians at the geriatric memory loss clinic stopped his doxepin and naproxen (an over-the-counter non-steroidal anti-inflammatory and pain killer); tapered off the lithium, clonazepam, and rivastigmine; and arranged in-home physical and occupational therapy. Within a month he no longer needed a wheelchair, he was no longer disoriented and confused, his MMSE score was 29 of 30, and he was able to perform all activities of daily living without assistance.
Currently, there is little information on the contribution of pharm-accumulation to overall levels of cognitive dysfunction, to aggregate age-related declines in cognitive function, or to its role linking physical inactivity, body fat, and their correlates with cognitive dysfunction. This needs to change. Thirty of forty years from now, it may be common for Americans to arrive at age 65 having taken drugs for attention deficit since childhood, depression since adolescence, anxiety and acid reflux since early adulthood, hypertension and cholesterol since entering middle age, and insulin resistance since sometime well before the end of middle age. It remains uncertain what effects this medical stew will have, but the threat to cognitive function is clear.
THE NEW MAJORITY: OLD OR OBESE
The size of the baby boom generation relative to those before and after tends to reshape culture and the standards of daily life. Soon, a much larger fraction of the total population will be old than has ever been the case before. In the next twenty years, the fraction of the U.S. population aged 65 years or older will increase from the current 13% (one person in eight), to around 20% (one person in five; U.S. Census Bureau, 2010). The percentage of the entire U.S. population age 65 years or older will be greater than in Florida today (17.8%), which is the state with by far the oldest population. When this happens, culture, buildings, products, and services will adapt to the lower average physical abilities.
As the physical demands of daily life decline, so do the levels of physical abilities. Business and technology provide substitutes for the vanishing abilities, which further reduce the physical demands. As the social norms and built environment adapt to ever lower physical abilities, they begin to discourage the activities that create the abilities. Walking and bicycling become impractical and dangerous (Lacayo, 2004; Lakdawalla & Philipson, 2002; Transportation Research Board, 2005). Desk work allows little movement (U.S. Department of Health and Human Services, 1996; Wyatt & Hecker, 2006). Stairways are designed and located for emergency use only (Mansi, Mansi, Shaker, & Banks, 2009). Maintaining physical function requires ever more determination to overcome the default lifestyle (Mirowsky & Ross, 2010). With ever diminishing demands, though, fewer individuals realize the extent of their own impairment.
The aging of the population may accelerate the trend toward lower physical capacities and thus lower cognitive function. As seniors become a larger fraction of the population, assistive technologies, architectures, and services may become the norm. Once common, they will reduce the physical demands on everyone in society, not just the elderly or impaired who absolutely need them. Elevators and electric dumbwaiters in private homes, shopping carts with seats and motors, and the like may accelerate a trend toward lower physical capacities. Population aging may interact with rising body weights among young and middle-aged adults. The overweight majority may readily adopt the physical norms of seniors.
There is no hard scientific data on trends in assistive products, but the media provide signs. The following quote comes from a Wall Street Journal article on the increasing fraction of new homes with elevators and on the innovative pneumatic designs that greatly reduce the costs of retrofitting existing homes.
Evelyn Thompson recently installed an elevator in her two-story single-family home in Port Orange, Fla., after her 84-year-old husband began having problems climbing the stairs after a series of back surgeries. Now she uses it regularly to transport laundry and cleaning supplies and to tote luggage upstairs when visitors arrive.
Besides, she adds: “My grandchildren think it’s the next best thing to Disney World.” (Tan, 2005)
As the quote illustrates, a product introduced to extend the function of impaired individuals readily displaces physical activity in others.
Mobility scooters provide another example. A mobility scooter is a chair on wheels with an electric motor. They are an offshoot of high-tech wheelchairs but designed for persons who can stand and walk, although with difficulty or limited range. Mobility scooters were introduced to extend the activity range of the injured, arthritic, or frail. Their manufacturers recognize that a large potential market exists among persons overweight or out of shape. Figure 3 shows a picture from one manufacturer’s online brochure (Trevelscoot.com, 2010). This particular scooter is made of aircraft materials and comes with a lithium ion battery, so it weighs only 35 pounds. The online brochure emphasizes the ease of transporting it, using it in airports, and carrying it past stairways if there is no wheelchair access. Other manufacturers target shopping malls, with scooters that can navigate store isles and carry purchases, and stack like shopping carts in rental kiosks (Articlebase.com, 2010). As the article notes, “the general population is aging and due to that and other factors, an increasing number of the people frequenting malls have mobility issues on one level or another. This has lead to mall wheelchair rental becoming a growth industry.”
