Abstract
Objectives.
To evaluate whether social contacts, support, and social strain/conflict are related to executive function and memory abilities in middle-age and older adults.
Methods.
Longitudinal data on social contacts, support, and strain/conflict were examined in relation to executive function and memory at ages 35–85 years using data from the national Midlife in the U.S. (MIDUS) study. Age-related differences in patterns of association were also examined.
Results.
Regression analyses, controlling for age, sex, race, education, chronic health conditions, and health behaviors, revealed significant positive associations between histories of greater social contacts and support and both executive function and episodic memory, whereas declines in social contacts were negatively associated with both outcomes. Greater average reported frequency of social exchanges characterized by strain or conflict was negatively associated with executive function but not episodic memory. Patterns were generally consistent across different age groups; where differences were seen, associations were stronger in younger age group.
Discussion.
Positive and negative aspects of social relationships are related to cognition throughout adulthood, consistent with the hypothesis that social factors have life-long influences on cognition. Positive and negative aspects of social engagement may thus be important factors to consider in relation to efforts to promote optimal cognitive development and cognitive aging.
Keywords: Executive function, Memory, Social support, Social engagement
WITH increasing longevity has come increased interest, among researchers and the lay public alike, in understanding the factors that influence risks for cognitive decline and impairment at older ages. One such factor appears to be one's level of social engagement —a term we use to refer broadly to both quantitative and qualitative aspects of social interaction, A growing body of evidence suggests that greater social engagement is associated with significantly lower risks for cognitive decline and dementia in older adults (Fratiglioni, Paillard-Borg, & Winblad, 2004). The question of whether such associations are evident across the life course (e.g., earlier in adulthood as well as at older ages) has not been examined.
Among older adults, longitudinal cohort studies have documented significant relationships between greater social integration (i.e., larger social networks) and less risk of cognitive decline (Barnes et al., 2007; Bassuk, Glass, & Berkman, 1999; Holtzman et al., 2004; Zunzunegui, Alvarado, Del Ser, & Otero, 2003). Two studies included longitudinal social engagement data and found greater cognitive declines in older adults who reported consistently low or decreasing levels of social engagement (Bassuk et al., 1999; Holtzman et al., 2004). Studies also have reported a significant relationship between greater social activity and lower subsequent risk of cognitive decline (Beland, Zunzunegui, Alvarado, Otero, & Del Ser, 2005; Bosma et al., 2002; Lovden, Ghisletta, & Lindenberger, 2005; Menec, 2003; Richards, Hardy, & Wadsworth, 2003; Yen, Yang, Shih, & Lung, 2004; Zunzunegui et al., 2003), although two studies did not find such a relationship (Aartsen, Smits, van Tilburg, Knipscheer, & Deeg, 2002; Hultsch, Hertzog, Small, & Dixon, 1999). Two studies of older adults also have linked greater reported support from others to lower risk of cognitive decline (Seeman, Lusignolo, Albert, & Berkman, 2001; Zunzunegui et al., 2003), whereas a third found that greater loneliness was associated with increased risk of decline (Tilvis et al., 2004). Notably, these studies reflect a range of populations and cultures from the United States (Barnes et al., 2007; Bassuk et al., 1999; Scarmeas, Levy, Tang, Manly, & Stern, 2001), Sweden (Fratiglioni, Wang, Ericsson, Maytan, & Winblad, 2000; Wang, Karp, Winblad, & Fratiglioni, 2002), France (Beland et al., 2005; Helmer et al., 1999), Germany (Seidler, Bernhardt, Nienhaus, & Frolich, 2003), Spain (Zunzunegui et al., 2003), and Taiwan (Yen et al., 2004) and show links between social engagement and better performance in multiple major cognitive domains, including recall/memory tasks (Bosma et al., 2002; Ertel, Glymour, & Berkman, 2008; Hultsch et al., 1999; Richards et al., 2003), verbal fluency (Bosma et al., 2002; Hultsch et al., 1999), executive function (Bosma et al., 2002), and processing speed (Hultsch et al., 1999).
The hypothesis that features of our social environments can impact on cognitive functioning, and do so not just in older age but throughout the life course, fits within a broader theoretical (and empirical) literature on the evolution of man as a social animal living in groups, resulting in a highly evolved “social brain”—a brain that both allows us (with varying individual degrees of success) to develop and maintain relationships with others (Burns, 2006; Dunbar, 2003; Grossmann & Johnson, 2007) and that is in turn attentive to and influenced by our interactions with others. Over the past several decades, a growing body of evidence has highlighted the salience of social relationships to multiple aspects of health and well-being (i.e., greater social integration/support being associated cross-sectionally and longitudinally with better physical and mental health and greater longevity (Baumeister & Sommer, 1997; Cohen & Syme, 1985; Seeman, 1996). Mechanisms through which social relationships are thought to affect health (including cognitive “health”) include social influences on brain development (Network, 2005; Smyke et al., 2007) as well as life-long influences on the brain's cognitive–emotional interpretation of (and responses to) stimuli and the resulting patterns of physiological activity in major biological regulatory systems that in turn affect risks for most major health conditions, including cognition (DeVries, Glasper, & Detillion, 2003; Hofer, 1987, 1995; McEwen, 2007; McEwen & Seeman, 1999; Seeman, McEwen, Rowe, & Singer, 2001). Similar to animal “enrichment” models (Diamond, 2001; Fillit et al., 2002), greater social interaction/engagement may also contribute to better cognitive functioning across the life course at least in part as a result of the cognitive demands associated with such interactions, including demands on cognitive processes such as attention, reasoning, language, executive function, and speed of processing.
