Abstract
Objectives.
Among the key targets of inquiry in cognitive aging are (1) the description of cognitive changes with advancing age and (2) the association of such cognitive changes with modulating factors in the changing epidemiological context.
Methods.
In the current study, we assemble multi-occasion (up to 12 years) cognitive (speed, episodic memory, and semantic memory) and self-reported health data from the Victoria Longitudinal Study (n = 988; ages 55–95 years).
Results.
The results from piecewise random effects models using age as a basis indicated that only selected measures of episodic memory and semantic memory showed evidence of significant declines prior to age 75. After age 75, all cognitive abilities showed evidence for statistically significant declines, although the magnitude of these changes varied considerably. Performance at age 75 was correlated with self-reported health for measures of processing speed and episodic memory. Changes in health status were related to changes in some aspects of processing speed.
Discussions.
The results indicated that (1) for many cognitive abilities declines in performance did not manifest until after age 75 and (2) self-reported health was related to level of performance more than changes over age.
Keywords: Cognition, Individual differences, Longitudinal change, Physical health
ALTHOUGH the overall fact of cognitive decline with normal aging is undisputed, continuing research is required to establish basic descriptive characteristics. These characteristics include (1) relative rates of decline across cognitive domains, (2) influence of modulating conditions, (3) whether there is an inflection point in late adulthood at which normal cognitive changes are accelerated, and (4) meeting methodological challenges (e.g., assembling long-term data sets, overcoming design limitations, and using change-structured analyses; see Dixon, in press; McArdle, 2009). Robust results from a host of cross-sectional reports and a growing number of longitudinal studies have confirmed general patterns of aging-related decline and have identified select covariates and predictors associated with (and differentiating) normal and accelerated decline (Hultsch, Hertzog, Dixon, & Small, 1998; McArdle, Fisher, & Kadlec, 2007; Wilson, Li, Bienias, & Bennett, 2006).
Among the common covariates and predictors, a growing emphasis has been to examine several aspects of health, as they relate to cognitive differences and changes in older adults. In this regard, researchers have addressed adults’ global health (e.g., chronicity), subjective (and instrumental) health, actual health conditions (e.g., type 2 diabetes), or various biological-health markers (Albert et al., 2009; Anstey, 2008; Wahlin, MacDonald, de Frias, Nilsson, & Dixon, 2006; Yeung, Fischer, & Dixon, 2009). In this study, we focus on the potential role of self-reported or subjective health in modulating cognitive changes with aging. All three forms of self-reports of health status are included in the database: (1) beliefs or ratings about health status, (2) personal impressions of comparative health status, and (3) judgments about functional or instrumental health (e.g., Sargent-Cox, Anstey, & Luszcz, 2010; Spiro & Brady, 2008). In previous research, we have found that such health measures both moderated and mediated cross-sectional age differences in cognitive performance (Wahlin et al., 2006). In addition, in a recent study, a similar subjective health composite mediated the effect of a serious health condition (type 2 diabetes) on cognitive neuropsychological performance among older adults (McFall, Geall, Fischer, Dolcos, & Dixon, in press). To our knowledge, however, no previous study has addressed the cognition–health and change issue with large-scale, multiwave longitudinal data in normal aging (Albert et al., 2009; Sargent-Cox et al., 2010).
The compelling advantages of examining actual two-construct (in this case, cognition–health) change trajectories through multiple-wave longitudinal studies are becoming better established (McArdle et al., 2007). Such intra-individual designs have both methodological–theoretical advantages (e.g., change is the focus, dual relationships in change are key aspects of aging) and methodological limitations (e.g., complexity of design, data requirements, attrition, or retest effects), all of which are much discussed in the literature (e.g., McArdle, 2009; Salthouse, 2009; Schaie & Hofer, 2001). In the present study, we generate a longitudinal gradient of changes between ages 55 and 95, with up to twelve years of longitudinal data for any individual. This creates a 40-year age span of adulthood with which to test differences and changes in cognition–health relationships. By conjecture, health decrements may have overall deleterious effects on cognitive aging but perhaps greater effects on cognitive changes for old–old adults than for more resilient young–old adults. We perform piecewise models that permit us to test this hypothesis. Given the age distribution in our sample, as well as the fact that the mid-70s have been identified as a likely period of accelerated normal age-related decline (Dixon, Small, MacDonald, & McArdle, in press), we selected age 75 as a pivot point. Subsequently, we tested whether (1) trajectories of cognitive changes vary before and after this age and (2) whether and how health may modulate these differential trajectories.
