EXECUTIVE SUMMARY
The University of Vermont College of Medicine and the Vermont Lung Center, with support of the National Heart, Lung, and Blood Institute (NHLBI), the Alpha-1 Foundation, the American Thoracic Society, the Emory Center for Respiratory Health, the Lymphangioleiomyomatosis (LAM) Treatment Alliance, and the Pulmonary Fibrosis Foundation, convened a workshop, “Stem Cells and Cell Therapies in Lung Biology and Lung Diseases,” held July 26–29, 2009 at the University of Vermont, to review the current understanding of the role of stem and progenitor cells in lung repair after injury and to review the current status of cell therapy approaches for lung diseases. These are rapidly expanding areas of study that provide further insight into and challenge traditional views of the mechanisms of lung repair after injury and pathogenesis of several lung diseases. The goals of the conference were to summarize the current state of the field, discuss and debate current controversies, and identify future research directions and opportunities for both basic and translational research in cell-based therapies for lung diseases.
This conference was a follow-up to two previous conferences held at the University of Vermont, “Adult Stem Cells, Lung Biology, and Lung Disease” sponsored by the NHLBI, the Cystic Fibrosis Foundation, the University of Vermont College of Medicine, and the Vermont Lung Center in 2005 and “Stem Cells and Cell Therapies in Lung Biology and Diseases sponsored by the NHLBI, Alpha-1 Foundation, American Thoracic Society, Pulmonary Fibrosis Foundation, University of Vermont College of Medicine, and the Vermont Lung Center in 2007. Those conferences have been instrumental in helping to guide research and funding priorities (1, 2).
Since the 2007 conference, investigations of stem cells and cell therapies in lung biology and diseases have continued to expand rapidly. However, there continue to be changes in focus and direction, particularly with respect to cell-based therapy approaches. Recent studies of immunomodulation and paracrine effects of adult stem and progenitor cells, notably adult mesenchymal stromal (stem) cells (MSCs) derived from bone marrow, adipose, and other tissues, have increasingly provided evidence of efficacy in animal models of acute and fibrotic lung injuries as well as in asthma, bronchopulmonary dysplasia, chronic obstructive pulmonary disease (COPD), sepsis, and other lung diseases. Although the mechanisms of MSC effects in these models are not yet fully understood, growing evidence implicates both soluble mediators released by the MSCs as well as cell to cell contact of MSCs with different inflammatory and immune effector cells. These studies have recently been extended to human lung explant models, and it is anticipated that clinical investigations of initial safety and efficacy of MSCs in acute lung injury will occur in the near future. In parallel, a 6-month interim analysis of a current clinical trial in the United States assessing systemic administration of MSCs in patients with moderate to severe COPD has demonstrated safety and has yielded promising results with respect to efficacy. This trial has completed its 2-year observation period, and data is expected to be released in late 2010 or early 2011. Circulating endothelial progenitor cells (EPCs) can contribute to regeneration of diseased pulmonary vasculature, and two recent clinical investigations in China have suggested the efficacy of autologous bone marrow–derived EPC administration in both adult and pediatric patients with pulmonary hypertension. A comparable trial of autologous EPC administration in pulmonary hypertension, the Pulmonary Hypertension: Assessment of Cell Therapy (PHaCET) trial, is ongoing in Canada. Circulating endothelial progenitor cells may also play roles in both acute lung injury and in fibrotic lung diseases.
Engraftment of systemically or intratracheally administered cells remains a controversial issue. Although most available evidence argues against significant engraftment, publications and abstracts presented at the conference suggest that several newly investigated cell types, including those derived from placental tissues or novel cell populations derived from adult bone marrow, may demonstrate a more robust ability to engraft and participate in lung repair. Further, significant advances continue to be made in novel areas of investigation including increasing exploration of 3-dimensional culture systems and bioengineering approaches to generate functional lung tissue ex vivo and in vivo. This has culminated in the first successful clinical use of a bioengineered trachea. In parallel, several recent reports demonstrate the potential feasibility of using decellularized whole lungs as scaffolds for recellularization and subsequent implantation. These areas are predicted to be of intense investigation over the next several years.
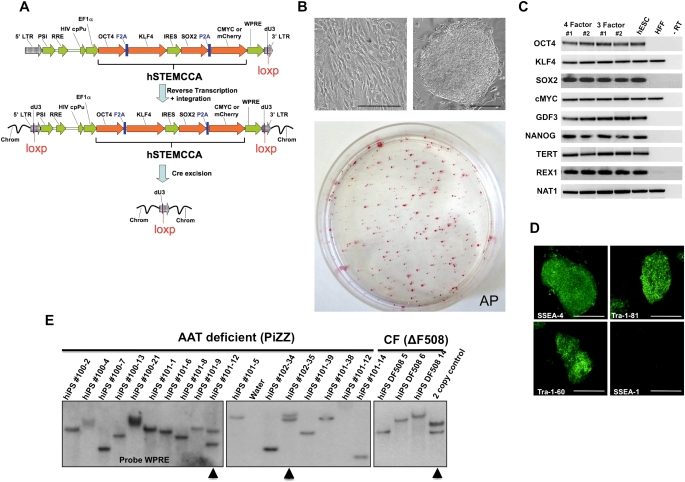
Comparably, progress continues in studies of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPS). Several groups have presented protocols for deriving definitive endoderm from either ESCs or iPS cultured in vitro and some have further demonstrated the ability to generate cells with phenotypic markers of type 2 alveolar cells from either mouse or human ESCs. In addition, a recent study demonstrated the potential ability of type 2 alveolar epithelial cells, derived in culture from human embryonic stem cells, to engraft in lung and ameliorate experimentally induced lung injury in a rodent model. Nonetheless, many challenges remain to generating functional lung cells from either ESCs or iPS. One potentially fruitful area of investigation derives from the recently demonstrated generation of disease-specific iPS from tissue samples obtained from patients with cystic fibrosis, α-1 antitrypsin deficiency, and other genetic or acquired lung diseases.
Significant progress continues to be made in investigations of local (endogenous) stem and progenitor cells residing in the lungs. Advances in lineage-tracing approaches and other techniques have provided important insights into understanding the identity and lineage expansion properties of previously identified putative endogenous progenitor populations and suggest an increasingly complex network of cellular repair after injury. However, the study of endogenous lung stem and progenitor cells remains complicated by the role of the specific micro-environmental niches in which these cells reside. Alteration of the niches with experimental protocols or removal of cells from the niches can change their identifying characteristics and biologic activities. One of the challenges continuing to face the field is to continue to devise more refined lineage tracing and other study mechanisms to define, characterize, and explore potential therapeutic and/or pathologic properties of endogenous lung progenitor cells. This includes studies of lung cancer stem cells, an area of increasing focus and high interest that remains incompletely understood. Another challenge is that most studies of endogenous progenitor cells continue to use mouse models. Correlative information in human lungs remains poorly defined.
A continuing issue of confusion is that of terminology. Despite suggested guidelines from previous conferences and from other sources, precise definitions and characterizations of specific cell populations, notably the putative endogenous cell populations in the lung as well as mesenchymal stromal cells and endothelial progenitor cells, are not agreed upon. The terms “stem cell” and “progenitor cell” are still used with varying degrees of clarity and precision by different investigators and in recent publications. This continues to complicate the comparison of different investigative approaches. A glossary of relevant working definitions applicable to the lung, originally presented in the report of the 2007 conference, is depicted in Table 1. This glossary does not necessarily reflect a consensus for the definition of each term and will undergo continuing revision as an overall understanding of the cell types and mechanisms involved in lung repair continue to be elucidated. Nonetheless, it is a useful framework.
TABLE 1.
GLOSSARY AND DEFINITION OF TERMINOLOGY
| Potency | Sum of Developmental Options Available to Cell |
|---|---|
| Totipotent | Ability of a single cell to divide and produce all the differentiated cells in an organism, including extraembryonic tissues, and thus to (re)generate an organism in total. In mammals only the zygote and the first cleavage blastomeres are totipotent. |
| Pluripotent | Ability of a single cell to produce differentiated cell types representing all three germ layers and thus to form all lineages of a mature organism. Example: embryonic stem cells. |
| Multipotent | Ability of adult stem cells to form multiple cell types of one lineage. Example: hematopoietic stem cells. |
| Unipotent | Cells form one cell type. Example: spermatogonial stem cells (can only generate sperm) |
| Reprogramming | Change in epigenetics that can lead to an increase in potency, dedifferentiation. Can be induced by nuclear transfer, cell fusion, genetic manipulation. |
| Transdifferentiation | The capacity of a differentiated somatic cell to acquire the phenotype of a differentiated cell of the same or different lineage. An example is epithelial–mesenchymal transition (EMT), a process whereby fully differentiated epithelial cells undergo transition to a mesenchymal phenotype giving rise to fibroblasts and myofibroblasts. |
| Plasticity | Hypothesis that somatic stem cells have broadened potency and can generate cells of other lineages, a concept that is controversial in mammals. |
| Embryonic stem cell | Cells isolated from the inner mass of early developing blastocysts. ES cells have the capacity for self renewal and are pluripotent, having the ability to differentiate into cells of all embryologic lineages and all adult cell types. However, ES cells cannot form extraembryonic tissue such as trophectoderm. |
| Adult stem cell | Cells isolated from adult tissues including bone marrow, adipose tissue, nervous tissue, skin, umbilical cord blood, and placenta that have the capacity for self renewal. In general, adult stem cells are multipotent, having the capacity to differentiate into mature cell types of the parent tissue. Some populations of adult stem cells, such as MSCs exhibit a range of lineage differentiation that is not limited to a single tissue type. Whether adult stem cells exhibit plasticity and can differentiate into a wider variety of differentiated cells and tissues remains controversial. |
| Adult tissue-specific stem cell | Same as adult stem cells, but with defined tissue specificity. A relatively undifferentiated cell within a given tissue that has the capacity for self-renewal through stable maintenance within a stem cell niche. Adult tissue-specific (endogenous) stem cells have a differentiation potential equivalent to the cellular diversity of the tissue in which they reside. The hematopoietic stem cell is a prototypical adult tissue stem cell. |
| Induced pluripotent stem cell | Reprogrammed adult somatic cells that have undergone dedifferentiation, such as dermal fibroblasts, reprogrammed by retroviral transduction to express four transcription factors: Oct 3/4, Sox2, c-Myc, and Klf4. iPS cells are similar to ES cells in morphology, proliferation, gene expression, and ability to form teratomas. In vivo implantation of iPS cells results in formation of tissues from all three embryonic germ layers. iPS cells have been generated from both mouse and human cells. |
| Progenitor cell | A collective term used to describe any proliferative cell that has the capacity to differentiate into different cell lineages within a given tissue. Unlike stem cells, progenitor cells have limited or no self-renewal capacity. The term progenitor cell is commonly used to indicate a cell can expand rapidly, but undergoes senescence after multiple cell doublings. Terminology that takes into account the functional distinctions among progenitor cells is suggested below. |
| Transit-amplifying cell | The progeny of a endogenous tissue stem cell that retain relatively undifferentiated character, although more differentiated than the parent stem cell, and have a finite capacity for proliferation. The sole function of transit-amplifying cells is generation of a sufficient number of specialized progeny for tissue maintenance. |
| Obligate progenitor cell | A cell that loses its ability to proliferate once it commits to a differentiation pathway. Intestinal transit-amplifying cells are obligate progenitor cells. |
| Facultative progenitor cell | A cell that exhibits differentiated features when in the quiescent state yet has the capacity to proliferate for normal tissue maintenance and in response to injury. Bronchiolar Clara cells are an example of this cell type. |
| Classical stem cell hierarchy | A stem cell hierarchy in which the adult tissue stem cell actively participates in normal tissue maintenance and gives rise to a transit-amplifying cell. Within this type of hierarchy, renewal potential resides in cells at the top of the hierarchy, that is, the stem and transit-amplifying cell, and cells at each successive stage of proliferation become progressively more differentiated. |
| Nonclassical stem cell hierarchy | A stem cell hierarchy in which the adult tissue stem cell does not typically participate in normal tissue maintenance, but can be activated to participate in repair following progenitor cell depletion. |
| Rapidly renewing tissue | Tissue in which homeostasis is dependent on maintenance of an active mitotic compartment. Rapid turnover of differentiated cell types requires continuous proliferation of stem and/or transit-amplifying cells. A prototypical rapidly renewing tissue is the intestinal epithelium. |
| Slowly renewing tissue | Tissues in which the steady-state mitotic index is low. Specialized cell types are broadly distributed, long-lived, and a subset of these cells, the facultative progenitor cell, retain the ability to enter the cell cycle. The relative stability of the differentiated cell pool is paralleled by infrequent proliferation of stem and/or transit amplifying cells. The lung is an example of a slowly renewing tissue. |
| Hematopoietic stem cell | Cell that has the capacity for self renewal and ability to differentiate into mature leukocytes, erythrocytes, and platelets. Whether HSCs exhibit plasticity and can differentiate into mature cells of other lineages remains controversial. |
| Endothelial progenitor cell | Circulating cells that have the potential to proliferate and differentiate into mature endothelial cells. Studies of EPCs have been complicated by the use of the same terminology to define at least two different cell populations that have different cell surface markers, different cell sources, and different abilities to differentiate into mature endothelial cells in vitro and in vivo. There is a critical need to develop a consensus definition of EPCs with particular emphasis on the functional capabilities of these cells. |
| Mesenchymal stromal (stem) cell | Cells of stromal origin that can self-renew and have the ability to differentiate into a variety of cell lineages. Initially described in a population of bone marrow stromal cells, they were first described as fibroblastic colony-forming units subsequently as marrow stromal cells, then as mesenchymal stem cells, and most recently as multipotent mesenchymal stromal cells or MSCs. MSCs have now been isolated from a wide variety of tissues, including umbilical cord blood, Wharton's jelly, placenta, adipose tissue, and lung. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has recently updated the minimal criteria for defining (human) MSCs (Table 3). MSCs have been described to differentiate into a variety of mature cells types and may also have immunomodulatory properties. |
| Fibrocyte | A cell in the subset of circulating leukocytes that produce collagen and home to sites of inflammation. The identity and phenotypic characterization of circulating fibrocytes is more firmly established than that for EPCs. These cells express the cell surface markers CD34, CD45, CD13, MHC II and also express type 1 collagen and fibronectin. |
| Bronchiolar stem cell | A term applied to a rare population of toxin (i.e., naphthalene)-resistant CCSP-expressing cells that localize to neuroepithelial bodies and the bronchoalveolar duct junction of the rodent lung. These cells proliferate infrequently in the steady-state but increase their proliferative rate following depletion of transit-amplifying (Clara) cells. Lineage tracing studies indicate that these cells have the differentiation potential to replenish specialized cell types of the bronchiolar epithelium. Human correlates have not yet been identified. |
| Bronchioalveolar stem cell | A term applied to a small population of cells located at the bronchoalveolar duct junction in mice identified in vivo by dual labeling with CCSP and SPC and by resistance to destruction with toxins (i.e., naphthalene). In culture, some of the dual labeled cells also express Sca1 and CD34, self renew, and give rise to progeny that express either CCSP, pro-SPC, or aquaporin 5 leading to speculation that a single cell type has the capacity to differentiate into both bronchiolar (Clara cells) and alveolar (type 1 and 2 pneumocytes) lineages. At present, the relationship of the cells studied in vitro to those observed by dual labeling in vivo is unclear. Human correlates have not yet been identified. |
Reprinted with permission from Reference 2.
The first session, “Endogenous Lung Progenitor Cells/Lung Cancer Stem Cells,” following an overview of the field by Paul Simmons (University of Texas) and respective presentations by Susan Reynolds (National Jewish), Ed Morrisey (University of Pennsylvania), Barry Stripp (Duke), Majd Mouded (University of Pittsburgh), and Kerstin Sinkevicius (Boston Children's Hospital), reviewed the current state of knowledge of endogenous progenitor cell populations, mechanisms regulating their behavior, and their potential to initiate or augment repair. This included lessons learned from lung development, the role of the local microenvironmental niches, and consideration of lung cancer progenitor cells. Key points emphasized during this session were that stem cells are operationally defined not solely by their intrinsic developmental potential but by their interaction with the microenvironments in which they reside. Further, the stem cell niche is a dynamic “temporal” niche with the capacity to modify stem cell behavior/readout in different contexts. Moreover, stem cell–associated markers are not uniquely expressed by stem cells and are unreliable predictors of the “stem” or “progenitor” cell potential of isolated cells. Validation by functional assays and lineage-tracing studies, particularly when interrogating isolated cells where histomorphometric spatial and positional cues are lost, are increasingly valid and necessary.