Data from the Midlife in the U.S. (MIDUS) study offer a unique opportunity to examine relationships between patterns of social engagement over a decade and subsequent levels of adult cognition and to do so for a cohort ranging in age from younger adults in their 30s to older adults in their 70s and 80s. We sought to test the hypothesis that patterns of social engagement would be related to adult cognitive functioning in all age groups. The MIDUS data offer the advantage of including assessments of quantitative aspects of social engagement (i.e., extent of social contacts/relationships) along with assessments of both positive and negative qualitative aspects of those relationships. The availability of information on not only positive/supportive qualities of individuals’ social relationships but also negative features of those same relationships (e.g., levels of social strain and conflict) provides a unique opportunity to assess the hypothesis that unlike supportive interactions (which are hypothesized to generate patterns of cognitive–emotional and physiological activation that will support better cognitive functioning), social strain/conflict will be associated with patterns of cognitive–emotional and physiological activation that will negatively impact on cognition. Existing evidence from both laboratory-based studies of the physiological and cognitive–emotional consequences of social conflict as well as population-based surveys linking reported levels of social conflict to profiles of physiological activity provides support for the hypothesis that social conflict/strain results in patterns of physiological arousal known to be associated with increased health risks (i.e., increases in blood pressure, inflammation, hypothalamic-pituitary-adrenal axis, and sympathetic nervous system arousal; Seeman & McEwen, 1996; Uchino, Cacioppo, & Kiecolt-Glaser, 1996). MIDUS also allows for controls for major potential confounders such as education, health status, and health behaviors that have previously been related to both social engagement (Glass, De Leon, Bassuk, & Berkman, 2006; Okun & Keith, 1998; Ryff, Singer, & Palmersheim, 2004; Seeman, Bruce, & McAvay, 1996; Zunzunegui et al., 2003) and cognition (Colcombe & Kramer, 2003; Potter & Steffens, 2007; Waldstein, 2000; Whitfield et al., 2000).
METHODS
The MIDUS study was initiated in 1994/1995 (MIDUS I). The original sample (N = 7,108) included respondents aged 25–74 years. Though national in scope, the original MIDUS cohort was not strictly speaking nationally representative. Like many such surveys, it underrepresented those at the extremes of the socioeconomic status (SES) continuum but did encompass a majority of the SES diversity within the U.S. adult population. A second wave of data collection was initiated in 2005/2006 (MIDUS II), including a new telephone protocol to assess cognitive function. As detailed in Radler and Ryff (2010), similar to other longitudinal studies, among the 4,963 participants reassessed at Wave 2, higher retention was found for Whites, women, and those with better health and higher education. Analyses also indicated that health status predicted retention more strongly among older individuals, whereas SES better predicted retention among those with lower functional health status (Radler & Ryff, 2010).
Cognitive Function
After a brief hearing check, cognition was assessed using the Brief Test of Adult Cognition by Telephone (BTACT), which includes six accuracy measures of key domains of cognitive aging: episodic memory (immediate and delayed word list recall), working memory (digits backward), executive function and semantic memory (category fluency), reasoning (number series completion), and speed of processing (backward counting). Additionally, the Stop and Go Switch Task (SGST) provides both accuracy and latency measures of cognitive function; in the following analyses, we focus on task-switching latencies (averaged over switch and nonswitch trials). In order to assess response latencies and ensure that participants were performing the task as directed, the latencies data were filtered to exclude individuals who did not meet 75% or better accuracy criteria on each task condition (for more detailed information, see Tun & Lachman, 2006, 2008).
The dependent variables reflect two summary measures of cognitive function based on exploratory and confirmatory factor analysis of the BTACT measures and task-switching latencies from the SGST (Lachman, Agrigoroaei, Murphy, & Tun, 2010). The episodic memory measure comprised scores on immediate and delayed word recall; the remaining BTACT items and SGST latency measures comprise an executive function score (Lachman et al., 2010; Tun & Lachman, 2006, 2008). Each summary score was computed as a mean of the z scores for the respective tests. The two means were also standardized to z scores, with a mean of 0 and a SD of 1. Cognitive outcome data were available for 3,195 participants for executive function and for 3,530 participants for episodic memory; those excluded from the current analyses tended to be older (executive function outcome only), non-White, less educated, and in poorer health (data not shown).
Social Engagement
Three aspects of social engagement were assessed at both MIDUS I and II: frequency of social contacts and extent of reported social support and social strain/conflict. Social contacts were assessed as the sum of the frequency of contact (never or hardly ever, less than once per month, about once per month, 2–3 times per month, once per week, about once a day, and several times per day) across two domains: family and friends. A sum score of social contacts was given only if information for both family and friends was available. Social support was created as the average of responses regarding “how much” spouse, other family, and friends are sources of understanding, caring, appreciation, and provide emotional, reliance, and esteem support based on response categories ranging from not at all, a little, and some to a lot (Cronbach's alpha = .90 for both M1 and M2 scale scores). Using the same range of responses, social strain/conflict was measured as the average of responses regarding how often spouse, other family, and friends are sources of demands, criticism, tension/arguments, or let you down or annoy you (Cronbach's alpha = .89 for both M1 and M2 scale scores). For both social support and social strain/conflict, mean scores were not created if more than two of the three domains queried (spouse, family, and friends) were missing data. Correlations among the three aspects of social engagement ranged from very low to moderate at most, with more contacts related to greater support (r[M1] = .32; r[M2] = .39) and greater conflict (r[M1] = .05; r[M2] = .02), whereas greater support was associated with lesser conflict (r[M1] = −.40; r[M2]= −.37).
To assess cumulative social engagement across these three domains, averages of MIDUS I and II scores for each domain were created. If one time point was missing data, the score from the available time point was imputed as the average score because those with data from both time points provided strong evidence that a majority of adults exhibited substantial stability over time in all domains with T1–T2 correlations of .44 for contacts, .59 for support, and .59 for conflict.