The overall purpose is to use newly assembled twelve-year (up to five wave) data from the archives of the Victoria Longitudinal Study (VLS) to examine age-based (as distinct from the more traditional wave-based) longitudinal changes in (1) several complementary domains of cognitive functioning that assess the domains of processing speed, episodic memory, and semantic memory and (2) several aspects of self-reported health. The first research goal is to examine descriptive characteristics of long-term cognitive changes. Our procedures and data permit us to test models that estimate change trajectories based on two “pieces” of the overall change range. Specifically, we compare trajectories from the 55- to 75-year segment with that of persons who were older than 75 years. The second goal is to examine the influence of traditional covariates (age, gender, and education) on cross-sectional differences and longitudinal changes in cognitive functioning. The third goal is to link changes in cognitive performance (prior to and including age 75 and post-75) to initial levels of self-reported health status as well as changes in health.
The VLS provides a fertile platform for examining these particular research questions, as it features large samples (in this case, initial n = 988), measured at regular intervals (about three years), across a longer term (up to twelve years), with multiple indicators of cognitive and health functions.
METHODS
Participants
Participants were drawn from two of the three independent samples of the VLS (Dixon & de Frias, 2004; Hultsch et al., 1998). The VLS follows a longitudinal sequential research design in which volunteer participants are repeatedly tested every three years on an extensive battery of cognitive, neuropsychological, physical, sensory, health, and psychological tests. As compared with the general population, the present overall sample was positively selected in terms of educational attainment. Intake exclusionary criteria ensure that general cognitive, physical, and mental health are initially good for similar-aged adults. Specifically, at intake, the criteria require exclusion for concurrent (or history of) serious health conditions that may affect mortality or cognitive health (e.g., cardiovascular, cerebrovascular, psychiatric, or neurodegenerative diseases).
We assembled VLS data from the first five waves for Sample 1 (twelve-year follow-up period) and the first three waves for Sample 2 (six-year follow-up period). Sample 1 began in the late 1980s with 484 White community-dwelling adults (288 women and 196 men) initially ranging between 55 and 85 years of age (M = 69.2 years). Sample 2 began in the 1990s with 530 participants (355 women and 175 men) initially ranging between 55 and 94 years of age (M = 68.25 years of age). The mean intervals between waves were similar (Sample 1 = 3.1 years and Sample 2 = 3.3 years). Selected demographic characteristics are shown in Table 1. Although the two samples were comparable in most respects, some minor differences in gender composition (Sample 2 had more men) and years of education (Sample 2 greater than Sample 1) were observed. Accordingly, gender and years of education were used as covariates in all statistical models. Among persons who were eligible to return for testing, the average rate of return for participants across all waves was more than 70%, and continuing participants showed a range of typical positive selection effects (Hertzog, Dixon, Hultsch, & MacDonald, 2003; Hultsch et al., 1998).
Table 1.
Demographic Characteristics of Sample 1 and Sample 2 of the Victoria Longitudinal Study
| Total | Sample 1 | Sample 2 | |
| n | 988 | 464 | 524 |
| Age | |||
| M | 68.80 | 69.13 | 68.51 |
| SD | 6.85 | 5.86 | 7.62 |
| Gender (% female) | 63.77 | 59.91 | 67.18* |
| Years of education | |||
| M | 14.19 | 13.45 | 14.86*** |
| SD | 3.18 | 3.08 | 3.13 |
| Sample size and average follow-up | |||
| Wave 1 | |||
| n | 988 | 464 | 524 |
| M | — | — | — |
| Wave 2 | |||
| n | 737 | 332 | 405 |
| M | 3.09 | 2.92 | 3.22 |
| Wave 3 | |||
| n | 586 | 250 | 336 |
| M | 6.32 | 5.90 | 6.62 |
| Wave 4 | |||
| n | 177 | 177 | — |
| M | 8.91 | 8.91 | — |
| Wave 5 | |||
| n | 129 | 129 | — |
| M | 12.28 | 12.28 | — |
*p < .05; ***p < .001.
Measures
The measures of cognitive performance from the VLS battery were selected to represent a continuum from basic neurocognitive processing speed to complex cognitive constructs. We have two measures each of processing speed, episodic memory, and semantic memory. In addition, we constructed a composite measure of health status based upon four self-report tasks. All tasks have been used and described in VLS research (Dixon & de Frias, 2004; Hultsch et al., 1998).
Processing speed.—
In the “lexical decision time” task (Baddeley, Logie, & Nimmo-Smith, 1985), participants decide as rapidly as possible whether a 5- to 7-letter stimulus appearing on the computer screen is an English word. Median latency across 60 trials was used here. In the “semantic decision time” task (Palmer, MacLeod, Hunt, & Davidson, 1985), participants decide whether a sentence appearing on the computer screen is plausible in the world as we know it. Median latency across 50 trials was the outcome.
Episodic memory.—
In the VLS “Word Recall” task, two categorized lists of English nouns (Battig & Montague, 1969; Howard, 1980), each consisting of 30 words from six taxonomic categories (five words per category), are presented at each wave. The number of words recalled, averaged across the two study lists, is the outcome measure. For the VLS “Story Recall,” two structurally equivalent narrative stories were administered at each wave (Dixon et al., 2004), with average proportion of correct gist recall of propositions across the two stories serving as the outcome measure. To reduce potential practice effects for these content-based memory tasks, new sets of stimuli are used in each of three consecutive waves, with no repetition until the fourth wave (nine years after baseline).