Figure 3.
Photo from an online brochure for a battery-powered mobility scooter.
What all this means for the future cognitive function of Americans remains uncertain, but the signs are not good. Some of the nations’ best epidemiologists, demographers, and health economists debate whether the obesity epidemic will begin to reduce U.S. life expectancy in the next 30 years. The pessimists think it will (Olshansky et al., 2005; Stewart, Cutler, & Rosen, 2009). The optimists think that continuing medical and technological innovation can keep Americans living longer and functioning in the community (Preston, 2005) or that broader medication for hypertension and cholesterol might avert a decline in life expectancy (Cutler, Glaeser, & Rosen, 2007). Even the optimistic scenarios suggest an increasing prevalence of the threats to cognitive function reviewed here. Projected growth in the population of diabetics is one sign of the problems to come. The aging of the baby boom generation, combined with the body mass trends, implies that the number of diabetics will increase by 86% in the next 25 years (Huang, Basu, O’Grady, & Capretta, 2009). Similar increases in metabolic syndrome and decreases in vascular and respiratory fitness seem plausible.
In the meantime, social movements urge making stairs more accessible and appealing (Mansi et al., 2009) and communities more walkable and bikeable (Brownson & Boehmer, 2004; Lacayo, 2004) and promote small changes in lifestyle that balance calories burned with calories eaten (Hill, 2009). The U.S. Centers for Disease Control, the Surgeon General, and public health policy makers encourage those and other community interventions to promote healthier diets and activities (Khan et al., 2009; Nestle & Jacobson, 2000; Office of the Surgeon General, 2001). These efforts should be endorsed. Clearly, public health scientists and officials want to change the default lifestyle to a healthier one. However, as Nestle and Jacobson (p 18) note, “Unintended consequences of our post-industrial society are deeply rooted cultural, social, and economic factors that actively encourage overeating and sedentary behavior and discourage alterations in these patterns … . ” When they wrote that, in the year 2000, the U.S. Public Health Service had, for two decades, set goals to reduce the prevalence of obesity and overweight. Instead, the prevalence increased. Now it has been three decades. In the year 2000, the U.S. Public Health Service set the ten-year goal of reducing to 15% the proportion of adults with a BMI of 30 or more. How close did we get? The most recent data (2007–2008) show 33.8% with BMIs that high (Flegal, Carroll, Ogden, & Curtin, 2010).
Within a few more decades, barring a massive turn around in survival or body weight, the majority of adult Americans will be either old or obese. That new majority will set the default lifestyle. Overriding the default will take ever greater determination and creativity on the part of individuals, groups, and communities seeking a healthier way of life. Until recently, the rising cognitive demands of daily life may have counteracted the effects on cognitive function of too much food and too little physical activity (and the resulting bio- and pharm-accumulations). The aging of the baby boomers together with the rise of body mass may push that balance into the negative zone.
FUNDING
This research was supported by the National Institute on Aging’s grant RO1-AG12393 (aging, status and the sense of control) to J. Mirowsky (Principle Investigator) and grant RO1-AG023380 (education, resource substitution, and health) to Catherine E. Ross (PI). It also was supported by the National Institute of Child Health and Human Development’s grant RO1-HD0536996 (educational differences in U.S. adult mortality) to Robert A. Hummer (PI) and grant R24-HD042849 to Mark Hayward (Director, Population Research Center) for administrative and computing support.