Covariates
Selection of covariates for inclusion in the current analyses was based on prior evidence, suggesting that they could be potential confounders (i.e., that they have been related to both social engagement; Glass et al., 2006; Okun & Keith, 1998; Ryff et al., 2004; Seeman et al., 1996; Zunzunegui et al., 2003; and cognition; Colcombe & Kramer, 2003; Potter & Steffens, 2007; Waldstein, 2000; Whitfield et al., 2000). Sociodemographics included age (in years), gender (coded 1 for female), education, and race (Black/African American, Native American/Aleutian Island/Eskimo, Asian or Pacific Islander, “other,” and multiracial compared with the reference category of “White”). Education was assessed based on a six-category degree-based measure ranging from “less than high school” to “PhD, EDD, MD, and other professional degree.” Health status was assessed based on (a) self-reports of major chronic conditions based on indicator variables for reports of heart disease, stroke, hypertension, and diabetes; (b) reported number of activities of daily living disabilities (0–6); and (c) a measure of depression based on the screening version of the major depression section of the World Health Organization’s Composite International Diagnostic Interview (Kessler, Mickelson, Walters, Zhao, & Hamilton, 2004), reflecting reports of being sad, blue, or depressed or loss of interest in most things all day or most of the day nearly every day for 2 weeks as well as at least four other associated symptoms, including problems with eating, sleeping, energy, concentration, feelings of self-worth, and thoughts of death; this variable is score present (1) or absent (0). Health behaviors included smoking status (coded as former smokers and current smokers, each compared with reference category of nonsmokers) and physical activity (assessed based on an ordinal measure indicating level of highest reported activity). Separate indices of activity were created for MIDUS I and II due to differences in available items with additional items at MIDUS II allowing for identification of light activity where only moderate and vigorous could be identified at MIDUS I (M1 index range = 0–3, no reported activity, only moderate activity, infrequent vigorous activity, and frequent vigorous activity vs. M2 index range = 0–4, no reported activity, only light activity, only light or moderate activity, infrequent vigorous activity, and frequent vigorous activity).
Analyses
We examined relationships for each of the three domains of social engagement to each of the cognitive outcomes using generalized linear mixed models (SAS 9.2 software) in order to account for clustering at the family level because 834 participants in the analytic sample were siblings of other participants. We examined both continuous and categorical (quartile)-based measures for the social engagement domains (examining indicator/flag variables for each quartile relative to the “best”—high contacts or support, low conflict) to evaluate possible nonlinearities in the relationships to cognition. Results are presented here only for the continuous social engagement measures as no evidence for nonlinearities was seen. Results are reported based on standardized coefficients (i.e., unit change in outcome per standard deviation difference on social engagement [or other] index). Covariates were added in steps to observe changes in the relationship between cognition and the social engagement variables, adjusting first for major sociodemographic characteristics, then for health status, and lastly for health behaviors. Hommel adjustment was applied to account for the multiple testing associated with primary analyses of average social influences (two outcomes and three major predictors), and analyses of effects of changes in social factors (four predictors and two outcomes; Hommel, 1988) tests for age interaction were treated as exploratory and were not adjusted for multiple testing.
Drawing on the unique range of adult ages within the MIDUS cohort, we tested for differences in relationships by age, with particular attention to whether patterns of association observed in previous work among older adults would be seen among younger MIDUS adults. For each cognitive outcome, tests for interactions between each social domain and age were examined, including age-by-social domain interaction terms with age centered at the sample mean.
We also assessed the effect of changes in social engagement over time in two ways. For our measure of social contacts, where change scores are integers reflecting changes across categories, we fit models where the change from M1 to M2 was categorized into dummy variables: down three points from M1 and up three points from M1 versus no change (reference group); difference of three points was selected based on both a priori desire to identify those with greatest reported change as well as examination of actual change distribution to ensure adequate sample sizes in both “change” groups. For the measures of social support and social strain/conflict, where continuous mean scores were available, we fit linear regression models using the social engagement score at M1 with a measure of continuous change in social engagement from M1 to M2 (as measured by M2 score − M1 score). Because methodologists have suggested that adjusting for the baseline level of a risk factor, when studying the effect of risk factor change on disease risk, can lead to overestimates of effect (Cain, Kronmal, & Kosinski, 1992), we repeated the multivariable analyses, adjusting for the mean of the baseline and final values—an approach that underestimates effects of change—to assess the stability of our findings relating to the effects of change.
RESULTS
Table 1 provides descriptive information for those included in the present analyses (i.e., with complete data on all variables used in the analyses) along with comparative statistics for the more complete group of all those seen at MIDUS II. There are few, if any, differences between our analysis sample and the overall MIDUS II cohort. At the time of their MIDUS II assessments, the analysis sample ranged in age from 32 to 84 years with an average age of 56.3 years. The sample was predominantly White (92.3%), with 55.3% women. Just over 38% reported attaining a Bachelor's degree or more with another 28.5% reporting some college or an Associates degree; only 5.4% had not attained at least a high school or General Education Development degree. Average reported levels of social contacts, support, and conflict show high levels of stability from MIDUS I to II: social contacts (M1: mean = 11.5, range = 2.0–16.0; M2: mean = 11.6, range = 2.0–16.0), social support (M1: mean = 3.4, range = 1.5–4; M2: mean = 3.5, range = 1.3–4.0), and social strain/conflict (M1: mean = 2.1, range = 1.0–4.0; M2: mean = 2.0, range = 1.0–3.8). Mean differences between MIDUS I and II were small: −0.14 for social contact score (SD = 2.58), 0.04 for social support (SD = 0.41), and −0.08 for social strain/conflict (SD = 0.39).
Table 1.