Semantic memory.—
“Fact recall” was measured by two sets of 40 diverse questions that tested individuals’ recall of world knowledge (Nelson & Narens, 1980). As with the episodic memory tasks, multiple lists of questions were counterbalanced across times of measurement. The number of correct items, averaged across the two lists, served as the outcome measure. The “Vocabulary” measure was a 54-item multiple-choice (recognition) vocabulary test drawn from the ETS Kit of Factor Referenced Tests (Ekstrom, French, Harman, & Dermen, 1976). The number of correct items was used as the outcome variable.
Self-reported health.—
This domain was indexed by five measures: self-rated health, illness episodes in the past four weeks, illness episodes in the past year, and chronic illness (Hultsch et al., 1998). Self-rated health consisted of two items that asked participants to rate their own health on a 5-point scale (very good, good, fair, poor, or very poor) compared with a perfect state of health and compared with other people their own age. For illness episodes, participants were asked about the frequency of visits to a doctor or hospitalizations over the previous four weeks or past year. For chronic illness, the presence and severity of 26 chronic conditions were assessed. The domain score of self-reported health was created on the basis of previous exploratory and confirmatory factor analyses (Hultsch, Hammer, & Small, 1993; Hultsch, Hertzog, Small, & Dixon, 1999, respectively), as well as because of the fact that the items were significantly correlated among each other (median r = .37, p < .001). Scores on each item were standardized and then summed to create a composite self-reported health score. Lower scores indicate poorer ratings of health.
Statistical Analyses
Prior to analysis, the cognitive test scores were converted to T-scores using the mean and standard deviation from the first assessment point. In order to examine longitudinal changes in cognitive performance and self-reported health, we applied random effects models using SAS Proc Mixed (Littell, Milliken, Stroup, Wolfinger, & Schabenberger, 2006). There are two important features of the statistical analyses. First, age (centered at age 75 and divided by 10) was used as the basis upon which changes in cognitive performance were observed. In this way, the resulting parameter estimates reflect change per decade of age. The advantage of using age (rather than wave) as the basis measure is that we are able to estimate an “accelerated” longitudinal design (Duncan, Duncan, & Hops, 1996; McArdle & Anderson, 1990; McArdle & Bell, 2000). Specifically, we can generate a longitudinal gradient of changes between the youngest age at the start of the study (i.e., age 55) to the older age at the end of the study (i.e., age 95 for one person in Sample 1). Although we have a maximum of twelve years of longitudinal data from any one individual, we are able to derive longitudinal change gradients that span over 40 years of adulthood (from age 55 to 95). Second, the models themselves are piecewise or spline models (McArdle, 2009), because we estimate two trajectories or pieces for each cognitive outcome, changes in performance prior to and including age 75 and changes in performance after age 75. Age 75 was selected as the inflection point because it was the approximate median of ages across the longitudinal follow-up period. As a result, we are able to optimize the data points that contribute to the estimation of changes before and after age 75. These models allow us to describe differences in the nature of longitudinal changes across age and enable us to derive conclusions as to whether the magnitude of change is greater in one age segment versus another. In the analyses, age at baseline, gender, and years of education (centered at twelve years) were included as predictors of performance at age 75 (intercept), as well as the two change parameters. Finally, a term specifying attrition was included in all of the models indicating whether the participant had completed all longitudinal assessments (0) or not (1).
In addition, using options in SAS, the Bayes estimates of the values at age 75, as well as the changes prior to age 75 and changes after age 75, were saved for the cognitive and health outcomes. Using these estimates, we correlated self-reported health at age 75 with cognitive performance at the same age. In addition, changes in self-reported health prior to age 75 were correlated with changes in cognitive performance across the same age period, as well as changes in cognition after age 75. For the latter, we are able to examine whether declines in health status prior to age 75 are related to accelerated cognitive declines after age 75. Finally, changes in self-reported health after age 75 were correlated with changes in cognitive performance over the same age period.
RESULTS
Longitudinal Changes in Cognitive Performance and Self-reported Health
The results of the analyses are shown in Table 2 and displayed in Figure 1. As a guide to Table 2, the top three rows show the basic model parameters for values at age 75 (intercept), as well as change per decade prior to and including age 75 and changes per decade after age 75. The next three rows specify the variance estimates for intercept, change less than or equal to age 75, and changes greater than or equal to age 75 and reflect whether there is evidence for statistically significant individual differences in these parameters. The next three sets of values represent age at baseline, gender, years of education (centered at 12), and attrition as predictors of performance at age 75 and the two change parameters. In all cases, the statistical test results evaluate whether the parameters are different from zero. We describe results for each domain separately.