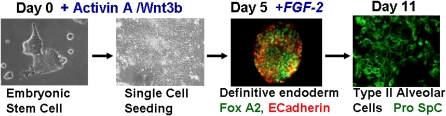
The second session, “Embryonic Stem Cells, iPS, and Lung Regeneration,” included a featured talk on nuclear reprogramming and pluripotency by Konrad Hochedlinger (Massachusetts General) that was followed by presentations from Carolyn Lutzko (Los Angeles Children's Hospital), Darrell Kotton (Boston University), Rick Wetsel (University of Texas) and Peter Lelkes (Drexel University), which highlighted developments in these areas. One of the notable advances in this area is the improved sophistication in directing ESCs in vitro through stages involved in generation of definitive endoderm and subsequently into cells with some phenotypic characteristics of type 2 alveolar epithelial cells. Comparable data demonstrates that iPS can be similarly manipulated toward definitive endoderm and potentially toward lung epithelial cells. However, full phenotypic and functional characterization of putative lung cells derived from ESCs or iPS remains controversial and is also an area where more rigorous methods are required. Novel data was presented demonstrating for the first time that in vivo administration of ESC-derived cells could mitigate experimentally induced lung injury. Whether this reflects engraftment of the cells in the lung or a heretofore unrecognized paracrine effect is not yet clear.
The third session, “Bioengineering Approaches to Lung Regeneration,” featured an overview by Dame Julia Polak (Imperial College London) followed by presentations from Christine Finck (University of Connecticut), David Hoganson (Massachusetts General), Edward Ingenito (Brigham and Women's), Viranuj Sueblinvong (Emory University), and Charles Vacanti (Brigham and Women's), which explored new and developing areas in bioengineering approaches for cell therapies of lung diseases. Advances in scaffold systems, understanding the role of cyclic mechanical forces, and other related areas were discussed. The fourth and fifth sessions, “MSC Immunomodulation of Immune and Inflammatory Responses,” and “EPCs and Clinical Trials in Lung Diseases” highlighted recent advances in cell therapy approaches for lung diseases. After the featured presentations in each session given by, respectively, Armand Keating (University of Toronto) and Mervin Yoder (Indiana University), presentations by Ryang Hwa Lee (Texas A and M), Conrad Liles (University of Toronto), Michael Matthay (UCSF), Daniel Weiss (University of Vermont), Serpil Erzurum (Cleveland Clinic), Asrar Malik (University of Illinois), Judith Shizuru (Stanford), and Duncan Stewart (University of Ottawa) highlighted different areas of advance.
The final session, “Summation and Direction,” featured a review of the current state of lung engraftment by Diane Krause (Yale) that was followed by perspectives given by representatives of the NHLBI, FDA, and each of the sponsoring nonprofit respiratory disease organizations, a presentation on ethics and policy issues in stem cell research by Jeffrey Kahn (University of Minnesota) and a summary by David Scadden (Harvard Stem Cell Institute). The conference concluded with vigorous discussion on future research and funding priorities led by Darwin Prockop (Texas A and M). As in previous conferences, discussion was spirited as to how and when to proceed to further clinical investigation in addition to the recent trials for COPD and for pulmonary hypertension. It was agreed that strong emphasis must continue be placed on animal models of human lung diseases, with a focus on studies that incorporate relevant functional outcome measures. Nonetheless, the safety and initial efficacy results obtained with the trial of MSCs in COPD and the increasing body of data demonstrating efficacy in other inflammatory and immune-mediated lung injury disease models suggests a potential role in inflammatory and immune-mediated lung diseases even in the absence of a comprehensive understanding of the mechanisms by which the MSCs are acting.
All participants acknowledged that the role of endogenous lung progenitor cells and of cell therapy approaches for lung diseases remains a timely and exciting area of study. Nonetheless, there are many areas in which our understanding of the processes and mechanisms remain poorly understood. Recommendations for areas of continued and future investigation are presented in Table 2. Following a review of the current literature, more extensive details on each session are presented below. The conference program, executive summaries for each speaker, and abstracts from the poster sessions are included in the on-line supplement.
TABLE 2.
OVERALL CONFERENCE SUMMARY RECOMMENDATIONS:
| Basic |
| • Strong focus must be placed on understanding immunomodulatory and other mechanisms of cell therapy approaches in different pre-clinical models. |
| • For studies evaluating putative engraftment, advanced histologic imaging techniques (e.g., confocal microscopy, deconvolution microscopy, electron microscopy, laser capture dissection, etc.) must be used to avoid being misled by inadequate photomicroscopy and immunohistochemical approaches. Imaging techniques must be used in combination with appropriate statistical and other analyses to maximize detection of rare events. |
| • Elucidate mechanisms of recruitment, mobilization, and homing of circulating or therapeutically administered cells to lung epithelial, interstitial, and pulmonary vascular compartments for purposes of either engraftment or of immunomodulation. |
| • Encourage new research to elucidate molecular programs for development of lung cell phenotypes. |
| • Investigate the mechanisms and potential roles of epithelial/endothelial/mesenchymal transitions (EMT, MET, etc.) in lung injury and/or repair/remodeling. |
| • Comparatively identify and study endogenous stem/progenitor cell populations between different lung compartments and between species. |
| • Develop robust and consistent nomenclature for the endogenous cell populations. |
| • Develop more sophisticated tools to identify, mimic, and study ex vivo the relevant microenvironments for study of endogenous lung progenitor/stem cells. |
| • Develop functional outcome assessments for endogenous progenitor/stem cells. |
| • Elucidate how endogenous lung stem and progenitor cells are regulated in normal development and in diseases. |
| • Identify and characterize putative lung cancer stem cells and regulatory mechanisms guiding their behavior. |
| • Elucidate mechanisms by which embryonic and induced pluripotent stem cells develop into lung cells/tissue. |
| • Develop disease specific populations of ES and iPS, for example for CF and α1-antitrypsin deficiency with the recognition that no strategy has yet been devised to overcome the propensity of ES and iPS cells to produce tumors. |
| • Explore lung tissue bioengineering approaches such as artificial matrices and three-dimensional culture systems for generating lung ex vivo and in vivo from stem cells, including systems that facilitate vascular development. This is predicted to be an area of rapid expansion. |
| • Evaluate effect of mechanical forces including stretch and compression pressure on development of lung from stem and progenitor cells. |
| • Identify additional cell surface markers which characterize lung cell populations for use in visualization and sorting techniques. |
| • Disseminate information about and encourage use of existing core services, facilities, and weblinks. |
| • Actively foster inter-institutional, multi-disciplinary research collaborations and consortiums as well as clinical/basic partnerships. Include a program of education on lung diseases and stem cell biology. A partial list includes NHLBI Production Assistance for Cellular Therapies (PACT), NCRR stem cell facilities, GMP Vector Cores, small animal mechanics and CT scanner facilities at several pulmonary centers. |
| Translational |
| • Support high-quality translational studies focused on cell-based therapy for human lung diseases. Pre-clinical models will provide proof of concept; however, these must be relevant to the corresponding human lung disease. Disease-specific models, including large animal models where feasible, should be used and/or developed for lung diseases. |
| • Basic/translational/pre-clinical studies should include rigorous comparisons of different cell preparations with respect to both outcome and toxicological/safety endpoints. For example, it is not clear which MSC or EPC preparation (tissue source, laboratory source, culture scheme, etc.) is optimal for clinical trials in different lung diseases. |
| • Incorporate rigorous techniques to unambiguously identify outcome measures in cell therapy studies. Pre-clinical models require clinically relevant functional outcome measures (e.g., pulmonary physiology/mechanics, electrophysiology, and other techniques). |
| Clinical |
| • Proceed with design and implementation of initial exploratory safety investigations in patients with lung diseases where appropriate, such as ARDS/ALI, asthma, and others. This includes full consideration of ethical issues involved, particularly which patients should be initially studied. |
| • Provide increased clinical support for cell therapy trials in lung diseases. This includes infrastructure, use of NIH resources such as the PACT program, and the NCRR/NIH Center for Preparation and Distribution of Adult Stem Cells (MSCs; http://medicine.tamhsc.edu/irm/msc-distribution.html), coordination among multiple centers, and registry approaches to coordinate smaller clinical investigations. |
| • Clinical trials must include evaluations of potential mechanisms and this should include mechanistic studies as well as assessments of functional and safety outcomes. Trials should include, whenever feasible, collection of biological materials such as lung tissue, BAL fluid, blood, and so on for investigation of mechanisms as well as for toxicology and other safety endpoints. |
| • Partner with existing networks, such as ARDSNet or ACRC, nonprofit respiratory disease foundations, and/or industry as appropriate to maximize the scientific and clinical aspects of clinical investigations. |
| • Integrate with other ongoing or planned clinical trials in other disciplines in which relevant pulmonary information may be obtained. For example, inclusion of pulmonary function testing in trials of MSCs in graft versus host disease will provide novel and invaluable information about potential MSC effects on development and the clinical course of bronchiolitis obliterans. |
| • Work with industry to have access to information from relevant clinical trials. |
BACKGROUND AND REVIEW
A comprehensive summary of relevant published literature since the 2007 workshop through the fall of 2010 is presented below. Please see the reports from previous workshops for a comprehensive review of previous literature in the field (1, 2). Readers are also referred to a number of general reviews (3–48) and specific reviews of each of the topics below that have been published over the past approximately 3 years.
Endogenous Lung Stem and Progenitor Cells
Endogenous tissue stem cells are thought to contribute to tissue maintenance and repair. Best characterized in the intestine, these cells are rare, undifferentiated, and are localized to specialized niches within each tissue. Tissue-specific stem cells exhibit self-renewal capacity and give rise to daughter cells, termed as transit amplifying cells, which in turn give rise to the more specialized or differentiated cells specific to that organ. It has also been proposed that some of the differentiated cell types can be induced to a mitotically active state. In this capacity, these cells have been termed facultative progenitor cells. Such facultative progenitor cells perform general tissue functions on a daily basis but can enter the mitotic cell pool for tissue injury repair. Thus, a facultative progenitor cell pool functions as a large and broadly distributed pool of reparative cells and can supplement the reparative capacity of the tissue stem cell. Alternatively, the facultative progenitor pool may serve for routine tissue homeostasis and regeneration whereas the tissue stem cells only come to play in more extreme situations of injury. These topics are further explored in several recent reviews (9, 10, 22, 25, 26, 28, 29, 34, 37, 46).
It remains unclear if paradigms and hierarchies described for endogenous stem and progenitor cells in organs such as the intestine also apply to the lung. The lung is a complex organ containing many distinct cell types that are distributed in several different regional microenvironments along the pulmonary tract (Figure 1) (49). Consequently, whereas the identification of cells that can proliferate under steady state or injury conditions has been relatively straightforward, characterization and classification of mitotically active putative endogenous stem and progenitor cells into a hierarchy has been challenging. The difficult questions are 1) if the cells should be arranged into a hierarchy; and 2) if there is a hierarchy, how the cells should be arranged. Finally, if there is a hierarchy, what are the defining characteristics of cells at different levels of the hierarchy: is it differentiation and proliferation potential as in the intestine? Or are there other more important issues that are specific to the lung that might include cell cycle time and frequency? Further, analyses and interpretations of potential stem and progenitor cells in different regions of the lung have been complicated by lack of agreement on definition, terminologies, and functions of the putative stem cells populations. This continues to complicate the field despite efforts to come to an agreement on terminologies. A proposed list of terminologies was included in the report of the 2007 conference and is repeated here (Table 1). Although there is some degree of consensus with the proposed definitions, there is still disagreement and ongoing debate and discussion. Nonetheless, analyses of lung stem and progenitor cells in animal models, particularly the mouse, have led to important advances over the past 5 years. It seems most likely that distinct stem and/or progenitor cell populations maintain specific anatomic regions of the lung (Figure 1).
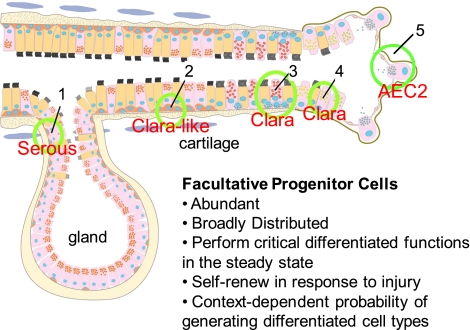
Figure 1.
A graphic representation of putative stem cell niches in the airway epithelium. (1) Basal cells in the gland duct; (2) surface basal cells typically present in the intercartilaginous zone; (3) variant Clara cells associated with pulmonary neuroendocrine cell (PNEC) bodies; (4) variant Clara cells present at the bronchiolar–alveolar duct junction. See text for further explanation. Reproduced with permission from Reference 49.
Evidence for distinct airway epithelial progenitor cell populations comes predominantly from studies in mice, but there is data from proliferation analyses in other species including hamster (50), rat (51), ferret (52), nonhuman primate (53, 54), and human (55). Much of the evidence for different airway progenitor cell populations and hierarchies has also come from studies in which selective ablation of epithelial cells was achieved through exposure of mice to toxic chemicals such as naphthalene or SO2. Naphthalene selectively depletes facultative bronchiolar progenitor populations such as Clara cells, whereas SO2 exposure can deplete upper airway cells allowing for assessment of underlying basal cell facultative progenitors. More recently these methods have been combined with genetic lineage tracing to evaluate differentiation potential of putative stem and progenitor cell populations (56–63). These analyses have identified five populations of airway epithelial cells in the mouse that have the ability to enter the cell cycle after injury to the lungs and thus be considered as facultative progenitor cells: basal (64), Clara-like, Clara, pulmonary neuroendocrine, and alveolar type 2 cells (56–58). Notably the difference between Clara cells and Clara-like cells further highlights the difficulty in identifying facultative progenitor cells. A Clara-like cell is a cell that expresses Clara cell secretory protein (CCSP) in the tracheobronchial epithelium rather than Max Clara's original definition for the Clara cell, which specified a terminal bronchiolar location. It is clear from ultrastructural analyses that there are epithelial secretory cells throughout the proximal to distal axis (54). However, they express a different repertoire of secreted proteins. Clara and Clara-like cells are also differentially sensitive to naphthalene as well as other toxic agents. Most importantly, the proximal Clara-like cells are derived from a different progenitor than the distal airway Clara cells (46).
Recent data suggests that, in mice, the facultative progenitor cell pool accounts for much of the airway epithelial cell replacement during normal homeostasis but can also significantly contribute to tissue repair after cellular injury (65). These studies have also been the basis for isolation of putative stem cells using flow cytometric analyses of lung homogenates to sort by cell surface markers. Cell populations isolated in this manner have been functionally tested in vitro (66, 67) or through ectopic transplantation approaches (68–70) and have suggested that these cells have the ability to regenerate various populations of airway and/or alveolar cells. However, reconstitution of the healthy or injured lung as a final, but critically important goal for the lung stem cell field, has not yet been achieved.
The trachea and large airway compartment contains two major epithelial cell lineages, basal and secretory/ciliated cells. A subpopulation of basal epithelial cells had been previously implicated as a tissue stem cell on the basis of morphology in combination with analysis of differentiation potential or label retention (69–71). Lineage tracing studies suggested that these cells express cytokeratins 5 or 14 and could serve as precursors for differentiated airway epithelial regeneration following injury (59–61). In contrast, pulse-chase studies have demonstrated that the Clara-like cell serves as a self-renewing cell type and as the progenitor for ciliated airway cells in rats (72–75). However, lineage tracing of Clara-like cells demonstrates that the Clara-like cells do not replenish all cell types in the tracheal and proximal airways (59, 60). This lack of consensus on whether a stem cell participates in repair of the upper airway epithelium reflects in part a failure to use all of the necessary stem cell analysis methods in a single study. This remains a significant limitation in the field. However, despite this, the use of multiple injury models in vivo, including exposure to SO2, detergent, and naphthalene, as well as in vitro analyses (61, 76–78), suggest that a subset of basal cells serve a role as either tissue stem cells or facultative progenitor cells of the upper airway in mice. Furthermore, similar conclusions have been derived using human cells in ex vivo or in vitro culture systems (59–61). Overall, the data for this compartment, while still limited, provides the strongest evidence for a lung tissue–specific stem cell to date. However, the question remains as to whether the classical hierarchies defined for the intestine and hematopoietic system can be applied to the lung. If constrained to the classical model, all the data at present cannot yet be accounted for. It seems reasonable to define a model that works for the lung and consider the possibility that different compartments of the lung use distinct types of hierarchies. For instance, in the tracheobronchial region there are at least two facultative progenitor cell pools: the basal cells and the Clara-like cells. In the steady state, they take care of their own lineages. However, after injury progenitor-progeny relationships change. Incorporating these data into a revised model, and recognizing the fact that the data supporting a role for a lung tissue stem cell in repair comes from experimental systems in which extensive injury was induced, may provide a more cohesive explanation of lung tissue repair.