References
- Abraham NG, Brunner EJ, Eriksson JW, Robertson RP. Metabolic syndrome—Psychosocial, neuroendocrine, and classical risk factors in type 2 diabetes. Annals of the New York Academy of Sciences. 2007;1113:256–275. doi: 10.1196/annals.1391.015. [DOI] [PubMed] [Google Scholar]
- Alwin DF, McCammon RJ, Hofer SM. Studying baby boom cohorts within a demographic and developmental context: Conceptual and methodological issues. In: Whitbourne SK, Willis SL, editors. The baby boomers grow up: Contemporary perspectives in midlife. Mahwah, NJ: Lawrence Erlbaum; 2006. pp. 45–71. [Google Scholar]
- Alwin DF, McCammon RJ, Wray LA, Rodgers WL. Population processes and cognitive aging. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging—Interdisciplinary perspectives. Thousand Oaks, CA: Sage Publications; 2008. pp. 69–89. [Google Scholar]
- Amenta F, Mignini F, Rabbia F, Tomassoni D, Veglio F. Protective effect of anti-hypertensive treatment on cognitive function in essential hypertension: Analysis of published clinical data. Journal of the Neurological Sciences. 2002;203–204:147–151. doi: 10.1016/s0022-510x(02)00281-2. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2008;(3) doi: 10.1002/14651858.CD005381.pub3. CD005381. [DOI] [PubMed] [Google Scholar]
- Articlebase.com. Mall wheelchair rental enhances the shopping experience. 2010 Retrieved from http://www.articlesbase.com/business-articles/transport-wheelchair-mall-wheelchair-rental-enhances-the-shopping-experience-1769317.html. [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer’s disease and decline in cognitive function. Archives of Neurology. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Beers HH, Jones TV, editors. Some drugs particularly likely to cause problems in older people. Merck manual of health and aging, Section 2, Chapter 6, Table 1. 2004. Retrieved from http://www.merck.com/pubs/mmanual_ha/tables/tb06_1.html. [Google Scholar]
- Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- Brody J. A mix of medicines that can be lethal. New York Times. 2007, February 27 p. F7. [Google Scholar]
- Brownson RC, Boehmer TK. Patterns and trends in physical activity, occupation, transportation, land use, and sedentary behaviors. 2004 Retrieved from trb.org/downloads/sr282papers/sr282Brownson.pdf. [Google Scholar]
- Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: What are the contributors? Annual Review of Public Health. 2005;26:421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- Campos P, Saguy A, Ernsberger P, Oliver E, Gaesser G. The epidemiology of overweight and obesity: Public health crisis or moral panic? International Journal of Epidemiology. 2005;35:55–60. doi: 10.1093/ije/dyi254. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of abnormal lipid levels among youths—United States, 1999–2006. Morbidity and Mortality Weekly Report. 2010;59:29–33. [PubMed] [Google Scholar]
- Chou S-Y, Grossman M, Saffer H. An economic analysis of adult obesity: Results from the Behavioral Risk Factor Surveillance System. 2002. National Bureau of Economic Research Working Paper, No. 9247. Retrieved from http://www.nber.org/papers/w9247. [DOI] [PubMed] [Google Scholar]
- Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic neurochemistry: Molecular, cellular and medical aspects, Sixth edition (Chapter 31) Lippincott, Williams and Wilkins; 1999. Retrieved from http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=bnchm&part=A2235#A2235. [Google Scholar]
- Cook A, Daponte BO. A demographic analysis of the rise in the prevalence of the US population overweight and/or obese. Population Research and Policy Review. 2008;27:403–426. [Google Scholar]
- Cotman CW, Berchtold NC, Christie L. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;9:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- Critser G. Generation Rx. Boston: Haughton Mifflin; 2005. [Google Scholar]
- Cutler DM, Glaeser EL, Rosen AB. Is the U.S. Population behaving healthier? 2007. National Bureau of Economic Research Working Paper, No. 13013. Retrieved from http://www.nber.org/papers/w13013. [Google Scholar]
- Cutler DM, Glaeser EL, Shapiro JM. Why have Americans become more obese? Journal of Economic Perspectives. 2003;17:93–118. [Google Scholar]
- Duany A, Plater-Zyberk E, Speck J. Suburban nation: The rise of sprawl and the decline of the American Dream. New York: North Point Press; 2000. [Google Scholar]
- Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O. Hypertension, cognitive decline and dementia. Archives of Cardiovascular Disease. 2008;101:181–189. doi: 10.1016/s1875-2136(08)71801-1. [DOI] [PubMed] [Google Scholar]
- Dziura J, de Leon CM, Kasl S, DiPietro L. Can physical activity attenuate aging-related weight loss in older people? The Yale Health Study, 1982–1994. American Journal of Epidemiology. 2004;159:759–767. doi: 10.1093/aje/kwh105. [DOI] [PubMed] [Google Scholar]
- Ewing R, Schmid T, Killingsworth R, Zlot A, Raudenbush S. Relationship between urban sprawl and physical activity, obesity, and morbidity. American Journal of Health Promotion. 2003;18:47–57. doi: 10.4278/0890-1171-18.1.47. [DOI] [PubMed] [Google Scholar]