Table 2.
Estimates From the Piecewise Models of Cognitive Performance
| Effect | Lexical decision time | Semantic decision time | Word recall | Story recall | Fact recall | Vocabulary | Self-reported health |
| Mean at age 75 | 49.38*** | 48.08*** | 51.59*** | 51.89*** | 48.13*** | 49.91*** | 50.76*** |
| Change per decade ≤ age 75 | 0.46 | 0.06 | −1.91* | 0.29 | −2.44** | −0.01 | −4.39*** |
| Change per decade > age 75 | 2.99 | 7.02* | −9.92*** | −5.61*** | −5.21*** | −2.51* | −4.69*** |
| σ2, age 75 | 41.01*** | 63.26*** | 60.28*** | 52.02*** | 66.51*** | 56.62*** | 39.22*** |
| σ2, change ≤ age 75 | 0.44 | 6.15 | 7.88 | 7.95* | 2.08 | 8.86* | 5.74 |
| σ2, change > 75 | 367.39*** | 793.51*** | 60.73*** | 14.54 | 18.64* | 43.26*** | 14.22 |
| Predictors of differences at age 75 | |||||||
| Baseline age | 0.15* | 0.15* | −0.10 | −0.28*** | 0.06 | −0.23*** | 0.36*** |
| Years of education | −0.25* | −0.43*** | 0.71*** | 0.82*** | 0.85*** | 1.09*** | −0.19 |
| Gender | −2.29** | 0.71 | −4.06*** | −3.79*** | 5.31*** | 0.24 | 0.84 |
| Attrition | 1.70* | 2.81*** | −3.64*** | −3.65*** | −4.31*** | −2.53*** | −2.26** |
| Predictors of change prior to age 75 | |||||||
| Baseline age | −0.09 | −0.03 | −0.13 | −0.02 | −0.05 | 0.02 | −0.08 |
| Years of education | −0.09 | −0.06 | 0.03 | 0.09 | 0.18 | 0.11 | −0.22* |
| Gender | −2.29** | −0.09 | −0.51 | −0.29 | −0.29 | −0.15 | 0.79 |
| Attrition | 0.05 | 0.69 | −1.39 | −0.84 | −0.51 | 0.33 | −0.43 |
| Predictors of change after age 75 | |||||||
| Baseline age | 0.23 | 0.36 | 0.25 | 0.29* | −0.03 | 0.04 | −0.01 |
| Years of education | 0.54 | 0.22 | −0.45 | −0.35 | −0.35 | −0.44* | 0.00 |
| Gender | 2.05 | −0.99 | 2.71 | 2.32 | −0.61 | −1.36 | −1.04 |
| Attrition | 5.94* | 1.83 | −2.10 | −3.46 | −2.82 | −2.06 | 0.45 |
*p < .05; **p < .01; ***p < .001.
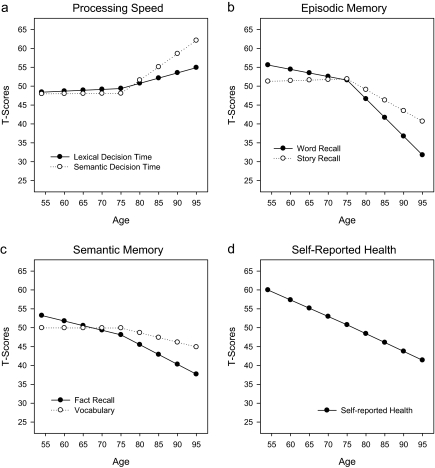
Figure 1.
Longitudinal changes in cognitive performance and self-reported health.
Processing speed.—
As seen in Figure 1a and the accompanying values in Table 2, neither lexical decision speed nor semantic decision speed showed evidence of statistically significant change prior to age 75. Semantic decision speed slowed at a rate of 0.75 SD per decade after age 75, whereas lexical decision time did not. Age, education, and attrition were predictors of response latency at age 75 with younger age, more years of education, and remaining in the longitudinal sample associated with faster response times. Male gender was associated with faster response times on the lexical decision time task. Female gender was associated with greater declines in response latency in lexical decision time for persons aged 75 years of age and younger and attrition modified declines in this measure after age 75
Episodic memory.—
The results for this outcome are shown in Figure 1b. For word recall, there was evidence of statistically significant change during both age periods, with the greater rate of change after age 75 of approximately 1 SD per decade of age. For story recall, the estimate of change prior to age 75 was not statistically significant, but the rate of change was just more than 0.5 SD units per decade after age 75. Younger age, female gender, more years of education, and remaining in the longitudinal sample were associated with better performance at age 75 on both tests of episodic memory. Age at baseline was associated with changes in story recall after age 75.