In mice, the predominant epithelial cell of the smaller airways is the nonciliated Clara cell, which exhibits characteristics of a facultative progenitor cell after injury to ciliated airway epithelial cells. However, unlike transit-amplifying cells in tissues with higher rates of epithelial turnover, such as intestine, Clara cells exhibit a low proliferative index in the steady state, are broadly distributed throughout the bronchiolar epithelium, and contribute to the specialized tissue function. Earlier pulse-chase studies identified the Clara cell as a progenitor for ciliated cells (72–75). More recently, these experiments were repeated using lineage tracing methods. The latter data set confirmed the injury/repair studies and provided the new observation that steady-state Clara cells self-renewed and that Clara cells functioned as a progenitor for distal airway ciliated cells during homeostasis (63). However, increasing data suggest fundamental differences between Clara-like and Clara secretory cells in proximal versus distal airways, respectively (60, 61), and thus the situation is more complicated than previously appreciated.
In another widely used approach, the Clara cell–specific toxin, naphthalene, has been used extensively to deplete the bronchiolar Clara cell pool. This toxin is metabolized by the cytochrome P450 isozyme found in Clara cells and parenteral treatment (i.e., intraperitoneal administration) results in dose-dependent depletion of the Clara cell population in mice. A population of naphthalene-resistant cells, termed variant Clara cells (vCE), was identified as a bronchiolar stem cell (56, 64, 65). vCE are located within discrete microenvironments that include the neuroepithelial body and bronchoalveolar duct junction (66, 67). In the bronchoalveolar duct junction, naphthalene-resistant Clara cells stain both for CCSP and for pro-Surfactant Protein C (SPC) (66). However, these cells were not shown to express the stem cell characteristic of label retention. Interestingly, the proSPC/CCSP dual positive cells increased in number after naphthalene injury and during compensatory lung growth following unilateral pneumonectomy in mice (66, 79). Further, when pro-SPC/CCSP dual positive cells were isolated using methods developed for enrichment of type 2 alveolar epithelial cells, some of the dual-labeled cells exhibited a unique cell surface phenotype, Sca1pos/CD34pos/CD45neg/CD31neg. These cells were found to proliferate in culture and give rise to progeny expressing CCSP, pro-SPC, or aquaporin 5 (66). As such, these cells were termed bronchioalveolar stem cells (BASCs). However, lineage tracing methods demonstrated that the bronchiolar and alveolar domains were distinct in the normal lung (80). Further, no contribution of lineage tagged Clara cells to alveolar epithelia was detected during steady state homeostasis or after hyperoxic injury (60, 63). These data suggest that cultured lung progenitor cells may assume a broader spectrum of cell phenotypes than their in vivo counterparts.
The complexity of the bronchiolar stem cell hierarchy is further confounded by the methods used to identify and evaluate the putative stem cell. Importantly, the presence of the abundant Clara cell facultative progenitor cell pool necessitates use of extreme levels of injury to activate the putative stem cell. Thus, the phenotype and function of the putative stem cell must be viewed in the context of epithelial injury and collateral damage to other tissue types and systemic effects. Further, the limited number of differentiated cell types found in the bronchiolar region, Clara, ciliated, and PNEC, has made it difficult to distinguish the putative stem cell from the facultative progenitor cell (29). A further complication is that the putative tissue-specific stem cell is quiescent during the response to some injuries such as ozone depletion of the ciliated cell pool (81) and the fact that bronchiolar stem cells did not play any greater role in normal airway epithelial homeostasis and turnover than did the abundant pool of facultative progenitor Clara cells (65). This study suggests that the neuroepithelial body–associated vCE likely function as a reserve population that can function in either normal maintenance or more relevantly following depletion of the facultative progenitor pool of vCE. Nonetheless, additional studies are needed to further confirm and clarify this hypothesis both in mouse as well as in human lungs.
Overall, it is possible that various cells thought to have stem or progenitor cell properties in the lung represent phenotypic variants of the same cell population(s). The diversity of interpretations highlight the widely recognized need for markers that are specific for the functionally distinct cell populations, more precise tools for lineage tracing, and further underscoring the importance of the in vivo microenvironment on cell behavior (82). For example, stem cell antigen (Sca-1), originally described as a marker of murine hematopoietic stem cells, has now been described as a marker for putative bronchiolar stem cells and fibroblastic progenitor cells in the lung (67, 83, 84). Notably, two recent studies used flow cytometric purification methods for enrichment of putative bronchiolar stem cells from mouse lung. The first of these suggested that naphthalene-resistant bronchiolar progenitor cells (the putative bronchiolar stem cell) have a Sca1+/CD34− cell surface phenotype and had low autofluorescence. However, this study did not use in vitro or in vivo functional analyses, thus limiting its interpretation (67). The second study suggested that clonogenic bronchiolar progenitor cells expressed EPcam and that co-culture of this cell with Sca1+ mesenchymal cells promoted differentiation to Muc1+ cells. The significance of Muc1 expression is unknown, as this marker is not typically used in in vivo analyses (83). Thus, although progress is being made in clarifying the identity and role of bronchiolar progenitor cells in mice, the role(s) of these cell populations in both normal homeostasis and in response to more severe injuries remains unclear. Moreover, little corresponding data as yet exists in other animal models or in human lungs.
In parallel with the identification of lung stem and progenitor populations, recent investigations have explored cell signaling and other regulatory mechanisms that manage putative airway progenitor populations in mice. For example, manipulations of Kras, p27, MAPK, p18, protein kinase C iota, or Pten have been shown to induce an expansion of bronchiolar progenitor and Bronchoalveolar Stem Cell (BASC) numbers and also to enhance lung tumorigenesis (85–94). Other cell signaling pathways such as Wnt/β-catenin, Hedgehog, and Notch cell are implicated in stem cell function in the lung and other tissues (80, 95–97). However, whereas stimulation of Wnt/β-catenin cell signaling appears to promote airway submucosal gland development, it inhibits differentiation of bronchiolar stem cells in the lung and does not appear to play a key role in maintenance or repair of the bronchiolar epithelium (80, 96, 97). The precise role of these and other pathways in endogenous lung stem and progenitor cells remains to be determined. The possibility remains that other endogenous stem or progenitor populations exist, and there is much room for additional information on regulatory mechanisms and pathways as have been elucidated in other epithelial progenitor cell populations (98).
Although it is attractive to speculate that lung diseases may in part be a consequence of endogenous airway stem cell failure, more studies are needed to draw direct connections. In particular, little is known of progenitor cell function in chronic diseases such as emphysema. More suggestive information is available for the genetic lung disease, cystic fibrosis (CF). The airway epithelium in patients with CF contains cuboidal cells that express primitive cell markers, including thyroid transcription factor and cytokeratin 7 (99). Neuroepithelial cells also express the CF transmembrane conductance regulator protein (CFTR), the defective protein in patients with CF that appears to play a role in neuropeptide secretion (100, 101). CFTR−/− mice contain fewer pulmonary neuroendocrine cells during embryonic development but increased numbers of these cells after birth (102). These observations suggest that endogenous airway progenitor cell pathways in CF lungs may be altered but this has not been extensively investigated or further clarified.
The question of an alveolar tissue-specific stem cell remains topical but less well explored than the airway. This deficiency is due in part to the vast reparative potential of this alveolar compartment and the critical role of gas exchange for organism viability. Alveolar epithelial reparative potential is centered on the alveolar type II cell (AECII) and the long held concept that AECII cells are precursors for AECI cells (74, 103, 104). The lack of an AECII-specific toxic agent is a further limitation to analysis of alveolar stem cells. These issues are being addressed through the use of stem cell markers and development of cell type cytotoxic genetic strategies (103, 105). In neonatal mice, a population of putative progenitor cells that expresses CCSP, stem cell antigen (SCA-1), stage-specific embryonic antigen 1 (SSEA-1), and the embryonic stem cell marker Oct-4 have been identified (106, 107). These cells were able to form epithelial colonies and differentiate into both type 1 and type 2 alveolar epithelial cells. However, the lineage relationship between alveolar type 2 and 1 cells has been challenged, and alveolar type 1 cells are a mitotic cell type in vitro (104). As indicated above, additional studies are needed to resolve these controversies.
Several studies suggest that tissue stem cells may be targets for environmental agents including pneumotrophic pathogens. Airway stem or progenitor-like cells were susceptible to infection with the severe acute respiratory syndrome (SARS) virus, raising the possibility that endogenous lung progenitor cells may be specific disease targets (106). Comparably, the basal epithelial cells of the trachea and upper airways appear more susceptible to infection with the common cold rhinovirus (108). However, it is likely that viruses such as SARS and rhinovirus target a wide range of respiratory epithelial cells in addition to progenitor cells. Endogenous progenitor cells may also be attractive candidates for targeting with gene transfer vectors that provide sustained expression. For example, intratracheally administered recombinant adeno-associated vectors may preferentially target vCE in adult mice whereas recombinant lentivirus vectors administered into the amniotic fluid may preferentially target airway progenitors in fetal mouse lungs (39, 109, 110).
Less information is available regarding the progenitor cell populations that maintain other cell populations such as interstitial, smooth muscle, or endothelial cells in the lung (24). Recently, several groups identified what appear to be resident mesenchymal stromal (stem) cells in mouse and sheep lung as well as in human nasal mucosa and in neonatal and adult human lungs (111–121). What role, if any, these cells might play in the repair of lung tissue, or in immune surveillance and immunomodulation, is unclear (115, 117, 120).
In addition to the role of endogenous lung stem and progenitor cells in repair from lung injury, increasing information suggests that mature differentiated lung cells may change their phenotype in response to environmental challenge and/or injury (122–124). This has been best described with alterations in the phenotype of AECII to AECI cells and vice versa (104). Epithelial–mesenchymal transition is a recognized phenomenon during development, however, its physiologic or pathophysiologic role in the adult lung remains unclear and controversial except perhaps for lung cancer and the development of metastases (125–129).
Overall, major challenges remain in this field. The obvious issues are development of adequate cell-specific markers and lineage tracing tools. Existing cell type–specific markers in particular are in need of refinement as increasing knowledge is obtained about the inherent plasticity of lung cell types and as previously identified lineages are deconstructed. These issues have been the focus of recommendations to the NIH and other funding agencies from previous conferences and continue to be at the forefront (Table 2). Also, problems remain with the terminology and methodologies used in different laboratories. For example, disagreement or lack of consistent interpretation and application of seemingly straightforward terminology, such as “differentiated versus undifferentiated” and “specialized versus unspecialized,” has continued to impede progress. As mentioned above, a list of suggested terminology is illustrated in Table 1, but even this is likely to need revision in the near future.
Lung Cancer Stem Cells.
There is intense interest in the connections between endogenous stem or progenitor cells and cancer stem cells. Cancer stem cells have been defined in transplantation assays as the cell subset that is capable of propagating disease. These cells are frequently termed tumor initiating cells and are hypothesized to be the cells that maintain tumor progression and disease resistance (130–132). Cancer stem cells are best described in leukemias, breast cancer, and brain cancer, but increasing evidence suggests lung cancers may contain rare populations of cancer stem cells (7, 131, 133, 134). These studies indicate that the different types of lung cancer are initiated from distinct cell types and that the lung tumor–initiating cell may or may not have the same identity as the cancer stem cells that maintain established tumors. Given the diversity of lung cancer subtypes, this may not be surprising. Purification and characterization of the tumor-initiating cell and/or the cancer stem cell is an important aspect of studies designed to test the cancer stem cell hypothesis (135, 136). CD45 negative side population cells have been identified in several human lung cancer cell lines and exhibit tumorigenic properties when subcutaneously implanted into immunotolerant mice (137). Side population cells have also been identified in clinical lung cancer specimens (137). Dual positive pro-SPC/CCSP positive cells, the bronchioalveolar stem cells (BASCs) discussed in the above section, have also been suggested as tumor-initiating cells (66) A number of recent reports implicate CD133+ cells as conferring resistance to chemotherapy and having tumor initiating properties (138–143). Recent studies have begun elucidating cell signaling and gene expression pathways including Pten, protein kinase C (iota), Wnt, hedgehog, c-kit, Akt, and others that may play roles in transformation of endogenous progenitor cells into lung cancer cells (85–93, 144). However, despite growing data, more work is needed to clarify the connections between endogenous lung progenitor cells, their potential roles as lung cancer stem cells, and most importantly, their potential role as therapeutic targets.
Bone marrow–derived or circulating MSCs, EPCs, and fibrocytes may contribute to development of primary and metastatic lung carcinoma and other malignancies in mouse models. These cells function, in part, by providing a supportive stroma for the cancers and/or by participating in tumor vascularization (145–159). In contrast, MSCs and EPCs have been demonstrated to home to areas of tumor development, and engineered EPCs and MSCs, as well as Hematopoietic Stem Cells (HSCs), have been used to suppress tumor growth in mouse tumor models of primary lung cancers, metastatic lung cancers, and of other cancers metastatic to the lung (148, 160–179). Cell based treatment may thus be useful in lung cancer therapeutics.
Structural Engraftment and Functional Effects of Circulating or Exogenously Administered Stem or Progenitor Cells
Structural engraftment.
A number of publications over the past approximately 10 years initially suggested that a variety of bone marrow–derived cells including hematopoietic stem cells (HSCs), mesenchymal stromal (stem) cells (MSCs), multipotent adult progenitor cells (MAPCs), and other populations, as well as stem and progenitor cells isolated from other tissues such as adipose, placenta, cord, blood, and others, could structurally engraft as mature differentiated airway and alveolar epithelial cells or as pulmonary vascular or interstitial cells. This literature was predominantly based on studies in mice using techniques that evaluated histologic demonstration of donor-derived marrow cells in recipient lungs after systemic administration of marked donor cells (green flourescent protein [GFP]-labeled cells, male cells to female recipients, and other approaches), usually, but not always, after myeloablation of the recipient mouse bone marrow (reviewed in References 1, 2). Previous lung injury was usually necessary to observe engraftment, although lung injury did not always result in an increase of apparent engraftment (180, 181). Furthermore, the myeloablative regimen used, usually total body irradiation, was also felt to contribute to lung injury and be required for evident engraftment (182, 183). A smaller body of literature in clinical bone marrow and lung transplantation also demonstrated varying degrees of apparent chimerism in lungs of the transplant recipients (reviewed in Ref. 1). However, whether epithelial engraftment does in fact occur to any significant degree remains controversial (20, 21, 184, 185). Several technical issues contributed to misinterpretation of results in the initial reports including inadequate microscopic techniques in which donor-derived cells superimposed on resident airway or alveolar epithelial cells were not effectively discriminated. Exquisite care and sophisticated microscopic approaches, including confocal and deconvolution techniques, must be used to effectively demonstrate potential engraftment (1, 184, 185). Furthermore, a variety of leukocytes, notably airway and alveolar macrophages, reside in the lung. Many of the early reports did not use antibodies directed against CD45 or other leukocyte markers to exclude the possibility that cells of donor origin detected in airway or alveolar epithelium were donor-derived leukocytes rather than epithelial cells. Other tools, such as the use of GFP as a marker of donor-derived marrow cells obtained from transgenic GFP mice in recipient mouse lungs can be subject to error in the presence of autofluorescent cells commonly found in the lung (186).
Nonetheless, with a better understanding of, and better approaches to, the possible confounding factors discussed above, some reports suggest that engraftment of donor-derived airway and/or alveolar epithelium can occur at low levels after perturbation of airway or alveolar epithelium in models of lung injury. This has been observed with MSCs of bone marrow or cord blood origin (187), side population cells (188, 189), plastic adherent marrow stromal cells (189–193), or full marrow transplantation after a myeloablative regimen (189, 194, 195). These studies have tended to use more sophisticated microscopic and other analytical techniques. Nonetheless, epithelial engraftment in general is rare except under conditions discussed below. In parallel, recent studies also continue to demonstrate rare apparent engraftment of pulmonary interstitium and vasculature after total marrow transplant in a variety of injury models (196–198).
These reports suggest that engraftment of lung tissues with circulating or donor-derived cells can occur under certain conditions, usually following previous perturbation through induction of lung injury. However, there are many variables yet to be explored that may increase epithelial, interstitial, or pulmonary vascular engraftment with circulating or donor-derived cells. More vigorous injury regimens, such as serial naphthalene administration to deplete airway epithelial cells, coupled with busulfan to suppress endogenous bone marrow, appear to have increased engraftment of exogenously administered bone marrow cells (199). Comparably, several reports suggest that chronic or progressive lung injury may result in more substantial engraftment of AECII cells and of interstitial and pulmonary vascular cells with donor-derived cells in mouse or rat models (191, 196). However, not all chronic lung injury models result in more substantial engraftment (200). The effect of age of either donor cells or of recipients is also less well-explored; although transplantation of whole marrow into 1-day-old mouse pups, using a variety of conditioning regimens, did not increase the number of bone marrow-derived cells over those observed after administration of total marrow to adult mice (195). The route of administration of donor-derived cells is also less well-characterized, as most studies have investigated engraftment after systemic administration of donor cells. Direct intratracheal administration of MSCs or other marrow-derived cells appears to result in apparent epithelial engraftment. However, levels of apparent engraftment are variable, and when initial engraftment is observed, it is not sustained (201, 202).