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Archives of Internal Medicine. 2003;163:2716, 2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong C, …, Princas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: The Iowa women’s health study. Archives of Internal Medicine. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among U.S. adults: Findings from the third National Health and Nutrition Examination Survey. Journal of the American Medical Association. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. American Journal of Clinical Nutrition. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- Grimshaw JM, Russell IT. Achieving health gain through clinical guidelines II: Ensuring guidelines change medical practice. Quality in Health Care. 1994;3:45–52. doi: 10.1136/qshc.3.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, Wilson RS, Chen J, Manson JE. Type 2 Diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- Haffner SM. Risk constellations in patients with the metabolic syndrome: Epidemiology, diagnosis, and treatment patterns. American Journal of Medicine. 2006;119:35–95. doi: 10.1016/j.amjmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. American Journal of Clinical Nutrition. 2009;89:477–484. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews| Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. 2009;32:2225–2229. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwuagwu CU, Steiner V, Raji MA. Medication-related cognitive impairments in the elderly. Clinical Geriatrics. 2008;16 Retrieved from http://www.clinicalgeriatrics.com/articles/Medication-Related-Cognitive-Impairments-Elderly-0. [Google Scholar]
- Jeffrey S. Anticholinergic drugs may increase cognitive decline. Medscape Medical News. 2008 Retrieved from http://www.medscape.com/viewarticle/573208. [Google Scholar]
- Kahng SK, Dunkle RE, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults. Research on Aging. 2004;26:31–61. [Google Scholar]
- Keck PE, Jr., Arnold LM. Serotonin syndrome. Psychiatric Annals. 2000;30:333–343. [Google Scholar]
- Kessler DA. New York: Rodale; 2009. The end of overeating: Taking control of the insatiable American appetite. [Google Scholar]
- Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, Zaro S. Recommended community strategies and measurements to prevent obesity in the United States. Morbidity and Mortality Weekly Report. 2009;58:1–29. RR-7. [PubMed] [Google Scholar]
- Kohn LT, Corrigan JM, Donaldson MS. To err is human: Building a safer health system. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:636–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends in Cognitive Sciences. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kumari M, Brunner E, Fuhrer R. Minireview: Mechanisms by which the metabolic syndrome and diabetes impair memory. Journal of Gerontology: Biological Sciences. 2000;55A:B228–B232. doi: 10.1093/gerona/55.5.b228. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Jones RN, Milberg WP, Tennstedt S, Talbot L, Morris JN, Lipsitz LA. Cognitive function in normal-weight, overweight, and obese older adults: An analysis of the Advanced Cognitive Training for Independent and Vital Elderly Cohort. Journal of the American Geriatric Society. 2006;54:97–103. doi: 10.1111/j.1532-5415.2005.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen LK, Lakka H.-M., Rauramaa R, Salonen JT, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25:1612–1618. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- Lacayo R. The walking cure: Car-centric city sprawl adds to weight gain, so a movement is afoot to put us within walking distance. Time Magazine. 2004, June 7 Retrieved from http://www.time.com/time/subscriber/covers/1101040607/article/the_walking_cure_car_ce01a.html. [Google Scholar]
- Lakdawalla D, Philipson T. The growth of obesity and technological change: A theoretical and empirical examination. 2002 doi: 10.1016/j.ehb.2009.08.001. National Bureau of Economic Research Working Paper, No. 8946. Retrieved from www.nber.org/papers/w8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi DM. Definition of drug-induced cognitive impairment in the elderly. Medscape Pharmacotherapy. 2000;2 Retrieved from http://www.medscape.com/viewarticle/408593. [Google Scholar]
- Mansi IA, Mansi N, Shaker H, Banks D. Stair design in the United States and obesity: The need for change. Southern Medical Journal. 2009;102:610–614. doi: 10.1097/SMJ.0b013e3181a4f67a. [DOI] [PubMed] [Google Scholar]
- Mensah GA, Mokdad AH, Ford E, Narayan KMV, Giles WH, Vinicor F, Deedwania PC. Obesity, metabolic syndrome, and type 2 diabetes: Emerging epidemics and their cardiovascular implications. Cardiology Clinics. 2004;22:485–504. doi: 10.1016/j.ccl.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Kim JY. Graphing age trajectories: Vector graphs, synthetic and virtual cohort projections, and cross-sectional profiles of depression. Sociological Methods and Research. 2007;35:497–541. [Google Scholar]
- Mirowsky J, Ross CE. Education, social status, and health. New York: Aldine de Gruyter/Transaction; 2003. [Google Scholar]
- Mirowsky J, Ross CE. Self-direction toward health: Overriding the default American lifestyle. In: Suls JM, Davidson K, Kaplan R, editors. The handbook of health psychology. New York: Guilford Press; 2010. pp. 235–250. [Google Scholar]