Semantic memory.—
The changes over age for fact recall and vocabulary are shown in Figure 1c. For fact recall, changes both prior to and after age 75 were statistically significant, declining at a rate of 0.23 SD per decade prior to age 75 and more than 0.5 SD per decade after age 75. For vocabulary, only the changes after age 75 were statistically significant, with declines of approximately 0.3 SD per decade. Among the predictors of performance at age 75, older age was associated with better performance for vocabulary. At age 75, more years of education was associated with better performance on both outcomes, and male gender was associated with higher scores on the fact recall task. Leaving the longitudinal sample was associated with poorer performance at age 75. More years of education were associated with lower rates of change in vocabulary after age 75.
Self-reported health.—
Changes in self-reported health are shown in Table 2 and Figure 1d. Analyses indicated that there was statistically significant change prior to age 75 and after age 75, with both rates of change exhibiting comparable trajectories of approximately 0.4 SD per decade. At age 75, age at baseline was associated with poorer ratings of self-reported health. Longitudinally, persons with fewer years of education exhibited greater declines in health status during the age period prior to age 75.
Correlations Between Self-reported Health and Cognitive Performance
The correlations between self-reported health and cognitive performance at age 75, as well as between the changes in these outcomes across the two age periods, are shown in Table 3. At age 75, poorer self-reported health was associated with longer response latencies for lexical decision time and semantic decision time as well as poorer performance on the two tests of episodic memory. The results also indicated that changes in self-reported health that reflected increasingly poorer health were associated with greater increases in response latency prior to age 75 for semantic decision time.
Table 3.
Correlations of Measures of Cognitive Performance With Self-Reported Health at Baseline and Changes Over Age.
| Cognitive measure | Self-reported health |
||
| Health at age 75 | Changes ≤ age 75 | Changes > age 75 | |
| Lexical decision time | |||
| At age 75 | −.10** | ||
| Changes ≤ age 75 | −.05 | ||
| Changes > age 75 | −.05 | −.01 | |
| Semantic decision time | |||
| At age 75 | −.14*** | ||
| Changes ≤ age 75 | −.09** | ||
| Changes > age 75 | .05 | −.01 | |
| Word recall | |||
| At age 75 | .06* | ||
| Changes ≤ age 75 | .05 | ||
| Changes > age 75 | .02 | −.02 | |
| Story recall | |||
| At age 75 | .11*** | ||
| Changes ≤ age 75 | .01 | ||
| Changes > age 75 | .04 | −.04 | |
| Fact recall | |||
| At age 75 | .01 | ||
| Changes ≤ age 75 | .01 | ||
| Changes > age 75 | −.02 | −.02 | |
| Vocabulary | |||
| At age 75 | .03 | ||
| Changes ≤ age 75 | .03 | ||
| Changes > age 75 | −.03 | −.03 | |
*p < .05; **p < .01; ***p < .001.
DISCUSSION
We examined changes in performance on multiple cognitive domains with an accelerated longitudinal design that estimated changes in cognitive performance and self-reported health from age 55 to 95. This 40-year band—together with our piecewise or spline models (McArdle, 2009)—provided us with a unique opportunity to examine several theoretically interesting questions. The main goals were to (1) test pre-/post-75 trajectories across a range of cognitive and health measures and (2) link cognitive and health differences and change.
There are several important results from the present study. First, a long-standing question in the field concerns the extent and timing of cognitive decline with aging and whether patterns may differ when followed longitudinally over longer bands of the older adult life span (Baltes & Nesselroade, 1979; Salthouse, 2009, 2010; Schaie, 2009). Our results contribute to this discussion by demonstrating that for most measures of cognitive performance, the onset of statistically significant declines occurred quite late, after age 75. Only word recall and fact recall showed evidence for statistically significant declines prior to age 75. By contrast, data from cross-sectional studies indicate that these declines happen much earlier, in the 20s or 30s for some cognitive abilities (Salthouse, 2009). Moreover, some of the criticisms of longitudinal estimates of age-related changes in cognitive performance, namely that positive practice effects help to maintain cognitive performance across the life span, are less relevant here. The measures of episodic memory and the fact recall task comprised parallel lists that were counterbalanced across occasions, thereby minimizing the effects of practice (see also Dixon et al., 2011). Nevertheless, the story recall task failed to exhibit statistically significant declines in performance prior to age 75. Moreover, although both word recall and fact recall declined significantly prior to age 75, the more sizable declines occurred after age 75. Taken together, the results indicate that even for speed-based indicators of neurocognitive integrity (e.g., Dixon et al., 2007; Salthouse, 1996), significant change did not occur until well into the late-life years.