The types of stem/progenitor cells or fully differentiated nonpulmonary cells that might engraft as lung epithelium, interstitium, or pulmonary vasculature, remain to be fully explored. In addition to existing studies of HSCs, MSCs (of bone marrow, cord blood, and placental tissues origin), EPCs, and fibrocytes, the possibility remains that there may be other cell populations that could be recruited to the lung or that localize to the lung after systemic or other route of administration. For example, two different populations of cells isolated from human amniotic tissue—human amniotic epithelial cells (hAECs) and a population of multipotent cells termed human amniotic fluid stem cells (hAFSCs)—have both been described to apparently engraft in limited amounts in mouse lungs (203, 204). The hAFSCs localized to distal airway where they expressed thyroid transcription factor 1 (TTF1) and surfactant protein C (SPC), whereas the hAECs localized to both distal airway and to areas of lung injury where they expressed a range of surfactant proteins. Notably, administration of hAECs to bleomycin-injured immunocompetent mice resulted in abrogation of lung injury without apparent host response to the cells (203). Comparably, hAECs administered to rats with experimentally induced myocardial infarction were able to reduce the extent of infarction and also apparently acquired phenotypic characteristics of cardiomyocytes (205). This suggests that these cells may be used in other xenogeneic models of lung injury and may also exhibit paracrine effects that modulate lung injury, a topic discussed further in the section on mesenchymal stromal cells below. A population of circulating bone marrow–derived CD45+/CXCR4+/cytokeratin+ cells has been described to participate in re-epithelialization of denuded tracheal xenografts (206). A recent report has also described a population of CCSP-expressing adult marrow cells that appear to more robustly lodge and engraft in lung after either systemic or intratracheal administration (Figure 2) (201). Other sources of stem or progenitor cells, such as adipose tissues, also have not been extensively characterized for their ability to engraft as lung tissue as have bone marrow and cord blood origin cells (187, 207). However, the ability to structurally engraft in adult lung may not solely be a property of stem or progenitor cells. Intratracheal administration of neonatal mouse lung fibroblasts resulted in apparent alveolar and interstitial engraftment, and engraftment was higher in areas of elastase-induced lung injury (208). Intratacheal administration of fibroblasts, transduced to express angiopoietin-1, mitigated acute lung injury and inflammation in mice (209). Comparably, intratracheal administration of AECII cells results in rare engraftment in areas of injured lung after administering bleomycin to rats (210). Notably, bleomycin-injured rats that received the AECII cells had less histologic injury and decreased hydroxyproline content. Most recently, AECII cells, derived in vitro from human embryonic stem cells, were able to both engraft and to mitigate bleomycin-induced lung injury in mice (211). These results suggest that lung injuries might be amenable to a variety of cell therapy approaches.
Figure 2.
Endogenous bone marrow Ccsp+ cells can repopulate the airway epithelium. (A–K) Representative double immunofluorescence staining for GFP (green) and Ccsp (red) of lung sections from bone marrow transplant recipient mice that received Ccsp+Sca-1+ cells from GFP+ donors. Asterisks indicate a GFP+ cell that was Ccsp–. White arrows point to donor-derived Ccsp+ cells. Red arrows point to Ccsp+ cells that were not donor derived. (A) GFP+ (positive control) lung. (B) Isotype staining of lung from bone marrow transplant recipient. (C–E) Bone marrow transplant recipients had a wild-type background. (C) Low-power image. (D and E) High-power images. (F–H) Bone marrow transplant recipients were Ccsp–/– 60 days following bone marrow transplant without naphthalene. (F) Low-power image. (G and H) High-power image. (I and K) Thirty days after bone marrow transplant with naphthalene injury. (I) Low-power image. (J and K) High-power image. Scale bars: 20 μm. Original magnification: ×60 (A, B, C, F, and I); ×90 (D, H, and J); ×120 (E, G, K). Reprinted with permission from Reference 201.
For those studies in which more robust evidence of engraftment has been suggested, the potential role of fusion is still not fully elucidated. Bone marrow-derived cells can be induced to fuse with lung epithelial cells in vitro, but in vivo investigations suggest that fusion is a rare occurrence (212, 213). One study suggests that fusion of donor-derived marrow cells with AECII cells can occur in mouse lungs but that the Y chromosome may be lost from the resulting heterokaryon cells (214). Nonetheless, fusion of exogenously administered cells with resident lung cells is thought to be a rare occurrence of uncertain physiologic significance.
Mechanisms by which circulating or systemically administered stem or progenitor cells might be recruited to the lung remain poorly understood. A number of studies demonstrate that after systemic administration, cells initially localize in the lung, and that lung injury results in increased localization and/or retention of marrow-derived cells in the lung (215–217). Whether this represents formation of cell emboli in the lung or a specific adherence to pulmonary vascular adhesion or other molecules remains unclear. In another study, embolization of systemically administered MSCs in lung was felt to result in secretion of an anti-inflammatory protein, TSG-6 (218). The timing of cell administration after lung injury can also influence recruitment and phenotypic conversion. Systemic administration of MSCs 4 hours after lung irradiation resulted in apparent engraftment of cells as epithelial and vascular endothelial cells (219). However, MSCs administered at later time points appeared to engraft as interstitial cells and participate in the development of fibrosis (215, 219). Recipient immune responses also play significant yet poorly characterized roles in retention of cells in lung (220). Commonly used approaches of sex-mismatched transplantation or cell administration may also result in clearance of cells (214). The range and identity of chemotactic soluble mediators released by injured lung cells and the role of up-regulation of adhesion molecules with which circulating cells might interact remains poorly understood (reviewed in 1 and 2, 215, 221–227). As with engraftment, a number of factors including age of donor or recipient, type of cell administered, route of administration, and so forth, might affect recruitment of cells to the lung.
Comparably, the mechanisms by which stem or progenitor cells isolated from adult tissues might be induced to acquire the phenotype of lung epithelial, interstitial, or vascular endothelial cells, remain poorly understood. In vitro studies demonstrate that soluble factors released from lung epithelial cells or from injured lung homogenates can induce the expression of lung epithelial markers in several types of marrow-derived cells, possibly through the activation of β-catenin and other cell-signaling pathways (228–230). Comparably, coculture of embryonic stem cells with fetal pulmonary mesenchyme can promote the development of cells expressing phenotypic markers of lung epithelial cells (231). One novel mechanism of inducing phenotypic change might involve the release of membrane-derived microvesicles, a recently appreciated means of intercellular communication that involves horizontal transfer of mRNA and proteins between cells (232, 233). Nonetheless, despite continuing interest in the possibilities of engraftment of exogenous cells in the lung, emphasis has moved to other areas, notably immumomodulatory effects of administered cells and ex vivo tissue engineering.
Endothelial progenitor cells.
In the past decade, circulating bone marrow–derived cells putatively similar to embryonal angioblasts have been identified (234, 235). Termed “endothelial progenitor cells, or EPCs,” these cells were reported to exhibit the potential to proliferate and differentiate into mature endothelial cells. Increasing evidence demonstrates that EPCs play a role in pathogenesis of a wide variety of lung diseases including pulmonary hypertension, pulmonary fibrosis, airway diseases, including asthma, COPD, acute lung injury, lung cancer, and most recently bronchopulmonary dysplasia and obstructive sleep apnea in children (236–256). However, studies of EPCs in lung diseases have been hampered by a lack of consensus regarding identification of these cells (257). Early investigations relied almost exclusively on the use of flow cytometry in conjunction with immunostaining to identify and enumerate these cells in bone marrow as well as in the circulation. Furthermore, when similar techniques were used in different studies, a different group of markers were used. The lack of a unique cell surface maker to identify an EPC continues to complicate comparative assessments for similar disease processes. As a result, a growing consensus in the field encourages the use of functional assays both in vitro and in vivo in conjunction with the use of flow cytometry and immunohistochemistry (IHC) to not only enumerate EPCs, but to better characterize their true ability to form functional endothelium. A critical area for future study remains to develop a consensus-based approach to definition and use of EPCs with particular emphasis on functional capabilities of these cells.
At present, there appear to be two major types of EPCs that can be isolated from human peripheral blood by differential culture, an approach that avoids having to have prior certainty of surface selection markers for EPCs (257, 259). The first, termed “early EPCs” are characterized by early growth in vitro, CD34/CD31/CD14 positivity, the inability to form tubes in a matrigel tube forming assay, and high levels of cytokine secretion. These cells are now known to be derivatives of the hematopoietic lineage differentiating along the myeloid lineage in response to certain cytokines and chemokines. The other type of EPC, termed “late outgrowth EPCs” or “outgrowth endothelial cells (OECs)” or “endothelial colony forming cells (ECFC)” is characterized by CD31, CD144, CD146, and CD105 positivity, lack of CD45, CD14, or CD115 expression, and the unique ability to spontaneously form human blood vessels when implanted in a gel into immunodeficient mice that inosculate with murine vessels to become a part of the systemic circulation. Each of these cell types may have a unique potential in lung microvascular repair, with early EPCs having a role as paracrine cells, and late EPCs functioning more specifically in restoring vascular structures of the lung. Intravenous infusion of each type of cell in an animal model has demonstrated their ability to preferentially localize to lung (259). The two EPC types may have a synergistic role in restoring vascular structure and function when infused together (258). Further characterization of these two EPC types, including methods to enhance their numbers ex vivo could have implications for the development of therapy specific to the phenotypic abnormalities of a given lung disease. In addition, the source of EPCs, for example adult peripheral blood versus umbilical cord blood, may also influence the differentiation potential of EPCs (260, 261). A schematic and summary of current classification and characterization of human EPCs is depicted in Figure 3.
Figure 3.
Schematics depicting current thinking in isolation and identification of endothelial progenitor cells. Reprinted with permission from Reference 19.
The number of circulating EPCs has been correlated with a variety of clinical variables in several lung disease states, demonstrating the potential utility of EPCs as biomarkers. Although increased circulating EPC numbers correlate with survival in and acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and are associated with less residual lung damage in patients with pneumonia (238, 239), increased numbers do not necessarily correlate with better outcomes or more normal physiology in all lung diseases. For example, an decrease in the number of circulating EPCs in patients with COPD was associated with more abnormal spirometry (241), although a different study showed that levels of circulating EPCs were inversely correlated with COPD disease severity (240). Increased numbers of circulating EPCs also portended worse survival among those with non–small cell lung cancer (146, 253, 254). In asthma, numbers of circulating EPCs were increased compared with nonasthmatic control subjects, but this did not correlate with clinical outcomes (237).
Several clinical factors have been implicated in the mobilization of EPCs, and mechanisms for their effects have begun to be elucidated (220). Hypoxia appears to be a stimulus for EPC mobilization and recruitment, whereas hyperoxia is correlated with decreased circulating EPCs, particularly in preterm EPCs (222, 260, 262). These features may play a role in bronchopulmonary dysplasia in premature infants and neonates exposed to high oxygen levels (255, 262). They also suggest that EPCs could contribute to lung repair after acute lung injury. A recent study demonstrated that systemic administration of a population of bone marrow–derived angiogenic cells improved lung alveolar architecture after neonatal hyperoxia exposure in rats (263). Some degree of engraftment was observed, but it is unclear if structural contribution of the engrafted cells, or rather paracrine or other growth stimulating effects, were responsible. Defective lung development or defective lung repair in the setting of protracted inflammation and injury may result in part from an inadequate contribution of local or circulating EPCs. Age has been previously reported to be inversely correlated both with EPC number and also in the ability of EPCs to home to ischemic tissues based on age (264, 265). This may be mediated through the inability of aged tissues to normally activate the hypoxia-inducible factor-1 α-mediated hypoxia response (264). Use of HMG-CoA reductase inhibitors has been demonstrated to have a beneficial effect on the mobilization of EPCs (266). This may be related to the effect of this class of drugs in the prevention of EPC apoptosis in response to noxious stimuli, including the effects of TNF-α and IL-1β, thereby enhancing EPC survival and differentiation (267). Other pathways recently implicated in mobilization of EPCs include circulating vascular endothelial growth factor (VEGF) and CXCL12 (268, 269). Hypoxia-induced release of insulin-like growth factor 2 may also play a prominent role in EPC homing (270).
Goals of increasing numbers of EPCs, or developing methods to enhance their mobilization may not be appropriate for all diseases that affect the lung, particularly for lung cancers (253, 254). Although levels of circulating EPCs may serve as biomarkers for disease progression or severity (253, 254, 271), EPCs may have an effect on the development of lung tumor vasculature and homing to sites of lung metastases as well as in other cancers (146, 149, 150, 155, 157, 272). Because neovascularization involves the recruitment of EPCs from the bone marrow, these cells are a logical target for antiangiogenesis therapy. For example, an investigational drug, TK 1–2 (the kringle domain of tissue-type plasminogen activator), was demonstrated to be useful in blocking adhesion, differentiation, and migration of ex vivo human EPCs in vitro and also in decreasing tumor growth and vascularity in a SCID mouse tumor model (156). These findings suggest that blocking EPCs could be an important therapy in the prevention of cancer progression. Additionally, after systemic injection, EPCs localize to the lung and appear to home to metastatic tumors in the lung through as yet poorly understood mechanisms (160, 162). This suggests that modification of EPCs to express suicide genes or other therapeutic molecules could be potentially used in cell-based therapy approaches for lung cancer (160, 162). Mechanisms controlling mobilization and homing of EPCs to the lung remain poorly understood and are the subject for more intense investigation.
A number of studies in mice and dogs have demonstrated a role for exogenously administered EPCs in vasculogenesis and vascular repair in experimental models of pulmonary hypertension (44, 197, 246, 247, 251, 252, 273–279). Furthermore, EPCs can be transduced to express proangiogenic factors such as endothelial nitric oxide synthetase (eNOS) or inhibitors of smooth muscle cell proliferation such as calcitonin gene-related peptide and appear to home to sites of endothelial damage and lung injury (276). EPCs can also preferentially localize to areas of injured lung after systemic administration and may also have paracrine effects to decrease inflammation (216, 277, 278). As such, two pilot trials of autologous EPC administration for primary pulmonary hypertension conducted at Zhejiang University in Hangzhou, China in adult and pediatric patients demonstrated increased 6-minute walk capacity and improved hemodynamic variables, including mean pulmonary artery pressure, pulmonary vascular resistance, and cardiac output, 12 weeks after systemic administration of autologous EPCs with conventional therapy when compared with patients receiving conventional therapy alone (280, 281). Importantly, no adverse effects of EPC administration were noted, although long-term follow-up is pending. A therapeutic trial of administration of autologous early outgrowth EPCs transduced to express eNOS for patients with pulmonary hypertension, the Pulmonary Hypertension and Cell Therapy (PHaCeT) trial, has been initiated at St. Michael's Hospital in Toronto and the Jewish General Hospital in Montreal. As of March 2010, the PHACeT trial has completed enrollment of the first two dose-panels with three patients receiving a total of 7 million early growth EPCs transfected to overexpress human eNOS in panel 1 and three more patients receiving 23 million cells in panel 2 (information courtesy of Duncan Stewart, M.D., F.R.C.P.C., University of Ottawa). The cell delivery procedure was well tolerated and there were no safety concerns. Notably, the first six patients showed a remarkable reduction in total pulmonary vascular resistance (PVR) over the course of the 3-day delivery period, which might represent the effect of increased NO release by the engineered EPCs within the pulmonary microcirculation. The trial's Data Safety Monitoring Board has approved moving to panel 3, which calls for a total of 50 million cells, in three divided doses over 3 days. Completion of the third dose panel will be followed by enrollment of an additional three patients at the highest tolerated cell dose, which should provide sufficient support to move forward with the design of a randomized controlled trial that can assess potential efficacy of this cell therapy approach in pulmonary arterial hypertension (PAH).
Going forward, clarification of the specific cell types involved in the process of neoangiogenesis will result in less confusion surrounding the term EPC. It is apparent that the early outgrowth cells are hematopoietic derivatives that can be identified by their classic cell surface markers, morphology, and in vitro hematopoietic colony-forming activity or in vivo reconstitution of the blood lineages in immunodeficient mice. In contrast, the ECFC or OEC are rare endothelial cells that display clonal proliferative capacity and in vivo vessel forming ability that can be discriminated by several cell surface antigens from the hematopoietic subsets. Roles for B lymphocytes, macrophages, dendritic cells, and red blood cells and/or their products in the neoangiogenesis process have also been described. Thus, going forward, rather than use the nondescript term “EPC,” the field may wish to consider use of the terminology that best describes the cell population under investigation to be more specific about which cell, in which patients, at which points in time, are involved in a particular aspect of the neoangiogenic process (19, 40, 41, 282).