- Morley JE. The metabolic syndrome and aging. Journal of Gerontology: Medical Sciences. 2004;59A:139–142. doi: 10.1093/gerona/59.2.m139. [DOI] [PubMed] [Google Scholar]
- Nestle M, Jacobson MF. Halting the obesity epidemic: A public health policy approach. Public Health Reports. 2000;115:12–24. doi: 10.1093/phr/115.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Surgeon General. The Surgeon General's call to action to prevent and decrease overweight and obesity. 2001. Retrieved from http://www.surgeongeneral.gov/topics/obesity/calltoaction/CalltoAction.pdf. [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, …, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. New England Journal of Medicine. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Petersen M. Our daily meds. New York: Sarah Crichton Books; 2008. [Google Scholar]
- Pollan M. The omnivore’s dilemma: A natural history of four meals. New York: Penguin; 2006. [Google Scholar]
- Prentice AM, Jebb SA. Beyond body mass index. Obesity Reviews. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- Preston SH. Deadweight?—The influence of obesity on longevity. New England Journal of Medicine. 2005;342:135–1137. doi: 10.1056/NEJMe058009. [DOI] [PubMed] [Google Scholar]
- Rashad I, Grossman M, Chou SY. The super size of America: An economic estimation of body mass index and obesity in adults. 2005 National Bureau of Economic Research Working Paper, No. 11584. Retrieved from www.nber.org/papers/w11584. [Google Scholar]
- Rogers J, Wiese BS, Rabheru K. The older brain on drugs: Substances that may cause cognitive impairment. Geriatrics and Aging. 2008;11:284–289. [Google Scholar]
- Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manouz A. Body mass index over the adult life course and cognition in late midlife: The Whitehall II Cohort Study. American Journal of Clinical Nutrition. 2009;89:601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. Historical processes and patterns of cognitive aging. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging—Interdisciplinary perspectives. Thousand Oaks, CA: Sage Publications; 2008. pp. 368–383. [Google Scholar]
- Schlosser E. Fast food nation. New York: Harper Collins; 2002. [Google Scholar]
- Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? International Journal of Epidemiology. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- Spiro A, Brady CB. Integrating health into cognitive aging research. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging—Interdisciplinary perspectives. Thousand Oaks, CA: Sage Publications; 2008. pp. 260–283. [Google Scholar]
- Stewart S, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. New England Journal of Medicine. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL. Passenger elevators invade suburban homes. The Wall Street Journal Online. 2005 Retrieved from http://realestatejournal.com/buildimprove/20050409-tan.html. [Google Scholar]
- Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Zeena T, Williams EJ, Brennan TA. Incidence and types of adverse events and negligent care in Utah and Colorado. Medical Care. 2000;38:261–271. doi: 10.1097/00005650-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Transportation Research Board. Does the built environment influence physical activity? Examining the Evidence: TRB special report 282. 2005 Retrieved from http://onlinepubs.trb.org/onlinepubs/trnews/trnews237activity.pdf. [Google Scholar]
- Trevelscoot.com. If you encounter a patch of rough, uneven terrain or there is no wheelchair ramp present between different levels, the TravelScoot can easily be carried over these kinds of obstructions. 2010 Retrieved from http://www.travelscoot.com/gallery_8.htm. [Google Scholar]
- Tsao J, Shah R, Leurgans S, Wilson R, Janos A, Wei P, …, Heilman K. Impaired cognition in normal individuals using medications with anticholinergic activity occurs following several years. 2008 American Academy of Neurology 60th Annual Meeting. Abstract S51.001. [Google Scholar]
- U.S. Census Bureau. U.S. Population Projections, State Interim Population Projections by Age and Sex: 2004–2030, Table 3. 2010. Retrieved from http://www.census.gov/population/www/projections/projectionsagesex.html. [Google Scholar]
- U.S. Department of Health and Human Services. Physical activity and health: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes, Obesity and Metabolism. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Groves T. Care for chronic diseases: The efficacy of coordinated and patient centered care is established, but now is the time to test its effectiveness. British Medical Journal. 2002;325:913–914. [Google Scholar]
- Wennberg JE. Unwarranted variations in healthcare delivery: Implications for academic medical centers. British Medical Journal. 2002;325:961–964. doi: 10.1136/bmj.325.7370.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. Journal of the American Medical Association. 2009;15:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt IA, Hecker DE. Occupational changes during the 20th century. Monthly Labor Review. 2006, March;3:35–57. [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, …, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. Journal of the American Medical Association. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]