A second notable result is that, despite the fact that all measures of cognitive performance showed evidence of statistically significant longitudinal declines after age 75, the magnitude of these decrements varied greatly. For example, performance on the measure of vocabulary declined by approximately 0.25 SD units after age 75, whereas performance on the measure of word recall declined by 1 SD per decade over that same period. The presence of modest declines in vocabulary is not surprising, given its status as a measure of crystallized intelligence, but the changes in word recall were substantial. Cumulatively, across the entire period from age 55 to 95, performance on the vocabulary measure declined by approximately 0.5 SD units, whereas word recall declined by almost 2.5 SD units! Influences from multiple sources (e.g., health, clinical status, samples, and methodological characteristics) can lead to individual differences or group shifting in longitudinal decline (or cross-sectional deficit) patterns (Dixon, 2011). Our results show that, for long-term longitudinal studies, not only do trajectories vary dramatically across a wide swath of older adulthood but also even in very late adulthood (post-75 years); when all trajectories are declining, they vary widely across cognitive domains. Accordingly, future researchers may wish to consider broader age ranges measured over longer periods across batteries representing multiple cognitive domains. Longitudinal change patterns may be quite variable, conditioned on a number of epidemiological and methodological factors.
A third main result indicated that self-reported health declined significantly over both age periods. This straightforward observation is likely conditioned on the facts that (1) our measure is a composite of several self-reported health markers covering the full construct domain (as recommended by Sargent-Cox, Anstey, & Luszcz, 2008) and (2) it is arguable that objective and subjective health track together in late life, so comprehensive reports showing decline, are reasonable and previously observed (e.g., Liang, Bennett, Whitelaw, & Maeda, 1991). In addition, such measures have been found to be notably sensitive in other aspects of cognition–health research in aging (McFall et al., in press; Sargent-Cox et al., 2010). Unsurprisingly, we also found in this study that self-reported health was related to the measures of processing speed and episodic memory at baseline, with poorer health being associated with poorer cognitive performance. Moreover, longitudinally, changes in health status, as measured by our composite, were actually predictive of changes in basic cognitive resources, as reflected both indicators of processing speed.
The lack of an association between changes in health status and changes in cognitive performance is somewhat surprising, given the literature that has linked the presence of specific health conditions or the measurement of overall medical health to cognitive performance in old age (Atkinson et al., 2010; Etnier et al., 2007). For example, Brady, Spiro, and Gaziano (2005) reported that uncontrolled hypertension was associated with greater age deficits on tests of category fluency and word recall. Similarly, Verdelho and colleagues (2010) reported that presence of diabetes was associated with greater decline in cognitive performance in a sample of older adults who had experienced white matter brain changes.
In the current study, we examined the relationship between concurrent changes in self-reported health and cognitive performance, whereas other studies have examined health in relation to cross-sectional differences in cognitive performance or subsequent changes in functioning. Thus, the fact that we examined how changes in health relate to changes in cognitive performance could help to explain the differences between our study and those reported above. Moreover, it may be the case that the subclinical health impairments, such as those that contribute to the self-reports of health observed here, are not sufficiently acute to have a great impact on changes in cognitive performance. The health assessment did not include measures of depression or specific medications, both of which could influence cognitive change. Future studies should include a combination of objective and self-report measures of health status, as well as a diversity of outcomes, in order to fully capture health status and its potential to influence cognition.
This study has several strengths, including the longitudinal design, the large sample, the broad 40-year age band, the wide representation of cognition, the merging of cognition and health constructs, and the statistical techniques for examining a unique question in cognitive aging. Although the results of the present study are informative, there are several limitations should also be acknowledged. First, by design, participants from the VLS represent initially healthy and generally well-educated older adults, although participants’ health did decline significantly across age. Second, we selected age 75 as the inflection point for our analyses, and this was done for a number of reasons. From a practical and empirical perspective, in this longitudinal data set, age 75 was selected because it optimized the number of data points (both before and after) that contributed to the estimation of changes in cognitive performance and self-reported health. Age 75 was approximately the median of ages across the follow-up period. A complementary theoretical perspective is that age 75 is roughly consistent with—and representative of—the age range at which the VLS has observed apparent accelerations of normal cognitive decline (e.g., in episodic memory; see Dixon et al., 2011). Other estimation procedures, such as change point analyses (Cohen, 2008), are available, and this would likely result in different results in terms of when the declines started across the cognitive ability domains examined here. However, the advantage of the standard inflection point allowed us to relate changes in cognitive performance to changes in self-reported health across the same age period. Finally, the acceleration of declines in cognitive functioning after age 75 could also reflect the influence of impending dementia (Laukka, Jones, Small, Fratiglioni, & Bäckman, 2004; Small, Fratiglioni, Viitanen, Winblad, & Backman, 2000) or impending mortality (MacDonald, Hultsch, & Dixon, 2008; Small & Bäckman, 1999), both of which have been shown to negatively affect cognitive performance in old age.
In summary, the results of the present study indicate that (1) for many cognitive abilities declines in performance did not manifest until after age 75, (2) there was significant heterogeneity in the magnitude of age-related declines observed across domains, and (3) self-reported health was related to cross-sectional differences in performance but were changes in self-reported health were only weakly associated with changes in cognitive functioning. Taken together, the results allow us to characterize the nature of change in cognitive functioning in late life but are unable to point to changes in self-reported health as a major contributor to the age-related cognitive declines.