Descriptive Statistics
| Study samplea | Total MIDUS II sample |
||
| n = 3,525 | n | ||
| A. Demographic measures | |||
| Age, years, mean (SD) | 56.3 (12.2) | 55.4 (12.5) | 4,963 |
| Male (%) | 44.7 | 46.7 | 4,963 |
| White (%) | 92.3 | 89.8 | 4,961 |
| Education (%) | |||
| <High school | 5.4 | 6.2 | 4,956 |
| High school diploma/General Education Development | 26.7 | 26.8 | |
| Some college | 20.7 | 22.1 | |
| AA | 7.8 | 7.9 | |
| BA/BS | 19.8 | 19.3 | |
| Graduate school | 19.6 | 17.7 | |
| B. Social measures | |||
| Social contact | |||
| Social contact, mean M1 (SD), range = 2–16 | 11.5 (2.4) | 11.4 (2.4) | 4,564 |
| Social contact, mean M2 (SD), range = 2–16 | 11.6 (2.5) | 11.6 (2.5) | 3,986 |
| Average social contact, mean M1 + M2 (SD), range = 2–16b | 11.5 (2.1) | 11.5 (2.2) | 4,746 |
| Difference in social contact M2 − M1 = down 3 point (%) | 12.7 | 12.8 | 3,804 |
| Difference in social contact M2 − M1 = same (<3 point difference; %) | 72.2 | 71.6 | 3,804 |
| Difference in Social contact M2 − M1 = Up 3 point (%) | 15.2 | 15.5 | 3,804 |
| Social support | |||
| Social support, mean M1 (SD), range = 1.5–4.0 | 3.4 (0.5) | 3.4 (0.5) | 4,631 |
| Social support, mean M2 (SD), range = 1.3–4.0 | 3.5 (0.5) | 3.6 (0.5) | 4,011 |
| Average social support, mean M1 + M2 (SD), range = 1.3–4.0b | 3.4 (0.4) | 3.4 (0.4) | 4,760 |
| Difference in social support M2 − M1, range = −2.3 to 2.5 | 0.04 (0.4) | 0.04 (0.4) | 3,882 |
| Social strain/conflict | |||
| Social strain/conflict, mean M1 (SD), range = 1.0–4.0 | 2.1 (0.4) | 2.1 (0.4) | 4,629 |
| Social strain/conflict, mean M2 (SD), range = 1.0 to 3.8 | 2.0 (0.4) | 2.0 (0.4) | 4,010 |
| Average social strain/conflict, mean M1 + M2 (SD), range = 1.0–3.7b | 2.0 (0.4) | 2.0 (0.4) | 4,760 |
| Difference in social strain/conflict M2 − M1, range = −2.2 to 1.6 | −0.08 (0.4) | −0.07 (0.4) | 3,879 |
| C. Cognitive measures | |||
| Executive function score, mean (SD), range = −3.0 to 3.4b | 0.1 (0.9) | 0.1 (0.9) | 3,251 |
| Episodic memory score, mean (SD), range = −3.1 to 3.8b | 0.03 (1.0) | 0.02 (1.0) | 3,616 |
| D. Health status | |||
| Heart disease (%) | 20.5 | 20.0 | 4,962 |
| Stroke (%) | 2.9 | 3.1 | 4,963 |
| Hypertension (%) | 39.4 | 38.9 | 4,961 |
| Diabetes (%) | 10.0 | 10.2 | 4,041 |
| Depression (M2) % | 6.1 | 6.4 | 4,963 |
| ADL M2, mean (SD), range = 0–6] | 0.5 (1.1) | 0.5 (1.2) | 4,018 |
| E. Health behaviors | |||
| Smoking (current vs. never;%) | 14.0 | 15.5 | 4,963 |
| Smoking (former vs. never;%) | 34.3 | 33.3 | 4,963 |
| Physical activity M1, mean (SD), range = 0–3 | 2.2 (1.0) | 2.2 (1.0) | 4,657 |
| Physical activity M2, mean (SD), range = 0–4 | 3.0 (1.3) | 3.0 (1.3) | 3,949 |
Note: ADL = activity of daily living.
Including all participants with nonmissing values for all variables of interest and either available score for executive function or episodic memory.
Reduced sample size for social contact (n = 3,524), social support (n = 3,523), social strain/conflict (n = 3,524), executive function (n = 3,180), and episodic memory (n = 3,514) scores.
Both executive function and episodic memory exhibited the expected age differences in cognitive performance, with poorer performance seen in the older age groups (see Figure 1)
Figure 1.
Age-related differences in cognition.
Regression models for executive function revealed the expected patterns of association for our measures of social engagement. Higher average reported social contacts and support were associated with better executive functioning, whereas higher average reported social strain/conflict was associated with poorer executive function. As shown in Table 2, these relationships remained significant for with sequential controls for age (Model 1) and other sociodemographic characteristics, including education (Model 2); contacts and conflict/strain also remain significant with further controls for major health conditions and health behaviors (Model 3), though estimates of the change in R2 associated with the social factors alone are small (ranging from 0.4 [social conflict] to 1.4 [social contacts]). Parallel analyses for episodic memory revealed significant positive associations for social contacts and support in all models (see Table 3), though again estimated changes in R2 are small (social contacts = 2.8; social support = 0.2). Reported levels of social strain/conflict were not significantly related to episodic memory in any models. When all three domains of social engagement were examined simultaneously, more social contacts and lower social strain/conflict each remained significantly associated with better executive function (B = 0.039, p = .01 for social contacts; B = −0.046, p = .003 for social strain/conflict). Both social contacts and support remained independently and positively associated with episodic memory (B = 0.035, p = .04 for social contacts; B = 0.037, p = .05 for social support)
Table 2.
Regression Models (standardized coefficients [SE]) for Executive Function by Social Engagement
| Model 1a | Model 2b | Model 3c | |
| n = 3,178 | n = 3,178 | n = 3,178 | |
| Average social contacts | |||
| Social contacts | 0.0340 (0.015)* | 0.0383 (0.038)** | 0.0356 (0.036)d* |
| Age (centered at 56.6) | −0.3806 (0.015)*** | −0.3458 (−0.346)*** | −0.3022 (−0.302)*** |
| Gender (male vs. female) | 0.0654 (0.065)*** | 0.0560 (0.056)*** | |
| White (vs. Black) | 0.0948 (0.095)*** | 0.0894 (0.089)*** | |
| Education | 0.3234 (0.323)*** | 0.3077 (0.308)*** | |
| Heart disease | −0.0139 (0.013) | ||
| Stroke | −0.0483 (0.012)*** | ||
| Hypertension | −0.0335 (0.014)* | ||
| Diabetes | −0.0366 (0.014)* | ||
| Smoking (current vs. never) | 0.0208 (0.015) | ||
| Smoking (former vs. never) | 0.0265 (0.015)∼ | ||
| Physical activity M1 | −0.0001 (0.015) | ||
| Physical activity M2 | 0.0395 (0.015)** | ||
| ADL M2 | −0.0612 (0.014)*** | ||
| Depression | −0.0127 (0.013) | ||
| Average social support | |||
| Social support | 0.0542 (0.054)*** | 0.0391 (0.039)** | 0.0274 (0.027)e |
| Age (centered at 56.6) | −0.3847 (0.016)*** | −0.3483 (0.014)*** | −0.3034 (0.014)*** |
| Gender (male vs. female) | 0.0636 (0.014)*** | 0.0530 (0.016)*** | |
| White (vs. Black) | 0.0933 (0.014)*** | 0.0881 (0.015)*** | |
| Education | 0.3219 (0.013)*** | 0.3073 (0.013)*** | |
| Heart disease | −0.0129 (0.014) | ||
| Stroke | −0.0480 (0.013)*** | ||
| Hypertension | −0.0337 (0.012)** | ||
| Diabetes | −0.0348 (0.014)** | ||
| Smoking (current vs. never) | 0.0036 (0.014) | ||
| Smoking (former vs. never) | 0.0258 (0.015)∼ | ||
| Physical activity M1 | 0.0001 (0.015) | ||
| Physical activity M2 | 0.0418 (0.015)** | ||
| ADL M2 | −0.0593 (0.015)*** | ||
| Depression | −0.0109 (0.014) | ||
| Average social strain/conflict | |||
| Social strain/conflict | −0.0552 (0.0153)*** | −0.0485 (0.0137)*** | −0.0426 (0.0139)f** |
| Age (centered at 56.6) | −0.3919 (0.0156)*** | −0.3549 (0.0144)*** | −0.3090 (0.0161)*** |
| Gender (male vs. female) | 0.0558 (0.0139)*** | 0.0461 (0.0148)** | |
| White (vs. Black) | 0.0935 (0.0127)*** | 0.0878 (0.0127)*** | |
| Education | 0.3251 (0.0142)*** | 0.3096 (0.0145)*** | |
| Heart disease | −0.0127 (0.0135) | ||
| Stroke | −0.0481 (0.0120)*** | ||
| Hypertension | −0.0328 (0.0142)* | ||
| Diabetes | −0.0340 (0.0144)* | ||
| Smoking (current vs. never) | 0.0046 (0.0150) | ||
| Smoking (former vs. never) | 0.0278 (0.0146)∼ | ||
| Physical activity M1 | 0.0019 (0.0150) | ||
| Physical activity M2 | 0.0462 (0.0146)** | ||
| ADL M2 | −0.0579 (0.0142)*** | ||
| Depression | −0.0094 (0.0133) | ||
Notes. ADL = activities of daily living.