Circulating fibrocytes.
Circulating fibrocytes were first described as a subset of circulating leukocytes that produced collagen and homed to sites of inflammation (283, 284). The identity and phenotypic characterization of circulating fibrocytes is more firmly established and these cells are described by the cell surface markers CD34, CD45, CD13, and MHC II and also express type 1 collagen and fibronectin. Circulating fibrocytes have been implicated in the pathogenesis of several lung diseases including both mouse and clinical models of pulmonary fibrosis, pulmonary hypertension, the subepithelial fibrosis that can develop in severe asthma, and in clinical bronchiolitis obliterans in patients with lung and bone marrow transplants (4, 8, 285–292). Further levels of circulating fibrocytes may be an indicator of worse prognosis in IPF (287, 293). Interestingly, numbers of circulating fibrocytes were highest in patients experiencing an acute exacerbation and the numbers returned to baseline with recovery (293). Several chemokines including stromal derived factor-1 (SDF-1) and the CCR2 and CCR5 axes have been implicated in recruitment of circulating fibrocytes to fibrotic lungs but overall mechanisms of fibrocyte recruitment to lung are poorly understood (286, 294–296). Matrix metalloproteinase expression may also be involved in recruiting fibrocytes to injured or post-transplant lungs (297). Similarly the mechanisms by which fibrocytes are induced to undergo phenotypic transformation into fibroblasts and myofibroblasts and contribute to fibrogenesis in lung are poorly understood although both haptoglobin and cysteinyl leukotrienes have been implicated (298, 299). Hypoxia is a potent stimulus for release of a variety of factors by pulmonary vascular endothelium that both serve to recruit fibrocytes as well as induce phenotypic conversion to fibroblasts or myofibroblasts (288). Notably, depletion of circulating fibrocytes abrogated hypoxia-induced perivascular remodeling in rats (288). Circulating fibroblasts may also be important in lung cancer development or metastasis. Circulating fibrocyte precursors found in blood of lung cancer patients contributed to tumor development when systemically administered to NOD SCID mice engrafted with human lung cancer xenografts (300). Bone marrow-derived cells may also contribute to fibroblasts and myofibroblasts in tumor stromal tissue (145). These results suggest that specific inhibition of fibrocytes or their use as drug delivery vehicles may also be important therapeutic targets in pulmonary vascular disease.
Mesenchymal Stromal (Stem) Cells.
MSCs were first described in 1,968 as an adherent, clonogenic, non-phagocytic, and fibroblastic-like population of bone marrow cells (301). The nomenclature has changed over the years as MSCs were initially termed fibroblastic colony forming units (302), subsequently as marrow stromal cells, then as mesenchymal stem cells (303), and most recently as multipotent mesenchymal stromal cells or MSCs (303). MSCs have now been isolated from a wide variety of tissues including umbilical cord blood, Wharton's jelly, placenta, and adipose tissue (304–311). More recently cells with characteristics suggesting identity as MSCs have been isolated from adult mouse lungs (113–116), human nasal mucosa (117) and from lungs of both human neonates and human lung transplant recipients (118–121). Human lung MSCs appear to have some immunomodulatory capabilities similar to those of bone marrow-derived MSCs (120) whereas ovalbumin sensitization and challenge increase the number of lung MSCs in mice (115). This suggests that the lung MSCs may be involved in regulation of local inflammatory immune responses. However, the exact identity and physiologic role of putative lung MSCs is not yet clear. Other multipotent progenitor populations have been described in lungs from other species but the exact identify and roles of these cells is also not well understood (111, 112, 312).
However, definition and investigation of MSCs continues to be confounded by several issues. MSCs isolated from different sources generally express comparable cell surface markers and differentiate along recognized lineage pathways. However, differences in gene expression, lineage tendencies, and other properties have been described among MSCs isolated from different sources (313–322). As with EPCs, many of the published studies have used different definitions and characterizations of MSCs. This has complicated comparative assessments of published studies. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has defined minimal criteria for defining (human) MSCs (303) (Table 3). It is hoped that rigorous adherence to these criteria will help to focus comparative investigations of their potential use in lung diseases. Furthermore, fibroblasts have also been described as capable of exhibiting properties consistent with MSCs, as have adult mouse lung–side population cells (113, 116, 323). This includes both differentiation ability as well as immunomodulatory activities, discussed below.
TABLE 3.
THE MESENCHYMAL AND TISSUE STEM CELL COMMITTEE OF THE INTERNATIONAL SOCIETY FOR CELLULAR THERAPY (ISCT) MINIMAL CRITERIA FOR DEFINING MESENCHYMAL STEM CELLS
| 1. Plastic adherence in standard tissue culture conditions |
| 2. Expression of CD73, CD90, and CD105 |
| 3. No expression of CD 11b, CD14, CD19, CD34, CD45, CD79α, or of HLA-DR |
| 4. Differentiation in vitro to osteoblasts, adipocytes, and chondroblasts |
Adapted with permission from Reference 303.
The field of MSCs is complicated by a number of other factors. One is significant differences among MSCs from different species. Mouse MSCs from bone marrow have been particularly difficult. Early cultures grow slowly and are heavily contaminated by hematopoietic cells that usually require immunoselection to remove (reviewed in Refs. 1, 2, 14, 36). With expansion, the cells pass through crisis, after which the cultures propagate rapidly as the few surviving cells are transformed and can become tumorigenic. In addition, MSCs from different mouse strains have different requirements of medium for optimal growth and different surface epitopes. These features present a significant obstacle for many potentially important experiments in mouse models for diseases and in transgenic mice. These features of murine MSCs were frequently not accounted for in publications in which cells termed “mesenchymal stem cells” or “MSCs” were used. As a result there are many important observations in a long list of publications that are difficult to interpret within the context of MSC biology and therapeutics. MSCs from rat bone marrow also present unusual features including strain differences, rapid initial growth, and a tendency of colonies to shed cells that generate new colonies (14, 36). Also, rat and mouse MSCs have a tendency to develop genomic instability with extensive expansion. At present, human MSCs from bone marrow are the best characterized MSCs but also present some challenging features. Early passage cells plated at low densities are enriched for small, rapidly replicating cells that are highly clonogenic and have a high potential for differentiation. However, the colonies formed by the MSCs have several surprising features. The cells within clonal, single-derived colonies become heterogeneous as the colonies expand with cells in inner regions expressing a different profile of genes than cells in the outer regions. The colonies formed on recloning cells from a single colony generate new colonies that are heterogeneous in size and differentiation potential. Also, the cells within clonal, single-derived colonies become heterogeneous as the colonies expand with cells in inner regions expressing a different profile of genes than cells in the outer regions. Human MSCs that are extensively expanded at high density retain some of the properties of low density cultures but are enriched for large, slowly propagating cells with diminished clonogenicity and differential potential. A further complication is that parallel preparations of MSCs from bone marrow aspirates isolated from the same normal donors in the same session can differ in features such as rate of propagation and potential to differentiate.
In addition, culture variables, including culture surface composition and stiffness (324–328), oxygen environment (329–335), mechanical forces (336–339), temperature (340), and other factors such as culture density can profoundly influence phenotype and behavior of MSCs. This includes culturing in three- versus two-dimensional scaffolds as discussed below in the section on lung bioengineering. As discussed below, research on MSCs is confused by the marked differences in MSCs from mice and human MSCs in terms of properties such as cell surface epitopes, ease of expansion, and genomic stability. It is also becoming increasingly apparent that different inflammatory environments can profoundly influence MSC behavior (341–343). Furthermore, there is growing evidence that MSCs are heterogeneous and that different MSC subtypes exist (344–346). To address some of the variations in properties of cultured MSCs, an NCRR/NIH-sponsored center offers standardized preparations of murine and human MSCs that investigators can use as benchmarks for preclinical experiments (http://medicine.tamhsc.edu/irm/msc-distribution.html).
Nonetheless, despite these issues, substantial progress continues to be made with MSCs in lung injury and repair. Initial emphasis was on the potential ability of MSCs to acquire epithelial phenotype and engraft as structural lung cells. However, despite the ability to induce expression of markers expressed by either airway or alveolar epithelial cells in in vitro culture systems, engraftment with MSCs, as with most other cell types investigated so far, is a rare event of uncertain physiologic significance in lung (1, 2, 14, 15, 36,). As such, emphasis has increasingly shifted toward the profound immunomodulatory, anti-inflammatory, and non-immunogenic properties of MSCs. MSCs have ability to regulate hematopoietic cells and to secrete multiple regulatory molecules such as growth factors and anti-inflammatory cytokines which can modulate immune and inflammatory responses (reviewed in 347–353). MSCs also inhibit the proliferation and function of a broad range of immune cells, including T cells, B cells, natural killer (NK) cells and dendritic cells (DCs) in in vitro models systems (Figure 4), (354–378). Notably, MSCs inhibit T lymphocyte proliferation, activation, and cytokine release in response to either alloantigens or to mitogenic stimuli through a dose-dependent direct suppressive effect on proliferation. The mechanisms of MSC inhibition of immune effector cell proliferation and function in vitro are only partly understood and both direct cell–cell contact as well as release of soluble mediators has been proposed (Figure 4) (354–378). MSCs have also been described to have capacity to act as antigen-presenting cells in in vitro model systems, a property influenced by exposure to IFNγ and to TGFβ (379). Furthermore, MSCs may also affect actions of cells involved in innate immune responses, notably macrophages (380–382).
Figure 4.
Schematic illustrating the range of in vitro immune-modulating effects described for mesenchymal stem cells (MSCs). DC = dendritic cell; HGF = hepatocyte growth factor; IDO = indoleamine 2,3-dioxygenase; IFN-γ = interferon γ; Ig = Immunoglobulin; IL = Interleukin; IL-1RA = Interleukin-1 receptor antagonist; Mac = Macrophage; NK = natural killer; PGE2 = prostaglandin E-2; SDF-1 = stem-cell derived factor 1; TNF-α = tumor necrosis factor-α; TGF-β1 = transforming growth factor- β1; TLR = Toll-like receptor; VEGF = vascular endothelial growth factor. Reprinted with permission from Reference 412.
It is also becoming increasingly apparent that different inflammatory environments can profoundly influence MSC behavior (341–343). MSCs express a wide variety of chemokine and cytokine receptors, including those for TNFα, IL-4, IL-17, and IFNγ. The IL-171 receptor in particular is expressed in high abundance and IL-17 has been described as a proliferative stimulus for MSCs (383). Expression of MHC and costimulatory molecules can be altered by exposure to inflammatory mediators commonly found in vivo such as IFNγ and TGFβ (376–379). Chemotaxis and migration toward a wide variety of mediators including SDF-1, TNFα, macrophage migration inhibitory factor (MIF), TGFβ, hepatocyte growth factor (HGF), and others has been described (223, 225, 226). Expression of specific cell surface molecules including CD44 and MARCKS (224, 227) have been implicated in directed MSC chemotaxis. MSCs also express toll-like receptors including the endotoxin receptor TLR4 (384–387). Activation of the TLRs in MSCs can produce a wide variety of effects, many of which are still being elucidated. Furthermore, expression of TRLs by MSCs can be influenced by a number of factors including exposure to bacterial toxins and to a variety of inflammatory mediators. Hypoxia, characteristic of many lung injuries and diseases, can also alter MSC expression of cell surface molecules and secretion of soluble mediators by MSCs (331, 335). Overall, this illustrates a rapidly growing body of evidence demonstrating that MSCs are malleable and can be significantly influenced by local inflammatory environments, including those found in lung injuries.
MSCs also seem to be relatively nonimmunogenic due to their low constitutive expression of major histocompatibility complex (MHC) type 1 and lack of constitutive expression of MHC type 2 and the costimulatory molecules CD80, CD86, and CD40 (reviewed in Refs. 347–353) This allows administration of allogeneic MSCs without significant host responses. Although the frequency of MSCs in the adult bone marrow is low (less than 0.1%), once isolated from bone marrow or from other tissues, MSCs can be expanded ex vivo, which makes it possible to manufacture these cells for potential therapeutic purposes. MSCs can also be relatively easily transduced or genetically manipulated to deliver or to secrete selected disease-modifying molecules (148, 161–179, 388–390). Overall, these properties of MSCs make them an attractive potential therapeutic tool as vectors for delivery of disease-specific treatment substances or as immunomodulatory agents. As such, MSCs have now been used in clinical trials for inflammatory and autoimmune diseases such as graft versus host and Crohn's disease as well as in acute myocardial infarction (391–396). One of the important findings to come out of the published data is that administration of MSCs appears to be safe and well tolerated, even in severely ill patients. Although the long-term effects of MSC administration are unknown, there appear to be no significant short-term effects.
In this context, a steadily increasing number of articles demonstrate efficacy of either systemic or intratracheal MSC administration in a growing spectrum of lung injury models in mice (31, 35, 42, 397–399). This includes mouse models of acute lung injury and fibrosis (230, 400–412), sepsis (413–417), pulmonary hypertension (418–420), ischemia re-perfusion injury (421, 422) bronchopulmonary dysplasia (423–425), bronchiolitis obliterans (426), asthma (427–433), COPD (434–442), and other pulmonary diseases (198, 200, 443, 444) (summarized in Table 4). MSCs have also been demonstrated to have efficacy in models of primary and metastatic lung cancer (164, 167–171, 177). Recent studies have also demonstrated efficacy of MSC administration in endotoxin-injured human lung explants (445). Although the mechanisms of the MSC effects are not completely understood, soluble mediators released by the MSCs appear to play important roles in amelioration of acute and fibrotic injuries in the different models. Some of those implicated in the different model systems include IL-1 receptor antagonist, IL-10, keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), angiopoietin 1, and TGFβ. Transduction of the MSCs to express either angiopoietin-1 or KGF further decreases endotoxin-mediated lung injury presumably through abrogation of endotoxin-mediated endothelial injury (403, 445). MSCs appear also to act in part by decreasing the increased endothelial permeability found in acute lung injury and also by secreting antibacterial peptides (400, 445–447). MSCs may also exert effects on lung inflammation and injury through primary interactions with the immune system rather than through direct actions in lung. For example, a growing body of evidence suggests that MSCs ameliorate allergic airways inflammation in mice by promoting a Th1 phenotype in vivo in antigen-specific CD4 T cells and in circulating antigen-specific immunoglobulins as a means of abrogating Th2-mediated lung injury (427, 428, 432). As such, MSCs appear to be capable of a spectrum of effects in different lung injuries.
TABLE 4.