FUNDING
This research was supported by a grant from the National Institutes of Health/National Institute on Aging (NIA; R37 AG008235) to R. A. Dixon, who also acknowledges support from the Canada Research Chairs program. B. J. Small was supported by a NIA grant to the National Alzheimer's Coordinating Center (U01 AG016976) to B. J. Small (P.I.) during the preparation of this manuscript. J. J. McArdle was supported by an NIA grant (R37 AG07137) during the preparation of this manuscript.
Acknowledgments
We thank the volunteer participants of the VLS for their time and effort and VLS staff members for their assistance in data collection and preparation. For further information about the VLS contact Roger A. Dixon (rdixon@ualberta.ca) or visit http://www.ualberta.ca/∼vlslab/.
References
- Albert ML, Spiro A, Sayers KJ, Cohen JA, Brady CB, Goral M, Obler LK. Effects of health status on word finding in aging. Journal of the American Geriatrics Society. 2009;57:2300–2305. doi: 10.1111/j.1532-5415.2009.02559.x. doi:10.1111/j.1532-5415.2009.02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ. Alcohol exposure and cognitive development: An example of why we need a contextualized, dynamic life course approach to cognitive ageing: A mini-review. Gerontology. 2008;54:283–291. doi: 10.1159/000161735. doi:10.1159/000161735. [DOI] [PubMed] [Google Scholar]
- Atkinson HH, Rapp SR, Williamson JD, Lovato J, Absher JR, Gass M, Espeland MA. The relationship between cognitive function and physical performance in older women: Results from the women's health initiative memory study. Journal of Gerontology: Medical Sciences. 2010;65:300–306. doi: 10.1093/gerona/glp149. doi:10.1093/gerona/glp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Logie R, Nimmo-Smith I. Components of fluent reading. Journal of Memory and Language. 1985;24:119–131. doi:10.1016/0749-596X%2885%2990019-1. [Google Scholar]
- Baltes PB, Nesselroade JR. History and rationale of longitudinal research. In: Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. New York, NY: Academic Press; 1979. pp. 1–39. [Google Scholar]
- Battig WF, Montague WE. Category norms of verbal items in 56 categories: A replication and extension of the Connecticut category norms. Journal of Experimental Psychology Monograph. 1969;80:1–46. Part 2. doi:10.1037/h0027577. [Google Scholar]
- Brady CB, Spiro A, III, Gaziano JM. Effects of age and hypertension status on cognition: The Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19:770–777. doi: 10.1037/0894-4105.19.6.770. doi:10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Cohen P, editor. Applied data analytic techniques for turning points research. New York, NY: Routledge; 2008. [Google Scholar]
- Dixon RA. Enduring theoretical themes in psychological aging: Derivation, functions, perspectives, and opportunities. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7th ed. San Diego, CA: Elsevier; 2011. pp. 3–23. [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating patterns and predictors of changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. doi:10.1080/13825580490511161. [Google Scholar]
- Dixon RA, Garrett DD, Lentz TL, MacDonald SWS, Strauss E, Hultsch DF. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. doi:10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Small BJ, MacDonald SWS, McArdle JJ. Yes, memory declines with aging—But when, how, and why? In: Naveh-Benjamin M, Ohta N, editors. Memory and aging. New York, NY: Psychology Press; in press. [Google Scholar]
- Dixon RA, Wahlin A, Maitland SB, Hultsch DF, Hertzog C, Backman L. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory & Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Duncan SE, Duncan TE, Hops H. Analysis of longitudinal data within accelerated longitudinal designs. Psychological Methods. 1996;1:236–248. doi:10.1037/1082-989X.1.3.236. [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC. Cognitive performance in older women relative to ApoE-episilon4 genotype and aerobic fitness. Medicine and Science in Sports and Exercise. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. doi:10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, MacDonald SWS. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? [Article] Psychology and Aging. 2003;18:755–769. doi: 10.1037/0882-7974.18.4.755. doi:10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Howard D. Category norms: A comparison of Battig and Montague (1969) norms with the responses of adults between the ages of 20 and 80. Journal of Gerontology. 1980;35:225–231. doi: 10.1093/geronj/35.2.225. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: Relationships to self-reported health and activity life style. Journal of Gerontology. 1993;48:P1–P11. doi: 10.1093/geronj/48.1.p1. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. New York, NY: Cambridge University Press; 1998. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Laukka EJ, Jones S, Small BJ, Fratiglioni L, Bäckman L. Similar patterns of cognitive deficits in the preclinical phases of vascular dementia and Alzheimer's disease. Journal of the International Neuropsychological Society. 2004;10:382–391. doi: 10.1017/S1355617704103068. doi:10.1017/S1355617704103068. [DOI] [PubMed] [Google Scholar]