Model 1 adjusted for social measure and age.
Model 2 adjusted for social measure, age, gender, education, and race.
Model 3 adjusted for social measure, age, gender, education, race, chronic conditions, smoking, and physical activity.
Hommel adjusted p value = 0.027.
Hommel adjusted p value = 0.12.
Hommel adjusted p value = 0.004.
*p = .05–0.01. **p = 0.01–0.001. ***p < 0.001. ∼p = 0.05–0.1.
Table 3.
Regression Models (standardized coefficients [SE]) for Episodic Memory by Social Engagement
| Model 1a | Model 2b | Model 3c | |
| n = 3,512 | n = 3,512 | n = 3,512 | |
| Average social contacts | |||
| Social contacts | 0.0941 (0.0162)*** | 0.0535 (0.0160)*** | 0.0493 (0.0160)d** |
| Age (centered at 56.6) | −0.3282 (0.0164)*** | −0.2975 (0.0161)*** | −0.2615 (0.0184)*** |
| Gender (male vs. female) | −0.2255 (0.0156)*** | −0.2396 (0.0165)*** | |
| White (vs. Black) | 0.0694 (0.0152)*** | 0.0626 (0.0153)*** | |
| Education | 0.1770 (0.0149)*** | 0.1648 (0.0156)*** | |
| Heart disease | −0.0027 (0.0162) | ||
| Stroke | −0.0293 (0.0154)∼ | ||
| Hypertension | −0.0004 (0.0164) | ||
| Diabetes | −0.0235 (0.0153) | ||
| Smoking (current vs. never) | 0.0218 (0.0162) | ||
| Smoking (former vs. never) | 0.0182 (0.0164) | ||
| Physical activity M1 | 0.0100 (0.0168) | ||
| Physical activity M2 | 0.0439 (0.0164)** | ||
| ADL M2 | −0.0671 (0.0157)*** | ||
| Depression | −0.0035 (0.0152) | ||
| Average social support | |||
| Social support | 0.1016 (0.0166)*** | 0.0615 (0.0160)*** | 0.0513 (0.0161)e** |
| Age (centered at 56.6) | −0.3351 (0.0165)*** | −0.3020 (0.0162)*** | −0.2655 (0.0186)*** |
| Gender (male vs. female) | −0.2273 (0.0154)*** | −0.2416 (0.0162)*** | |
| White (vs. Black) | 0.0659 (0.0152)*** | 0.0598 (0.0152)*** | |
| Education | 0.1742 (0.0149)*** | 0.1634 (0.0156)*** | |
| Heart disease | −0.0009 (0.0162) | ||
| Stroke | −0.0284 (0.0154)∼ | ||
| Hypertension | −0.0001 (0.0164) | ||
| Diabetes | −0.0208 (0.0154) | ||
| Smoking (current vs. never) | 0.0227 (0.0162) | ||
| Smoking (former vs. never) | 0.0170 (0.0164) | ||
| Physical activity M1 | 0.0089 (0.0168) | ||
| Physical activity M2 | 0.0473 (0.0164)** | ||
| ADL M2 | −0.0632 (0.0158)*** | ||
| Depression | −0.0004 (0.0153) | ||
| Average social strain/conflict | |||
| Social strain/conflict | −0.0080 (0.0165) | −0.0198 (0.0157) | −0.0166 (0.0158)f |
| Age (centered at 56.6) | −0.3268 (0.0169)*** | −0.2996 (0.0165)*** | −0.2613 (0.0187)*** |
| Gender (male vs. female) | −0.2358 (0.0154)*** | −0.2504 (0.0162)*** | |
| White (vs. Black) | 0.0689 (0.0152)*** | 0.0619 (0.0153)*** | |
| Education | 0.1791 (0.0149)*** | 0.1663 (0.0156)*** | |
| Heart disease | −0.0022 (0.0162) | ||
| Stroke | −0.0284 (0.0155)∼ | ||
| Hypertension | −0.0005 (0.0164) | ||
| Diabetes | −0.0208 (0.0153) | ||
| Smoking (current vs. never) | 0.0227 (0.0162) | ||
| Smoking (former vs. never) | 0.0178 (0.0164) | ||
| Physical activity M1 | 0.0144 (0.0167) | ||
| Physical activity M2 | 0.0481 (0.0165)** | ||
| ADL M2 | −0.0664 (0.0158)*** | ||
| Depression | −0.0018 (0.0152) | ||
Notes. ADL = activities of daily living.