MESENCHYMAL STEM CELL TABLE
| Injury Model | Experimental Model, Route, and Timing of Treatment | MSC Source | MSCs Modified | Syn or Allo | Outcome Compared to Injury Effects | Potential Mechanisms of MSC Actions | Cell Controls? |
|---|---|---|---|---|---|---|---|
| Acute Lung Injury | |||||||
| Gupta 2007 (400) | Mouse intranasal LPS Intratracheal MSCs 4 h after LPS |
Mouse BM Plastic Adherent |
No | Syn | • Improved survival • Improved histologic inflammation and edema • Decreased BALF TNF-α, MIP-2 • Increased BALF and serum IL-10 |
None specified (soluble mediators) | Apoptotic MSC, 3T3 fibroblasts • Did not mimic effects on survival or inflammation |
| Xu 2007 (230) | Mouse intraperitoneal LPS Intravenous MSCs 1 h after LPS |
Mouse BM Plastic Adherent CD11b,CD45-depleted |
No | Syn | • Decreased histologic injury and lung edema • Decreased BALF neutrophilia but no change in BALF cytlokines • Decreased systemic inflammation (serum IL-1β, IFN-γ, IL-6, MIP-1α, KC) |
None specified (soluble mediators and cell-cell contact) | Primary lung fibroblasts • Did not mimic effects on histology or edema |
| McCarter 2007 (209) | Rat intratracheal LPS Intravenous cells 24 hr prior to LPS |
Primary skin fibroblasts | Plasmid transfection to express Ang1 or PFLAG | Syn | • Decreased histologic injury and edema • Decreased BALF inflammation • Increased Ang1 levels in lung |
Ang1 secretion | Pflag-transduced fibroblasts Did not mimic effects on histology, underedema, or inflammation |
| Mei 2007 (403) | Mouse intratracheal LPS Intravenous MSCs 30 min after LPS |
Mouse BM Plastic Adherent Texas (Tulane) MSC Core |
Plasmid transfection to express Ang1 or PFLAG | Syn | • Decreased histologic injury • Decreased BALF total cell count • Decreased BALF cytokines and chemokines • Decreased LPS-induced protein leak • Decreased apoptosis • In general, MSC-Ang1 more effective than MSCs |
Ang 1 secretion (other soluble mediators) | No |
| Xu 2008 (404) | Mouse nebulized LPS for 7 successive days Intravenous MSCs 2 h after 1st LPS dose |
Mouse BM Plastic Adherent from Ficoll gradient |
Lentivirus-transduced to express Ang1 | Syn | • Decreased BALF total protein, neutrophils, TNFα at 7 but not 3 d • Decreased lung edema and MPO activity at 7 but not 3 d • Decreased histologic injury • Effects only seen with MSC-Ang1. Control MSCs had no effects. |
None specified (soluble mediators) | No |
| Lee 2009 (445) | Explant human lung intratracheal LPS Intratracheal MSCs or conditioned media 1 h after LPS |
Human BM Plastic adherent Texas (Tulane) MSC Core |
No | Allo | • Decreased histologic injury • Decreased lung permeability/improved alveolar fluid clearance |
KGF secretion | No |
| Iyer 2010 (408) | Mouse intraperitoneal LPS Intravenous MSCs 1 h after LPS |
Mouse BM Plastic Adherent CD11b, CD45-depleted |
No | Syn | • Decreased histologic injury and lung edema • Prevent oxidative stress induced by systemic LPS • Decreased systemic inflammation (serum IL-1β, IFN-γ, IL-6, MIP-1α, KC |
None specified (soluble mediators and cell-cell contact) |
Primary lung fibroblasts • Did not mimic effects on histology or edema |
| Asthma | |||||||
| Cho 2008 (427) | Mouse ovalbumin-induced allergic rhinits Intravenous MSCs on Days 18–20 (during antigen challenge) |
Mouse adipose Plastic adherent |
No | Syn | • Decreased nasal inflammation • Decreased ova-specific IgE, IgG1 • Increased ova-specific IgG2a • Decreased IL4, IL-5, increased IFNγ in cultured splenocytes |
None specified (soluble mediators) | No |
| Goodwin 2011 (432) | Mouse ovalbumin-induced allergic airways inflammation Intravenous MSCs on Days 1 and 7 (during antigen sensitization) |
Mouse BM Texas (Tulane) MSC Core |
No | Syn and Allo | • Decreased airways hyperresponsiveness • Decreased eosinophilic Th2 lung inflammation • Skewing of ova-specific CD4 T lymphocytes from Th2 to Th1 • Decreased ova-specific IgE and IgG1, increased IgG2a |
None specified (soluble mediators) | EDCI-fixed MSCs and primary dermal fibrolasts • partially mimic MSC effects |
| Park 2010 (428) | Mouse ovalbumin-induced allergic airways inflammation Intravenous MSCs on Days 18–20 (during antigen challenge) |
Mouse adipose Plastic adherent |
No | Syn | • Decreased airways hyper-responsiveness • Decreased eosinophilic Th2 lung inflammation • Skewing of OVA-specific CD4 T lymphocytes from Th2 to Th1 • Decreased OVA-specific IgE and IgG1, increased IgG2a |
None specified (soluble mediators) | No |
| Nemeth 2010 (429) | Mouse ragweed pollen-induced allergic airways inflammation Intravenous MSCs on Day 14 (at onset of antigen challenge) |
Mouse BM Plastic adherent CD11b, 45 depleted |
No | Syn and Allo | • Decreased histologic injury and mucus metaplasia • Decreased BALF inflammation (total cell counts, eosinophils, IL-4, IL-13) • Decreased serum IgE and IgG1 • Allo had same effect as syn MSCs |
TGF-β1secretion | Primary dermal fibroblasts • partially mimic MSC effects |
| Bonfield 2010 (430) | Mouse ovalbumin-induced chronic allergic airways inflammation Intravenous MSCs during last week of 4-wk challenge |
Human BM Plastic adherent |
No | Xenogeneic | • No obvious lung inflammatory effects of xenogeneic MSCs in immunocompetent mice • Improved histologic injury • Decreased BALF inflammation (total cells, eosinophils, IL-5, IL-13, IFN-γ) • Decreased serum IgE • Decreased BALF macrophage iNOS mRNA |
None specified (soluble mediators) | Syngeneic mouse bone marrow-derived macrophages • Do not mimic effects on histology, BALF total cell counts, serum IgE, body weight • Other endpoints not assessed |
| Bronchiolits obliterans | |||||||
| Grove 2010 (426) | Mouse subcutaneous trachea allografts Intravenous MSCs at time of transplant |
Mouse BM Plastic Adherent Texas (Tulane) MSC Core |
No | Syn and Allo | • Decreased allograft fibrosis and occlusion • Decreased allograft TGFβ and G-CSF • Increased allograft IL-10 |
None specified (soluble mediators) | • No |
| Bronchopulmonary dysplasia | |||||||
| Aslam 2009 (424) | Neonatal mouse hyperoxia (75% FiO2) Intravenous MSCs 4 d after initiation of hyperoxia |
Mouse BM Plastic adherent or conditioned media Neg selected for: CD11b, 14, 19, 31, 34, 45, 79α Pos selected for: CD73, 90, 105, cKit, Sca-1 |
No | Syn | • Decreased histologic injury • Decreased BALF inflammation • Decreased RV hypertrophy, pulmonary arteriole muscularization • MSC-conditioned media produced same effects |
Heat labile secreted molecule(s) suggested for conditioned media | Pulmonary artery smooth muscle cells or mouse lung fibroblasts • PAMSC, PASMC-CM, or MLF did not mimic effects on RV hypertrophy or BALF inflammation • PAMSC or PAMSC-CM did not mimic effects on histologic injury or vascular remodeling |
| Chang 2009 (425) | Neonatal rat hyperoxia (95% FiO2) Intravenous or intraperitoneal MSCs 5 d after initiation of hyperoxia |
Human umbilical cord blood Plastic adherent |
No | Xenogeneic | • Decreased histologic injury (IP and IT) • Decreased apoptosis (IP and IT) • Decreased myeloperoxidase and IL-6 (IP and IT) • Decreased collagen, α-SMA, TNF-α, TGF-β (IT only) |
None specified (soluble mediators) | Primary human foreskin fibroblast (IT only) • Did not mimic effects on histology • Other endpoints not assessed |
| van Haaften 2009 (423) | Neonatal rat hyperoxia (95% FiO2) Intratracheal MSCs 4 or 14 d after initiation of hyperoxia |
Rat BM plastic adherent | No | Syn | • Decreased histologic injury • Improved capillary density • Improved RV hypertrophy and hemodynamics |
MSC-conditioned media had protective effects in several in vitro assays | Pulmonary artery smooth muscle cell • Did not mimic effects on histology, capillary density, RV hypertrophy, hemodynamics |
| COPD | |||||||
| Shigemura 2006a (435) | Rat elastase following lung volume reduction surgery Direct topical application of ASC- seeded polyglycolic acid at time of surgery |
Rat adipose Plastic adherent |
No | Syn | • Improved histologic repair | HGF secretion | No |
| Shigemura 2006b (436) | Rat elastase Intravenous ASCs 1 wk after elastase |
Rat adipose Plastic adherent |
No | Syn | • Decreased apoptosis, improved histologic repair • Improved gas exchange and exercise tolerance |
HGF secretion | No |
| Yuhgetsu 2006 (437) | Rabbit elastase Intratracheal MSCs 24 hr after elastase |
Rat BM mononuclear cells from Ficoll gradient of total BM | No | Syn | • Improved histology and lung function • Decreased BALF fluid inflammation • Decreased tissue MMP2 and MMP9 expression |
None specified (soluble mediators) | No |
| Zhen 2008 (439) | Rat papain ± 7.5 Gy TBI Intravenous MSCs after papain (timing not specified) |
Rat BM Plastic adherent mononucelar cells from Percoll gradient |
No | Syn | • Decreased histologic injury • Decreased alveolar cell apoptosis |
None specified | No |
| Schweitzer 2010 (440) | Mouse cigarette smoke for 4 mo Intravenous ASCs every other week for 3rd and 4th months of CS exposure | Human or mouse adipose plastic adherent | No | Syn or Xenogeneic | • Decreased histologic injury and caspase activation • Decreased BALF inflammation • Decreased CS-induced MAPK signal transuction • Decreased weight loss • Decreased BM suppression |
None specified | No |
| NOD-SCID mouse (for xenogeneic) human ASCs 3 d after VEGFR blockade | Human or mouse adipose plastic adherent | No | Syn or Xenogeneic | • Human ASCs decreased histologic injury and caspase 3 activation following VEGFR blockade • Emboli if used > 5 × 105 MSCs or if > passage 3 |
None specified | No | |
| Zhen 2010 (442) | Rat papain Intravenous MSCs 2 h after papain |
Rat BM Plastic adherent mononucelar cells from Percoll gradient |
No | Syn | • Decreased histologic injury • Partial restoration of VEGF expression in lung homogenates |
TNF-α–stimulated VEGF secretion by MSCs | No |
| Katsha 2010 (441) | Mouse intratracheal elastase Intratracheal MSCs 14 d after elastase |
Mouse BM Plastic Adherent |
No | Syn | • Decreased histologic injury • Decreased IL-1β • Transient increase in lung EGF, HGF, and secretory leukocyte protein inhibitor |
None clarified (soluble mediators) | No |
| Fibrosis | |||||||
| Ortiz 2003 (401) | Mouse bleomycin Intravenous MSCs immediately or 7 d after bleomycin |
Mouse BM Plastic adherent CD11b, 34, 45-depleted |
No | Syn | • Decreased histologic and inflammatory injury • Decreased hydroxyproline, MMP2, MMP9 content |
None specified (soluble mediators) | No |
| Rojas 2005 (568) | Mouse bleomycin Intravenous MSCs immediately after bleomycin |
Mouse BM Plastic Adherent CD11b, CD45-depleted |
No | Syn | • Increased osteopontin • Decreased histologic injury and lung fibrosis • Decreased inflammatory cytlokines |
None specified (soluble mediators) | No |
| Ortiz 2007 (402) | Mouse bleomycin Intravenous MSCs immediately after bleomycin |
Mouse BM Plastic Adherent CD11b, 34, 45-depleted |
No | Syn | • Decreased systemic inflammation (serum IL-1β, IFN-γ, IL-6, MIP-1α, KC) | None specified | No |
| Zhao 2008 (405) | Rat bleomycin Intravenous MSCs 12 h after bleomycin |
Rat BM plastic adherent mononuclear cells isolated from Percoll gradient of total BM | No | Syn | • Decreased histologic injury • Decreased hydorxyproline, laminin, hyaluronan • Decreased TGF-β, PDFG-A, PDGF-B, IGF mRNA |
None specified (soluble mediators) | No |
| Aguilar 2009 (408) | Mouse bleomycin Intravenous MSCs 8 h and 3 d after bleomycin |
Mouse BM Texas (Tulane) MSC Core Mouse HSCs |
Lentivirus transduced to express KGF | Syn | • MSCs: Decreased lung collagen mRNA and protein, no change in histologic injury • HSCs: decreased collagen, histo injury, aSMA, TNFa, CCL-2, CCL-9 • HSCs: ncreased type 2 cell proliferation |
KGF secretion | No |
| Kumamoto 2009 (409) | Mouse bleomycin Intravenous MSCs 3 d after bleomycin |
Mouse BM Plastic adherent for 2 hours or 9 days CD11b, 31, 45-depleted |
No | Syn | • Decreased histologic injury and hydroxyproline • Decreased inflammatory cells |
None specified (soluble mediators) | No |
| Moodley 2009 (406) | SCID mouse bleomycin Intravenous MSCs 24 h after bleomycin |
Human umbilical cord (Wharton's jelly) Plastic adherent |
No | Xenogeneic | • Decreased histologic injury • Decreased hydroxyproline, type 1 collagen mRNA • Increased MMP2, decreased TIMP-2 |
None specified (soluble mediators) | Primary human lung fibroblasts • No effect on bleomycin induced collagen mRNA or MMP-2 expression • other endpoints not clarified |
| Lee 2010 (411) | Rat bleomycin Intravenous MSCs 4 d after bleomycin |
Rat BM Plastic Adherent |
No | Syn | • Decreased neutrophilic lung inflammation • Decreased BALF proinflammatory cytokines, nitrite, nitrate • Decreased collagen accumulation |
None specified (soluble mediators) | No |
| Cargnoni 2010 (410) | Mouse bleomycin Intravenous, intraperitoneal, or intratracheal cells 15 min after bleomycin |
BALB/c mouse placenta | No | Allo and Xenogeneic | • Improved body weight (allo and xeno, IP and IT) • Decreased histologic fibrosis at D14 but not D9 (allo IV, IP, IT) |
None specified (soluble mediators) | Allogeneic Mouse BCF fetal membrane-derived cells • No effects on lung inflammation at 9 or 14 days (IP or IT) |
| Human term placenta amnion and chorion-derived stromal cells human term placenta-derived epithelial cells used as a mix of 50% hAMCS + hCMSCs and 50% hAECs | No | Allo and Xenogeneic | • No change in lung inflammation at D9 or 14 (allo IV) • Decreased tissue neutrophils on D14 (allo IP, IT) • No change in lung inflammation D3,7,9,14 (Xeno IP,IT) • Decreased severity of histologic injury at D14 (Xeno IP, IT) • Decreased tissue neutrophils on D14 (Xeno IP but not IT) • Increased lung inflammation at D14 in control mice (Xeno IP, IT) |
None specified (soluble mediators) | • Decreased lung fibrosis at D14 (IP or IT) • Stimulated inflammation in PBS control mice at D9 IIP and IT) and D14 (IT) |
||
| Ischemia-reperfusion injury | |||||||
| Manning 2010 (421) | Rat left lung ischemia for 2 h Intravenous MSCs 2 h after injury |
Rat BM Plastic adherent |
Retrovirus-transduced to secrete IL-10 | Syn | • Improved oxygenation • Decreased histologic injury and apoptosis • Improved microvascular permeability • MSC-IL10 generally better than MSC-vector control |
IL-10 secretion other paracrine effects-soluble mediators | No |
| Obstructive Sleep Apnea | |||||||
| Carreras 2010 (443, 444) | Induced obstructive apneas rat | Rat BM Texas (Tulane) MSC Core |
No | Syn | • Increased MSC chemotaxis and adhesion to endothelial cells in presence of apneic serum | None specified (soluble mediators) | No |
| Pulmonary Hypertension | |||||||
| Baber 2007 (426) | Rat monocrotaline Intratracheal MSCs 2 wk after injury |
Rat BM Plastic adherent |
No | Syn | • Improved cardiopulmonary physiology | None specified (soluble mediators) | No |
| Sepsis | |||||||
| Gonzalez-Rey 2009 (414) | Mouse cecal ligation and puncture or intraperitoneal LPS Intraperitoneal MSCs 2 d after injury |
Human adipose | No | Xenogeneic | • Decreased mortality, PLF inflammatory cells and bacterial CFU • Decreased serum/lung/liver/intestine inflammatory cytokines and MPO activity |
None specified | No |
| Nemeth 2009 (413) | Mouse cecal ligation and puncture Intravenous MSCs 1 h prior, concomittant, or 24 h after surgery |
Mouse BM Plastic adherent CD11b, 45 depleted |
No | Syn and Allo | • Improved survival and organ function • Decreased circulating TNF-α, IL-6 • Increased circulating IL-10 |
LPS and TNFα-stimulated MSCs stimulated macrophages to produce IL-10 through cell-cell contact and iNOS-dependent release of PGE2 |
Whole bone marrow, heat-killed MSCs, skin fibroblasts • No effects on survival • Other endpoints not assessed |
| Mei 2010 (415) | Mouse cecal ligation and puncture Intravenous MSCs 6 h after surgery |
Mouse BM Plastic Adherent Texas (Tulane) MSC Core |
Yes | Syn | • Improved survival and organ function • Decreased pulmonary and systemic inflammation • Decreased BALF IL-1β, IL-6, JE, KC, CCL5 • Enhanced bacterial killing by host immune cells • Decreased inflammatory gene expression by microarray • Increased phagocytic gene expression by microarray |
None specified (soluble mediators) | No |
| Yagi 2010 (416) | Rat intraperitoneal LPS or 30% burn Intramuscular MSCs immediately after LPS or burn |
Human MSC Texas (Tulane) MSC Core |
No | Xenogeneic | • Improved tissue injury (lung/liver/kidney) | None specified (soluble mediators) | No |
| Yagi 2010 (417) | Rat intraperitoneal LPS Intramuscular MSCs immediately after LPS |
Human MSC Texas (Tulane) MSC Core |
No | Xenogeneic | • Improved tissue injury (lung/liver/kidney) • Decreased circulating TNF-α, IFN-γ, IL-6 |
soluble TNFR1 secretion | No |
Definiton of abbreviations: Allo = allogenic; BALF = bronchoalveolar lavage fluid; BM = bone marrow; COPD = chronic obstructive pulmonary disease; KC = keratinocyte chemoattractant; KGF = keratinocyte growth factor; LPS = lipopolysaccharide; MIP = macrophage inhibitory protein; MPO = myeloperoxidase; MSCs = mesenchymal stem cells; SCID = severe combined immunodeficiency; Syn = syngeneic; TIMP = tissue inhibitor of metalloproteinase.