- Liang J, Bennett J, Whitelaw N, Maeda D. The structure of self-reported physical health among the aged in the United States and Japan. Medical Care. 1991;29:1161–1180. doi: 10.1097/00005650-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd ed. Cary, NC: SAS Press; 2006. [Google Scholar]
- MacDonald SWS, Hultsch DF, Dixon RA. Predicting impending death: Inconsistency in speed is a selective and early marker. Psychology and Aging. 2008;23:595–607. doi: 10.1037/0882-7974.23.3.595. doi:10.1037/0882-7974.23.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. doi:10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Anderson E. Latent variable growth models for research on aging. In: Birren JE, Schaie KW, editors. The handbook of the psychology of aging. New York, NY: Plenum Press; 1990. pp. 21–43. [Google Scholar]
- McArdle JJ, Bell RQ. Recent trends in modeling longitudinal data by latent growth curve methods. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multi-group data: Practical issues, applied approaches, and scientific examples. Mahwah, NJ: Erlbaum; 2000. pp. 69–108. [Google Scholar]
- McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the Health and Retirement study, 1992–2004. Psychology and Aging. 2007;22:525–545. doi: 10.1037/0882-7974.22.3.525. doi:10.1037/0882-7974.22.3.525. [DOI] [PubMed] [Google Scholar]
- McFall GP, Geall BP, Fischer AL, Dolcos S, Dixon RA. Testing covariates of type 2 diabetes-cognition associations in older adults: Moderating or mediating effects? Neuropsychology. 2010;24:547–562. doi: 10.1037/a0019246. doi: 10.1037/a0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Norms of 300 general information questions: Accuracy of recall, latency of recall, and feeling of knowing ratings. Journal of Verbal Learning and Verbal Behavior. 1980;19:338–368. doi:10.1016/S0022-5371%2880%2990266-2. [Google Scholar]
- Palmer J, MacLeod CM, Hunt E, Davidson JE. Information processing correlates of reading. Journal of Memory and Language. 1985;24:119–131. doi:10.1016/0749-596X(85)90016-6. [Google Scholar]
- Salthouse TA. The processing speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. doi:10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The paradox of cognitive aging. Journal of Clinical and Experimental Neuropsychology. 2010;32:622–629. doi: 10.1080/13803390903401310. doi:10.1080/13803390903401310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, Luszcz M. Determinants of self-rated health items with different points of reference: Implications for health measurement of older adults. Journal of Aging and Health. 2008;20:739–761. doi: 10.1177/0898264308321035. doi:10.1177/0898264308321035. [DOI] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, Luszcz MA. The choice of self-rated health measures matter when predicting mortality: Evidence from 10 years follow-up of the Australian longitudinal study of ageing. BMC Geriatrics. 2010;10:18. doi: 10.1186/1471-2318-10-18. doi:10.1186/1471-2318-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. “When does age-related cognitive decline begin?” Salthouse again reifies the “cross-sectional fallacy.”. Neurobiology of Aging. 2009;30:528–529. doi: 10.1016/j.neurobiolaging.2008.12.012. doi:10.1016/j.neurobiolaging.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Hofer SM. Longitudinal studies in aging research. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. New York, NY: Academic Press; 2001. pp. 53–77. [Google Scholar]
- Small BJ, Bäckman L. Time to death and cognitive performance. Current Directions in Psychological Science. 1999;8:168–172. doi:10.1111/1467-8721.00040. [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Archives of Neurology. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. doi:noc90093 [pii] [DOI] [PubMed] [Google Scholar]
- Spiro A, III, Brady CB. Integrating health into cognitive aging research and theory. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Los Angeles, CA: Sage; 2008. pp. 260–283. [Google Scholar]
- Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T, LADIS Study White matter changes and diabetes predict cognitive decline in the elderly: The LADIS Study. Neurology. 2010;75:160–167. doi: 10.1212/WNL.0b013e3181e7ca05. doi:10.1212/WNL.0b013e3181e7ca05. [DOI] [PubMed] [Google Scholar]
- Wahlin A, MacDonald SWS, de Frias CM, Nilsson L.-G., Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: Moderating, mediating, or both? Psychology and Aging. 2006;21:318–332. doi: 10.1037/0882-7974.21.2.318. doi:10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: Separating retest effects from the effects of growing older. Psychology and Aging. 2006;21:774–789. doi: 10.1037/0882-7974.21.4.774. doi:10.1037/0882-7974.21.4.774. [DOI] [PubMed] [Google Scholar]
- Yeung SE, Fischer AL, Dixon RA. Exploring effects of type 2 diabetes on cognitive functioning in older adults. Neuropsychology. 2009;23:1–9. doi: 10.1037/a0013849. doi:10.1037/a0013849. [DOI] [PMC free article] [PubMed] [Google Scholar]