Model 1 adjusted for social measure and age.
Model 2 adjusted for social measure, age, gender, education, race.
Model 3 adjusted for social measure, age, gender, education, race, chronic conditions, smoking, physical activity, ADL, and depression.
Hommel adjusted p value = 0.004.
Hommel adjusted p value = 0.003.
Hommel adjusted p value = 0.60.
*p = 0.05–0.01. **p = 0.01–0.001. ***p < 0.001. ∼p = 0.05–0.1.
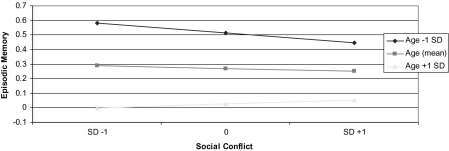
Tests for age interactions revealed significant interactions with respect to executive function for social support (B = −0.271, p = .03) and social strain/conflict (B = 0.212, p = 0.002) and for social strain/conflict (B = 0.185, p = 0.02) with respect to episodic memory. Figure 2 (executive function) and Figure 3 (episodic memory) illustrate the patterning of these age interactions, plotting the associations at age 55 years (mean age) and those at 1 SD (approximately 12 years) above and below the mean. As shown, these age interactions reflected stronger patterns of associations among younger participants.
Figure 2.
Age-related differences in associations between social support and social strain/conflict and executive function.
Figure 3.
Age-related differences in associations between social strain/conflict and episodic memory.
A final set of analyses examined whether and how changes in reported social engagement were related to the two cognitive outcomes. As shown in Table 4, once adjusted for multiple comparisons, only declines in social contacts were significantly related to both executive function and episodic memory outcomes, independent of all covariates. Those reporting a decrease in frequency of contacts from MIDUS 1 (baseline) to MIDUS II exhibited poorer executive function and poorer memory performance. Analyses controlling for average social engagement rather than baseline yielded weaker though basically similar results (data not shown).
Table 4.
Regression Models (standardized coefficients [SE]) Examining Associations Between Changes in Social Engagement From MIDUS 1 (M1) to MIDUS II (M2) and Cognitive Outcomesa
| Executive function | Episodic memory | |
| Social contacts | ||
| M1 − baseline contacts (continuous) | 0.0285 (0.0162) | 0.0472 (0.0175)** |
| Difference M2 − M1 = up 3 point | −0.0101 (0.0152) | 0.0089 (0.0164) |
| Difference M2 − M1 = down 3 point | −0.0423 (0.0139)b | −0.0512 (0.0161)c |
| Social support | ||
| M1 support (continuous) | 0.0302 (0.0161) | 0.0582 (0.0181)** |
| Difference M2 − M1 | 0.0315 (0.0153) | 0.0374 (0.0178) |
| Social strain/conflict | ||
| M1 conflict (continuous) | −0.0457 (0.0153)** | −0.0196 (0.0177) |
| Difference M2 − M1 | −0.0169 (0.0157) | −0.0149 (0.0170) |
Notes. aModel adjusted for age, gender, education, race, chronic conditions, smoking, physical activity, activities of daily living, and depression.
Hommel adjusted p value = 0.015.
Hommel adjusted p value = 0.01.
*p = 0.05–0.01. **p = 0.01–0.001. ***p < 0.001.
DISCUSSION
Analyses of MIDUS social engagement and cognition data revealed significant positive associations between average reported contacts and support and measures of both executive function and episodic memory, independent of age, education, gender, and race as well as major health conditions and health behaviors. Extending prior work, examination of possible links between reported frequency of negative social exchanges such as excessive criticism and demands or perceptions that others “let you down” and cognition also revealed that greater frequency of such social strain/conflict was significantly and negatively associated with executive function but not episodic memory. Longitudinal social engagement data also revealed that declines in social contacts over the decade between MIDUS I and II were associated with poorer executive function and episodic memory performance.
The wide age range in the MIDUS cohort (35 years and older at the time of the cognitive assessments) also allowed us to test the hypothesis that associations between social engagement and cognition previously documented for older adults would be evident at younger ages as well. Consistent with this hypothesis, we found significant positive associations between social contacts and support and both cognitive outcomes for those younger than the age of 65 years. Social strain/conflict was also significantly associated with poorer executive function and marginally associated with poorer episodic memory in this younger age group. For those older than the age of 65 years, we also found significant positively associations between social contacts and both executive function and episodic memory. For episodic memory, effect sizes for social support and conflict were similar to those seen among the younger age group but were not statistically significant due to larger standard errors. Formal tests for age interactions revealed that associations were significantly stronger among the younger adults for social support and conflict with respect to executive function and stronger for social strain/conflict with respect to episodic memory.
The overall pattern of these findings is consistent with a life course model for social engagement influences on cognition wherein social influences are evident not only at older ages but across the life course. MIDUS data show that among adults aged 32 years and older, those reporting higher average social contacts and social support (based on assessments approximately a decade apart) performed significantly better on tests of executive function and episodic memory, whereas higher average reported frequency of social strain/conflict was associated with significantly poorer performance. Changes over time were also associated with differences in cognitive performance with declines in contact (i.e., reductions in exposure to “positive factors”) associated with poorer performance. Though not statistically significant when accounting for multiple comparisons, there was suggestive evidence that increases in support (i.e., increased exposure to a “positive factor”) were associated with better performance.
Though the MIDUS data provide support for our original hypothesis that social engagement would be associated with better cognition at younger as well as older ages, the fact that a number of the associations were stronger among the younger member of the MIDUS cohort was unexpected. Possible reasons for the stronger associations among those younger than the age of 65 years may include the greater attrition among the older adults from baseline to MIDUS II (when the cognition assessments were made) such that those with the poorest social engagement histories were less likely to remain in the cohort by the MIDUS II follow-up and are thus not included in the current analyses. Had they remained, one might hypothesize that they would have exhibited the poorest cognition and might have contributed to stronger associations between poor social engagement and poor cognition among the older adults. Our pattern of weaker associations at older ages parallels findings for multiple other risk factors (e.g., Berry, Ngo, Samelson, & Kiel, 2010; Tate, Manfreda, & Cuddy, 1998) and may, like these others, also reflect in part the greater competing risks at older ages wherein even those with good profiles of social engagement are, for example, more likely than their younger counterparts to have other risk factors that will increase their risks for poorer cognition. Thus, though we controlled for a number of known risk factors for poorer cognition (e.g., health conditions, health behaviors), it is likely that among the older adults, even those with better profiles of social engagement have other age-related physiological or other changes that increase their risks for poorer cognition. Our inability to control more completely for such factors may have reduced our ability to demonstrate the benefits of greater social engagement in the older age groups. Importantly, the current analyses are, to our knowledge, the first to examine patterns of association between social engagement and cognition for those younger than the age of 65 years, so the findings reported here, though intriguing, require further investigation to determine their ultimate import.