However, as noted earlier in the discussion on MSCs, many of the studies discussed above used different preparations of MSCs. As detailed in Table 4, these ranged from populations of heterogenous plastic adherent adipose stromal cells to purified well-characterized bone marrow–derived MSCs obtained from core facilities such as the NCRR/NIH- sponsored Texas (formerly Tulane) Center for Preparation and Distribution of Adult Stem Cells (MSCs) (http://medicine.tamhsc.edu/irm/msc-distribution.html). Furthermore, differences between syngeneic, allogeneic, and xenogeneic MSC administration have been less well explored in lung injury models. As such, whereas the overall consistency of studies demonstrating ability of MSCs to ameliorate different types of lung injuries is encouraging, further rigor must be applied to understand the mechanisms of MSC effects when comparing MSCs isolated by different protocols and obtained from different tissue sources. The specificity of MSC effects compared with potential anti-inflammatory or immunomodulatory effects of other stromal cells such as fibroblasts must be carefully considered (323, 448) (Table 4). For example, systemic administration of primary dermal fibroblasts similarly decreased ragweed pollen or ovalbumin-induced allergic airways inflammation as did MSCs (429, 432). Fibroblasts transduced to express angiopoietin-1 decreased acute endotoxin-induced lung injury (209). However, intratracheal administration of 3T3 fibroblasts did not mimic effects of MSCs in ameliorating endotoxin-induced acute lung injury (400). These results suggest that MSCs and stromal cells such as fibroblasts may share similar anti-inflammatory mechanisms. However, fibroblasts are also heterogeneous depending in part on tissue source, and thus potential anti-inflammatory effects may differ depending on their origin. Further, fibroblasts are less likely to have the same degree of low immunogenicity as do MSCs and can provoke lung inflammation (432). Nonetheless, comparison of MSCs with fibroblasts in different injury models will likely provide important insights as to the anti-inflammatory mechanisms of MSCs and it is important to include appropriate cell controls such as fibroblasts when assessing MSC effects in different lung injury models.
MSCs are also increasingly described as vehicles for delivery of therapeutic genes and proteins (148, 161–179, 388–390). Notably, MSCs can home to tumors, through unclear mechanisms, and serve as vehicles for delivery of chemotherapeutic and other antitumor agents (148, 161–179, 388). This has recently been described in mouse lung tumor models and may provide a viable therapy for lung cancers (164, 167–171, 177, 449, 450). Notably, human bone marrow–derived MSCs engineered to express tumor necrosis factor–related apoptosis inducing ligand (TRAIL) were found to significantly clear tumor metastases in a xenograft model of metastatic human lung cancer in mice (171). Comparably human umbilical cord blood MSCs engineered to express IFNβ were found to significantly attenuate human bronchioalveolar carcinoma xenografts in mice (177). In contrast, MSCs may also contribute to tumor stroma and influence behavior of cancer cells (145, 148, 151, 154). A recent report demonstrated that human MSC administration could promote growth and pulmonary metastases in a mouse model of primary human osteosarcoma (174). Whether marrow-derived cells contribute to development of epithelial cancers remains an active area of investigation (147, 153).
Additional cautions with regard to systemic administration of MSCs have been raised in previous conference reports and are repeated here (1, 2, 16). Most culture strategies use fetal or bovine calf serum. Despite washing of the cells prior to systemic administration, some bovine antigens may remain adherent to cell surfaces and trigger immune reactions as well as decrease potential engraftment in recipient mice or patients (451). Culture of MSCs in medium with lower calf serum content, use of heterologous species–specific serum, or alternative serum substitutes such as platelet lysate, and removal of calf serum antigens before administration are proposed strategies to decrease these potential adverse effects (452–455). Additionally, after intravenous administration, MSCs initially lodge in the lung vasculature before moving through the pulmonary capillary system and on to other organs. However, depending on the preparative regimens used, MSCs can clump and potentially lodge as emboli in lung capillaries (456, 457). Pretreatment of mice with the vasodilator sodium nitroprusside has been proposed as a mechanism of decreasing MSC cell trapping in pulmonary capillaries (458).
Whether MSCs may undergo malignant transformation remains a topic of considerable debate and concern (16, 459–461). Murine MSCs that were extensively expanded in culture through many passages developed chromosomal instability and produced lung sarcomas in mice (456, 457). The results emphasize the greater propensity of mouse MSCs to acquire chromosomal abnormalities with serial passages in culture (456, 462–464). However, extensive culture of almost any mammalian cell in culture can lead to crisis, followed by immortalization and then transformation to tumorigenic cells, as has been well documented for mouse fibroblasts (465). Concerns about the potential tumorigenicity of human MSCs were raised by three reports that described escape from senescence and generation of malignant cells as the MSCs were expanded in culture (466–468). The reports were unexpected insofar as emergence from senescence had not been observed in laboratories that had studied the cells for over a decade. The discrepancies were largely explained by reports from two of the laboratories indicating that their cultures of human MSCs had been cross contaminated with human fibrosarcoma or osteosarcoma cell lines (469). This further highlights the need to develop better markers for defining MSCs and the important need to verify the identity of cells even if received from established sources. It is anticipated that additional strategies to maximize therapeutic use of MSCs while decreasing chance of any adverse effects will develop over the next several years.
The pros and cons of moving toward clinical trials with MSCs and other cell types (EPCs) have been vigorously debated at all three conferences to date. It is anticipated that this debate will continue. Nonetheless, clinical data is accumulating. In a Phase I, double-blind, placebo-controlled trial of PROCHYMAL (ex vivo cultured adult human mesenchymal stem cells) conducted by Osiris Therapeutics Inc. (Columbia, MD) in patients with acute myocardial infarction, an improvement in both FEV1 and FVC was noted in treated patients (396). Although the mechanisms of improvement in pulmonary function in this patient population are not yet understood, these observations stimulated a multicenter, double-blind, placebo-controlled Phase II trial of PROCHYMAL (Osiris Therapeutics Inc., Columbia, MD) for patients with moderate to severe COPD (FEV1/FVC < 0.70; 30% ≤ FEV1 ≤ 70%). The primary goal of the trial, which was initiated in May 2008, is to determine safety of MSC infusions in patients with lung disease. The secondary goal is initial estimation of the potential efficacy of MSCs for decreasing the chronic inflammation associated with COPD thus improving both pulmonary function and quality of life. The trial has recruited 62 patients in six participating U.S. sites. In the 6-month interim data analysis, no infusional toxicities or significant adverse events were reported (470). Notably, a significant decrease in the circulating inflammatory marker C-reactive protein, commonly elevated in patients with COPD, was noted in treated patients as was a trend toward improvement in quality of life indicators. The trial ends in the fall of 2010 and further results will be forthcoming.
Lung tissue bioengineering.
One rapidly growing area of investigation is that using three-dimensional (3D) matrices or other artificial scaffolding for growth of functional lung tissue from stem cells ex vivo and in vivo. These approaches have been increasingly successful in regenerating other tissues including skin, vasculature, cartilage, bone, and more recently, heart and liver (471, 472). Given the complex 3D architecture and structure–function relationships of the lung, this is a daunting task, Nonetheless, there has been significant progress in several areas. Notably, MSCs isolated from amniotic fluid, umbilical cord blood, or bone marrow can be seeded on biodegradable polyglycolic acid or other biosynthetic scaffolds and also onto de-cellularized cadaveric scaffolds for generation of tracheal cartilage for use in repair of congenital tracheal defects and also tendon tissue for use in congenital diaphragmatic defects (321, 328, 473–483). This has recently culminated in successful clinical use of a bioengineered bronchus and more extensive use of bioengineered trachea and bronchi is expected (484).
Three-dimensional culture systems have also been used as matrices for ex vivo lung parenchymal development and for study of growth factors and mechanical forces on lung remodeling (111, 312, 485–492). For example, culture of fetal rat lung suspensions in a 3D glycosaminoglycan scaffold resulted in formation of alveolar-like structures in the scaffold (489). Fetal mouse cells cultured in 3D hydrogels and in synthetic polymer scaffolds resulted in the generation of alveolar-like units (485). Stimulation of the fetal mouse cells in polymer scaffolds with different isoforms of fibroblast growth factor stimulated different patterns of development (486). Fetal rat lung cells cultured in a biosynthetic gelatin matrix and subsequently injected into normal rat lungs induced formation of branching, sacculated epithelial structures reminiscent of lung parenchymal architecture (492). Comparable scaffold approaches have been used to study creation of pulmonary vascular networks and to investigate the effects of vascular endothelial cells on the development of airway and alveolar epithelial tissues (493, 494).
These studies demonstrate the power of 3D culture systems to evaluate lung development and repair. However, many of these studies were not designed to demonstrate functionality of the engineered lung tissue, such as the potential for ventilation and gas exchange. It is also not clear if artificial scaffolds provide the best framework for generating functional lung tissues. Artificial scaffolds neither fully replicate the complexity of the lung architecture nor do they contain all the matrix components essential for normal lung development and function. As such, recent work has focused on the use of more natural models including nasal septa and decellularized whole lung as a more physiologic scaffold in which the native structure of the lung and relevant extracellular matrix components, including collagens, elastin, and laminin, are left intact after removal of cellular material with detergents and other agents (45, 495–500) (Figure 5). In particular, the use of decellularized lungs for growing alveolar cells in culture was first described 25 years ago (496) and has been rediscovered for testing in in vivo applications. The decellularized lungs can be both mechanically ventilated and undergo vascular perfusion to provide more physiologic study of ex vivo lung generation. Two recent studies demonstrated that inoculating decellularized rat lungs with different mixes of fetal rat lung homogenates, endothelial cells, and A549 carcinoma cells resulted in recellularization of the decellularized scaffolds (499, 500). Although the resulting cellular architecture did not fully resemble that of native lung, the recellularized lungs were able to be surgically implanted in rats that had previously undergone unilateral pneumonectomy with short-term survival of the rats and some degree of gas exchange and vascular perfusion achieved (499,500; Figure 5). These are important proof of concept studies that are expected to stimulate a number of comparable studies utilizing de-cellularized lungs, including human lungs.
Figure 5.
(A) Massome's trichrome and pentachrome staining of extracellular matrix proteins collagen and elastin in native and de-cellularized mouse lung. Reprinted with permission from Reference 497. (B, A) Gross image of AC rat lung (left) next to mESC-recellularized lung (right) after culture for 14 days showing contraction of the ECM. (B–D) Two-photon imaging: three-dimensional reconstructions of (B) normal fresh rat lung tissue, (C) AC lung, and (D) recellularized rat lung tissue. Green color corresponds to SHG showing collagen and red to autofluorescence of cells, elastin, and other ECM. (D) Recellularized lung tissue imaged at a depth of 22 mm. Reprinted with permission from Reference 498. (C, A) Tissue-engineered left lung was implanted into adult Fischer 344 rat recipient and photographed approximately 30 minutes later. (B) X-ray image of rat showing the implanted engineered left lung (white arrow) and the right native lung. (C) Hematoxylin and eosin stain of explanted lung. Red blood cells perfusing septa are evident, and some red blood cells are present in airspaces. Scale bar = 50 μm. Reprinted with permission from Reference 499. (D, a) Photograph of left rat chest after left anterior thoracotomy, left pneumonectomy, and orthotopic transplantation of a regenerated left lung construct. Recipient's left pulmonary artery (A), left main bronchus (B), and left pulmonary vein (V) are connected to regenerated left lung pulmonary artery (a), bronchus (b), and pulmonary vein (v). White arrowheads, the recipient's right lung (infracardiac and right lower lobe); black arrowheads, the regenerated left lung construct. (D, b) Radiograph of rat chest after left pneumonectomy and orthotopic transplantation of a regenerated left lung construct. White arrowheads, recipient's right lung; black arrowheads, regenerated left lung construct. Reprinted with permission from Reference 500.
However, there are few studies as yet evaluating whether stem or progenitor cells isolated from adult bone marrow, cord blood, or other sources, including the lung itself, can also comparably form airway or alveolar-like structures when cultivated in 3D matrices, including decellularized whole lungs, or other scaffolding material, or further whether stem or progenitor cells cultured in a such fashion that can be used for functional lung regeneration in vivo. Populations of cells described as adult lung somatic progenitor cells isolated from adult sheep lungs,and cultured in synthetic polymer constructs resulted in the expression of airway and alveolar epithelial markers by the cells (111). Structures resembling lung airways and parenchyma developed when impregnated constructs were implanted subcutaneously in nude mice or inserted into the wound cavity after wedge lung resection in sheep. Implantation of adult sheep lung mesenchymal stem cells cultured in synthetic fibronectin-hydrogel scaffolds into lungs of sheep with experimentally induced emphysema resulted in improved tissue mass and lung perfusion (312, 501). However, it is not clear whether the MSCs differentiated or had paracrine effects in this model. Adipose-derived MSCs, cultured ex vivo in sheets of polyglycolic acid and then applied to wound edges after lung volume reduction surgery in rats, accelerated alveolar and vascular regeneration (436). Data presented in abstract form at this meeting and also at the 2009 and 2010 ATS meetings demonstrate that intratracheal inoculation of murine MSCs into decellularized whole mouse lungs results in growth of the cells along both airways and in parenchymal lung regions with some of the cells demonstrating expression of pro-SPC (502). Intratracheal inoculation of decellularized rat lungs with murine ESCs resulted in apparent differentiation into several epithelial and vascular structures (498). Lung tissue bioengineering with stem cells is projected to be an area of intense investigation.
In addition to genetic regulatory programs and culture approaches, including use of 3-dimensional scaffolds, mechanical forces also play a role in how differentiating tissues respond to gene instructions (503–505). During breathing, lung cells are subjected to complex mechanical loading that includes shearing due to air movement, compression due to pressurization, and stretch due to the expansion of the lung tissue during inhalation (490). In utero, the mechanical forces are, in part, generated by fetal breathing-like movements produced by rhythmic contractions of the respiratory muscles with varying frequency and amplitude (506–509). The normal movement is essential in the development of fetal lung, differentiation of AECII cells, and synthesis of surfactant protein (510–513). Several in vitro studies have demonstrated that application of cyclic mechanical stretch to cultures of mixed fetal rodent lung cells increases SPC mRNA expression compared with cells cultured under static conditions (510–514). The influence of stretch induced by mechanical ventilation in clinical and experimentally induced lung injuries has been an area of growing investigation (515–517). Stretch and other mechanical influences such as fluid shear have been intensely investigated for influence on development of tissues such as cartilage, bone, and vascular structures from MSCs and EPCs as well as from ESCs (336, 518–520). However, there are few studies evaluating effects of physical forces on development of lung epithelial cells or on development of 3D lung structures from endogenous lung progenitor cells or from either embryonic or adult stem cells. Preliminary data presented at this meeting demonstrates that cyclic bi-axial mechanical stretch of MSCs can induce generation of both airway and alveolar epithelial proteins (521).
Further investigation of the effects and mechanisms of mechanical forces on development of lung from stem and progenitor cells is expected to be an area of intense investigation. The obvious challenges in developing complex 3D functional lung tissues ex vivo will be in recapitulating the normal dynamic integrated 3D network of epithelial cells, endothelial cells, fibroblasts, neuronal cells, and inflammatory cells in the appropriate environment and architecture with the correct effector molecules and mechanical forces, all of which are vital for proper function. A thorough an understanding of the synergistic interactions between cells in physiologically relevant conditions is critical for the development of tissues that recapitulate the structure and function of the parental tissue in vivo. Use of bioreactor systems may provide significant inroads into this area (522–524). Thus far, relatively limited data is available for modeling the lung. Several recent studies have demonstrated the power of rotating bioreactor systems to assess interactions of pathogenic bacteria such as P. aeruginosa with lung epithelial cells (525, 526). Comparably, growth of lung epithelial cells, dendritic cells, and macrophages on porous filters has been shown to mimic some of the innate immune pathways of lung airways (527). A more recent study demonstrated that human lung cells and human blood capillaries were able to be coated onto porous polydimethylsiloxane chips mimicking the alveoli of the lungs (528). This “lung-on-a-chip” device was further used to evaluate how nanoparticles and bacteria enter the lungs and may also be useful for high throughput screening of drugs. Clearly, the field is ripe for further comparable technologic advances in bioengineering approaches for lung regeneration (Figure 8).