Interpretation of the overall findings must also include consideration of several limitations of the MIDUS data. Foremost is the lack of longitudinal cognition data. With only a single assessment of cognition at MIDUS II, analyses presented here provide evidence for “associations” between histories of social engagement and executive function and episodic memory as of MIDUS II. Further investigation of the temporal sequencing of changes in social engagement and changes in cognition must await future longitudinal cognitive data. Such data will allow (as the current data do not) for evaluation of the relative strengths of what are, in all likelihood, reciprocal relationships between social engagement and cognition with cognition and changes in cognition impacting on patterns of social engagement even as social engagement itself influences cognition and trajectories of cognitive aging. The current analyses only allow us to determine the presence of associations between these two important domains without being able to assess the relative strength of the directionality of their respective influences. For the majority of the current MIDUS cohort, however, available cognitive data show performance levels that are sufficiently high that reverse causation—with cognitive function being sufficiently poor as to negatively impact on social engagement—would seem less plausible as the primary explanation for the observed association. Only among the oldest of the MIDUS participants, where cognitive performance is poorest, might one speculate that patterns of social engagement were potentially more affected by cognitive functioning. Whether and how any reciprocal effects come to be manifest over the life course, however, awaits further longitudinal data for both cognition and social engagement. Also, the social engagement data are based on self-reports and thus could be subject to differential response bias (e.g., if those with better cognition are more likely to bias their self-reports toward more positive reporting of social engagement); if so, this could result in an upward bias in estimates of the association between social engagement and cognition. Finally, as noted previously, the MIDUS is a national sample with substantial socioeconomic diversity but is not strictly nationally representative, and the cognitive data were collected only for those remaining in the longitudinal cohort, which, like all longitudinal studies, is positively selected for those who remained healthier and more interested in continuing their participation in the study. Thus, the generalizability of our findings remains to be determined.
These analyses, however, also have a number of significant strengths. First, the age range of the cohort allowed for the first formal tests of the hypothesis that relationships between social engagement and cognition are not restricted to older ages but rather are evident much earlier in life. MIDUS data allowed for testing of these associations among adults as young as 35 years at the time of the cognitive assessments. Second, the MIDUS data provide particularly rich information on social engagement, including assessments of more quantitative aspects such as social contacts as well as more qualitative features, including frequency of both positive social exchanges (social support) and negative exchanges (social strain/conflict). Also, the social engagement data are available at two time points approximately a decade apart, allowing for evaluation of more cumulative aspects of social engagement (i.e., social histories) as they relate to cognition. Third, although the cognitive data are only available at a single time point, the range of measures provides assessments for key cognitive domains known to be important in cognitive aging across the life span. Furthermore, the innovative telephone-based SGST provided enhanced data on speed of processing and executive function through assessments of latencies in task switching.
In summary, MIDUS data provide evidence linking three aspects of social engagement—social contacts, support, and conflict—to both executive function and episodic memory in adults ranging in age from 35 to 84 years. Those reporting greater average contacts and support performed better on both types of cognitive tasks, whereas those reporting greater average frequency of negative social exchanges performed did more poorly. The fact that these associations were evident in younger adults and that changes in these features of social engagement are related to performance points to the potential value of considering more positive aspects of social engagement such as social contacts and support in the context of any future efforts to improve or bolster levels of cognition across the life course. These data also suggest the importance of taking account of risks stemming from higher levels of social strain/conflict. Clearly, further research is needed to confirm these initial findings regarding the associations at younger ages and the impact of negative social exchanges. The current findings, however, will hopefully encourage attention to these questions as we seek to understand how patterns of social engagement impact on cognitive function across the life course and how we might best leverage such knowledge to enhance cognitive development earlier in life and reduce risks for cognitive declines in later life.
FUNDING
This work was supported by (grant numbers AG-032271 and AG-020166) from the Behavioral and Social Research Program in the National Institute on Aging and (grant number M01-RR00865) from the National Institutes of Health funding for the General Clinical Research Centers Program.
References
- Aartsen MJ, Smits CH, van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: Cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. Journal of Gerontology Series B Psychological Sciences. 2002;57:P153–P162. doi: 10.1093/geronb/57.2.p153. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Cauley JA, Lui LY, Fink HA, McCulloch C, Stone KL, Yaffe K. Women who maintain optimal cognitive function into old age. Journal of the American Geriatric Society. 2007;55:259–264. doi: 10.1111/j.1532-5415.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Sommer KL. What do men want? Gender differences and two spheres of belongingness: Comment on Cross and Madson (1997) Psychological Bulletin. 1997;122:38–44. doi: 10.1037/0033-2909.122.1.38. Discussion 51–35. [DOI] [PubMed] [Google Scholar]
- Beland F, Zunzunegui MV, Alvarado B, Otero A, Del Ser T. Trajectories of cognitive decline and social relations. Journal of Gerontology Series B Psychological Sciences. 2005;60:P320–P330. doi: 10.1093/geronb/60.6.p320. [DOI] [PubMed] [Google Scholar]
- Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: An important consideration in studies of older adults. Journal of the American Geriatric Society. 2010;58:783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MP, Ponds RW, Jelicic M, Houx P, Metsemakers J, Jolles J. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: A reciprocal association? Zeitschrift für Gerontologie und Geriatrie. 2002;35:575–581. doi: 10.1007/s00391-002-0080-y. [DOI] [PubMed] [Google Scholar]
- Burns JK. Psychosis: A costly by-product of social brain evolution in Homo sapiens. Progress in Neuropsychopharmacology & Biological Psychiatry. 2006;30:797–814. doi: 10.1016/j.pnpbp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cain KC, Kronmal RA, Kosinski AS. Analysing the relationship between change in a risk factor and risk of disease. Statistics in Medicine. 1992;11:783–797. doi: 10.1002/sim.4780110609. [DOI] [PubMed] [Google Scholar]
- Cohen S, Syme SL. Issues in the study and application of social support. In: Cohen S, Syme SL, editors. Social support and health. New York, NY: Academic Press; 1985. pp. 3–22. [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiology and Behavior. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Diamond MC. Response of the brain to enrichment. Anais da Academia Brasileira de Ciências. 2001;73:211–220. doi: 10.1590/s0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- Dunbar R. Psychology. Evolution of the social brain. Science. 2003;302:1160–1161. doi: 10.1126/science.1092116. [DOI] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. American Journal of Public Health. 2008;98:1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit HM, Butler RN, O’Connell AW, Albert MS, Birren JE, Cotman CW, Tully T. Achieving and maintaining cognitive vitality with aging. Mayo Clinic Proceedings. 2002;77:681–696. doi: 10.4065/77.7.681. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- Glass TA, De Leon CF, Bassuk SS, Berkman LF. Social engagement and depressive symptoms in late life: Longitudinal findings. Journal of Aging and Health. 2006;18:604–628. doi: 10.1177/0898264306291017. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25:909–919. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Helmer C, Damon D, Letenneur L, Fabrigoule C, Barberger-Gateau P, Lafont S, Dartigues J. Marital status and risk of Alzheimer's disease: A French population-based cohort study. Neurology. 1999;53:1953–1958. doi: 10.1212/wnl.53.9.1953. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early social relationships: A psychobiologist's view. Child Development. 1987;58:633–647. [PubMed] [Google Scholar]
- Hofer MA. Hidden regulators: Implications for a new understanding of attachment, separation, and loss. In: Goldberg S, Muir R, Kerr J, editors. Attachment theory: Social, developmental, and clinical perspectives. Hillsdale, NJ: Analytic Press; 1995. pp. 203–230. [Google Scholar]
- Holtzman RE, Rebok GW, Saczynski JS, Kouzis AC, Wilcox Doyle K, Eaton WW. Social network characteristics and cognition in middle-aged and older adults. Journal of Gerontology Series B Psychological Sciences. 2004;59:P278–P284. doi: 10.1093/geronb/59.6.p278. [DOI] [PubMed] [Google Scholar]
- Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Walters EE, Zhao S, Hamilton L. Age and depression in the MIDUS survey. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we? A national study of well-being at midlife. Chicago, IL: University of Chicago Press; 2004. pp. 227–251. [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. American Journal of Geriatric Psychiatry. 2010;18:4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Menec VH. The relation between everyday activities and successful aging: A 6-year longitudinal study. Journal of Gerontology Series B Social Sciences. 2003;58:S74–S82. doi: 10.1093/geronb/58.2.s74. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Predicting individual differences in attention, memory, and planning in first graders from experiences at home, child care, and school. Developmental Psychology. 2005;41:99–114. doi: 10.1037/0012-1649.41.1.99. [DOI] [PubMed] [Google Scholar]
- Okun MA, Keith VM. Effects of positive and negative social exchanges with various sources on depressive symptoms in younger and older adults. Journal of Gerontology Series B Psychological Sciences. 1998;53:P4–P20. doi: 10.1093/geronb/53b.1.p4. [DOI] [PubMed] [Google Scholar]
- Potter GG, Steffens DC. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist. 2007;13:105–117. doi: 10.1097/01.nrl.0000252947.15389.a9. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS National Study of Health and Well-Being. Journal of Aging Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Social Science and Medicine. 2003;56:785–792. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer BH, Palmersheim KA. Social inequalities in health and well-being: The role of relational and religious protective factors. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we?: A National Study of Well-Being at Midlife. Chicago, IL: University of Chicago Press; 2004. pp. 90–123. [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE. Social ties and health: The benefits of social integration. Annals of Epidemiology. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Bruce ML, McAvay GJ. Social network characteristics and onset of ADL disability: MacArthur studies of successful aging. Journal of Gerontology Series B Social Sciences. 1996;51:S191–S200. doi: 10.1093/geronb/51b.4.s191. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology. 2001;20:243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine regulation. Psychosomatic Medicine. 1996;58:459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler A, Bernhardt T, Nienhaus A, Frolich L. Association between the psychosocial network and dementia–A case-control study. Journal of Psychiatric Research. 2003;37:89–98. doi: 10.1016/s0022-3956(02)00065-1. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox NA, Marshall PJ, Nelson CA Bucharest Early Intervention Project. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Tate RB, Manfreda J, Cuddy TE. The effect of age on risk factors for ischemic heart disease: The Manitoba Follow-Up Study, 1948–1993. Annals of Epidemiology. 1998;8:415–421. doi: 10.1016/s1047-2797(98)00011-8. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. Journal of Gerontology Series A Biological Sciences and Medical Sciences. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: The Brief Test of Adult Cognition by Telephone. Age Ageing. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Developmental Psychlogy. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Waldstein SR. Health effects on cognitive aging. In: Stern PC, Certensen LL, editors. The aging mind: Opportunities in cognitive research. Washington, DC: National Academy Press; 2000. pp. 189–217. [Google Scholar]
- Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. American Journal of Epidemiology. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Whitfield KE, Fillenbaum GG, Pieper C, Albert MS, Berkman LF, Blazer DG, Seeman TE. The effect of race and health-related factors on naming and memory. The MacArthur Studies of Successful Aging. Journal of Aging and Health. 2000;12:69–89. doi: 10.1177/089826430001200104. [DOI] [PubMed] [Google Scholar]
- Yen YC, Yang MJ, Shih CH, Lung FW. Cognitive impairment and associated risk factors among aged community members. International Journal of Geriatric Psychiatry. 2004;19:564–569. doi: 10.1002/gps.1131. [DOI] [PubMed] [Google Scholar]
- Zunzunegui MV, Alvarado BE, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. Journal of Gerontology Series B Social Sciences. 2003;58:S93–S100. doi: 10.1093/geronb/58.2.s93. doi:10.1093/geronb/58.2.S93. [DOI] [PMC free article] [PubMed] [Google Scholar]