Figure 8.
Schematic of challenges and issue for lung bioengineering. Slide courtesy of Dame Julia Polak DBE, MD, DSc.
Embryonic Stem Cells and Induced Pluripotent Stem Cells
Progress using embryonic stem cells for lung regeneration or repair has been slower to develop (27, 47). In studies over the past approximately 5 years, several laboratories have demonstrated that both mouse and human ESCs can be induced in culture to acquire phenotypic markers of type 2 alveolar epithelial cells, including expression of surfactant proteins and lamellar bodies, and even form pseudoglandular structures (27, 231, 529–534). However, in general, this occurred at a low level unless the ESCs were transduced to select for surfactant protein-expressing cells (535). More recent protocols and manipulation of cell signaling pathways guiding embryologic lung development and development of definitive endoderm have yielded more robust in vitro derivation of cells with phenotypic characteristics of AECII cells from murine ESC (Figure 6) (536, 537). Derivation of airway epithelial cells from ESCs has proven even more elusive, although development of cells with phenotypic markers of airway epithelial cells has been demonstrated after culturing ESCs under air–liquid interface conditions (538, 539). More recently, effect of matrix proteins, 3D scaffolds, and mechanical forces on ESC differentiation to lung epithelium has been investigated (498, 519, 523, 540–542). This is projected to be an area of increased investigation.
Figure 6.
Schematic of protocol used to differentiate esc into cells with phenotypic characteristics of type 2 alveolar epithelial cells (537). Embryonic stem cells (ESCs) can be effectively manipulated in vitro to differentiate into type 2 alveolar epithelial cells using lung development cell signaling pathway to guide ESCs differentiation. FGF-2 = Fibroblast growth factors-2, Pro Spc = Pro-surfactant protein C. Adapted with permission from Reference 537.
Moreover, there are still only limited available studies of effects of ESC administration to lung in vivo. Endotracheal administration of mESC-derived AECII cells to fetal mice resulted in survival of the cells in the lungs and maintenance of pro-SPC expression over a 24-hour period (27). However, whether the cells truly engrafted or had functional significance was not determined. More recently, it has been demonstrated that intratracheal administration of the AECII cells derived from hESCs (hES-ATII cells) (535) 1 or 2 days after induction of acute lung injury resulting from intratracheal bleomycin administration to immunocompromised SCID mice resulted in a substantial number of hES-ATII cells appearing to have engrafted in lung with persistent SPC expression up to 9 days later (211). Up to 20% of the total SPC-expressing cells appeared to be of hES-ATII origin. A small number of hES-ATII cells also appeared to have differentiated into type 1 cells in vivo. No apparent engraftment was observed if hES-ATII cells were administered to naïve uninjured mice or if a control monocyte population was administered. Notably, bleomycin-induced lung injury was significantly reduced in mice receiving hES-ATII cells but not monocytes or vehicle control. Reduced injury was measured by both qualitative and quantitative measures, including, importantly, functional measurements of tidal volumes and arterial oxygen saturations (211).
This is the first published investigation demonstrating amelioration of lung injury by ESC administration. Notably, these studies reflect the importance of assessing functional physiologic outcomes in parallel with changes in lung injury endpoints such as histologic inflammation and hydroxyproline content. Whether the observed amelioration of lung injury resulted from structural engraftment of the administered cells or reflected a previously unsuspected paracrine effect of the hES-derived cells is not yet clear. Nonetheless, these results have a number of ramifications, including the study and potential use of ESCs in genetic lung diseases, as human deltaF508 embryonic stem cell lines have been established (543–545). These cells exhibit normal morphology and protein expression compared with other hES cell lines but have not been studied in detail.
The American Thoracic Society issued a statement in 2006 calling for expanded human embryonic stem cell research and a follow-up statement in 2010 after the temporary injunction on the use of human stem cells in the United States (3, 546). It is hoped that human embryonic stem cell research will continue to expand in the U.S. and that there will be further rapid advances in the study of ESCs for lung injury and repair.
Rapid advances in techniques for generating, and studying induced pluripotent stem cells have raised hope that these cells will also be useful tools for generating lung epithelial and other lung cells ex vivo (547–551). An exciting recent advance was the generation of disease-specific human-induced pluripotent stem cells (iPS) cell lines from patients with both genetic and acquired lung diseases including cystic fibrosis, α 1 anti-trypsin disease, sickle cell, and scleroderma (552) (Figure 7). However, while iPS cells can be readily differentiated into cells with characteristics of primitive endoderm, generation of cells expressing phenotypic markers of lung epithelial cells has thus far proved elusive. Also, there is no strategy yet developed to ensure that cultures of extensively differentiated iPS or ESCs do not contain a remnant of undifferentiated tumorigenic cells. Nonetheless, it is expected that rapid advances in this field will occur over the next few years.
Figure 7.
Derivation of transgene-free iPS cell line from tail tip of a CFTR knockout (KO) mouse. (A) The human floxed STEMCCA reprogramming vector. (B–D) generation of iPS cells by reprogramming human dermal fibroblasts, and their characterization by RT-PCR and immunostaining. (E) Southern blot of iPS cell clones generated from dermal fibroblasts from CF (DF508 CFTR) and α1-antitrypsin deficient (PiZZ) volunteers. The Southern blot shows high frequency of single copy STEMCCA integrants in the resulting clones prior to Cre-mediated excision of this vector to generate human transgene-free iPS cells. Adapted with permission from References 551 and 552.
SCIENTIFIC PRESENTATIONS
Session 1: Endogenous Lung Progenitor Cells and Lung Cancer Stem Cells
This session focused on recent developments in the characterization, organization, and regulation of regenerative cells in the adult lung in the steady state and in lung disease, as well as on the relationship of normal endogenous lung stem cells to lung cancer stem cells.
Paul Simmons (University of Texas) presented an overview of models of stem cell organization, comparing and contrasting the functional properties of stem and progenitor cell hierarchies in rapidly renewing tissues with those in complex solid organs such as the lung where tissue maintenance and repair involves the coordinated interaction of diverse cell types of differing proliferative and differentiative potentials. He pointed out that although stem and progenitor cell fate in all organ systems is specified by their complex interaction with the extracellular matrix proteins, soluble and insoluble factors and stromal cell elements that comprise their niche (553), their organization, behavior, and functional properties may not conform to a classical stem cell model. He then discussed the fidelity, or lack thereof, of biomarkers in classifying different cell types in the lung. For example, although lung mesenchymal cells are characterized by the expression of the Sca-1 antigen, and can be resolved from lung epithelial cells on the basis of their differential expression of Sca-1 (84), work in his laboratory has identified Sca-1neg lung fibroblastic perivascular cells in the distal lung, which are defined by their expression of PDGFα receptor.
Susan Reynolds (National Jewish Health, Denver) described experiments analyzing the organization of tracheobronchial stem and progenitor cell compartments in the normal steady-state and in naphthalene treated mice. These studies have revealed the existence of three related cytokeratin 14pos tracheobronchial epithelial stem/progenitor cells of differing potentiality that are defined by the differential expression of cytokeratins 5 and 15 (64). Her data suggests that tracheobronchial Clara and ciliated cell lineages are maintained by unipotent cytokeratin 14pos cells in the steady state but by multipotent cytokeratin 14neg5pos15pos cells, which up-regulate cytokeratin 14 after naphthalene-mediated ablation of tracheobronchiolar epithelial cells. This work highlights the power of perturbation models in revealing the organization and regenerative potential of adult lung stem and progenitor cells and how they are recruited to repair the lung after injury.
Ed Morrisey (University of Pennsylvania) spoke about the critical role of the Wnt signaling pathways: not only in lung endodermal progenitor cell fate specification during development, but also in regulating and maintaining the integrity of epithelial and mesenchymal cell lineages in the adult lung in the steady state and in response to injury or disease. In particular, he highlighted the role of Wnt7b in promoting and regulating smooth muscle cell differentiation by transcriptional activation of the extracellular matrix protein, Tenascin C, which in turn up-regulates PDGFα and β receptor expression (554). Significantly, loss of Wnt7b results in defective lung vascular and airway smooth muscle cell development, whereas up-regulated Wnt7b-Tenascin C-PDGFR signaling characterizes allergen-induced asthma in mice and human pulmonary hypertension. The presentations that followed analyzed the response of bronchoalveolar stem and progenitor cell pools in response to injury or insult, and their potential role as candidate lung cancer stem cells when mutated or transformed.
Barry Stripp (Duke University) described studies in chimeric and transgenic reporter mouse models that combined lineage tracing, clonal analysis, and halogenated thymidine analog labeling of proliferating cells to measure the cycling and turnover of lung stem and progenitor cell pools in the steady state, and after naphthalene-induced airway injury, or perturbation, of stem cell pool size and differentiation potential by manipulation of canonical Wnt signaling (65, 80, 96). These studies confirm that epithelial stem and progenitor cell pools that maintain the integrity of airway epithelium in the steady state are functionally distinct from those that are recruited to repair the lung after injury: bronchiolar epithelial stem cells with the highest regenerative potential being relatively quiescent, less susceptible to cytotoxic insult, and only recruited in response to severe injury.
Majd Mouded (University of Pittsburgh) described the analysis of the behavior of candidate bronchioalveolar stem cells (BASC) in the course of elastase or cigarette smoke–induced emphysema using an airway lineage (CC-10) tagged ROSA26 reporter mouse model. Preliminary experiments show increased proliferative activity in the BASC compartment after injury together with augmented alveolar Type 2 cell numbers near the bronchioalveolar duct junction consistent with the participation of BASC or a related precursor in alveolar repair. Although promising, he identified a need for better and more informative animal reporter models and definitive biomarkers for the delineation and characterization of cells participating in alveolar repair in lung disease.
Kerstin Sinkevicius (Boston Children's Hospital) shared her data on the analysis of cancer propagating cells in lung tumors established by orthotopic transplantation of primary adenocarcinomas from Kras and Kras-p53-deficient mice, which recapitulate key features of human non–small cell lung cancers. These experiments compared the immunophenotypic staining pattern of candidate lung cancer–initiating and propagating cells with that of their normal counterparts and tested the hypothesis that they share a common cell surface phenotype. This work (555) demonstrates the fidelity of the orthotopic transplant model in recapitulating the properties of primary lung tumors from which they are derived, but also reveals that a difference in genotype at a single locus significantly influences the marker repertoire that defines cancer propagating cells in different tumors. Briefly, whereas cancer-propagating cells derived from Kras-p53-deficient tumors were highly enriched in the Sca-1pos cell fraction, this was not the case for Kras tumors where Sca-1pos and Sca-1neg cells were equally tumorigenic. On the other hand, analysis of only Sca-1neg cells are able to propagate tumors in EGFR mutant tumors. Although the study demonstrates the significance of confounding factors in determining the nature and properties of aberrant cells in disease pathophysiology, it also highlights the limitations of phenotypic analysis in the absence of robust functional assays in assigning stemness.
The panel discussion that followed raised questions about the nature and properties of stem cell niches in the lung and the role of the niche in specifying stem cell fate and regulating regenerative potential. A discussion of the prerequisite functional characteristics that must be met to assign stemness in the steady-state and after perturbation or injury was also debated, questioning if regenerative and reparative cells differ in different contexts. The concept of the temporal niche was also raised. The fact that the properties of the stem cell niche alter during disease progression poses a challenge in the development of strategies to harness the potential of endogenous regenerative cells, or to deliver exogenous reparative cells. Consequently, the repair or normalization of the niche is a critical consideration in developing successful stem cell–based or stem cell–directed therapies for lung repair. In summary, cell lineage tracing studies, the interrogation of gain-of-function, loss-of-function, and the temporal analysis of lung perturbation models have significantly enhanced our understanding of the organization and regulation of regenerative cells in the lung. However, there remains a pressing need for the identification and validation of biomarkers and biomarker repertoires for classifying lung stem and progenitor cells and the stromal elements that comprise their niches, as well as robust functional assays predictive of their regenerative potential.
Session 2: Embryonic Stem Cells, iPS, and Lung Regeneration
The theme of this session focused on the regenerative potential for lung cell lineages and tissue. Various types of stem cells were discussed, including ESCs and iPS cells as well as the importance of devising and providing the correct functional niche.
Konrad Hochedlinger of the Harvard Stem Cell Institute pointed out that although the properties of ESC and iPS are similar, the efficiency of reprogramming iPS is low. Key bottlenecks in this field are that actual reprogramming of individual stem cells is a rare event, that viral infection is inefficient, that there is a risk of insertional mutagenesis, and that reprogramming is a stochastic process requiring several events to occur in the correct sequence (556). Furthermore, Hochedlinger pointed out that most differentiated cells have limited replication potential in culture and ultimately undergo senescence, which may be a critical barrier to scalable reprogramming. Additionally, iPS cells may actually be derived from adult progenitor cells as opposed to mature cells, as had been previously assumed. As a corollary of that, cellular immortalization may therefore eliminate a key roadblock during cellular reprogramming. Thus, he suggested INK4a/ARF and p53 as key putative molecular targets, the deletion or inhibition of which may remove major roadblocks to cellular reprogramming.
Darrell Kotton from Boston University focused the discussion of ESC and iPS technology on driving differentiation of endoderm and of lung epithelium from endoderm. He pointed out the caveat that iPS cells still have many issues to be worked out, including the necessity for multiple integrations, as well as the far-from-negligible potential for oncogenesis of iPS cells in vivo, not least because of the requirement for integration of oncogenes such as Myc. Nevertheless, he showed that postnatal mouse fibroblasts can be induced into an iPS phenotype using a lentiviral “stem cell cassette” (STEMCCA), which expresses Oct4, Sox2, Kif4, and cMyc simultaneously. These cells were validated in puripotency assays including the formation of teratomas in vivo, as well as chimera derivation from mouse blastocysts, including the derivation of lung endoderm, as shown by the expression of Sox2-GFP in lung epithelium. He then showed elegant proof of principle that subsequent Cre-mediated excision of the STEMCCA does not cause reversion of the iPS phenotype previously induced by STECCA expression (550, 551). Taking a lesson from endoderm derivation during gastrulation, which is driven by spatial information imparted by BMP4, Wnt, and Nodal signaling, among other factors, including FGF, he then showed that lung-specific endoderm TTF1-GFP reporter gene expression can be driven within STECCA iPS-derived endoderm using a cocktail of fibroblast growth factor (FGF) and Nodal.
Carolyn Lutkzo from the Saban Research Institute at Children's Hospital in Los Angeles shared her protocol for differentiating definitive endoderm from hESC, which follows from developmental principles. She finds that she is able to sequentially induce expression of mesoderm, definitive endoderm, and primitive gut tube markers including Gata6, Sox17, Foxa2 and Ttf1 over the course of 7days from cultures of hESC using sequential exposure to Activin A/Wnt3a, Activin A/0.2% serum, and KGF/2% serum. Then the introduction of FGF10 to the mix induces Ttf1, CC10, and cytokeratin 18, but not SpC. This system requires further optimization, but it shows distinct promise for the feasibility of deriving respiratory endoderm from hESC in vitro.
Rick Wetsel from the University of Texas Health Science Center in Houston described a genetic strategy using an SPC promoter-driven neomycin resistance cassette–based selection system to drive purification of AEC2 derived from hESC to greater than 90% purity (535). He now finds that not only are his hESC-derived AEC2 capable of acting as progenitors for AEC1, but that they also ameliorate bleomycin-induced lung injury after in vivo engraftment, which results in a complete rescue of lethality in mice (211).
Lastly, Peter Lelkes from Drexel University pointed out the importance of the 3D tissue niche in directing differentiation of murine ESC into alveolar-like constructs. He demonstrated examples of organotypic recombination of fetal mouse lung cells within matrigel/collagen gels. Furthermore, he showed the presence of vascular endothelial vascular structures within these constructs and indicated that FGF and VEGF signaling, as well as Shh and tenascin, play important roles in this process. He then demonstrated the potential for synthetic electrospun collagen matrix (versus elastin matrixes that mimic decellularized human lung) to some extent support morphogenesis of fetal lung cell-derived organotypic epithelial and vascular structures.
Taken together, these presentations demonstrate significant progress not only in the derivation of definitive endoderm as well as respiratory endoderm from both mouse and human ESC, but also in the potential for the derived cell lineages to contribute to regenerative medical applications in the lung in vivo. Moreover, some excitement was generated about the potential for matrix-driven tissue engineering using organotypic recombination of stem/progenitor cells in matrix. Nevertheless it is evident that much remains to be accomplished, particularly in terms of scalability, toxicity, tolerance, and connectivity in translating these undeniably exciting potential approaches to human disease applications.
Session 3: Bioengineering Approaches to Lung Regeneration
The goal of this session was to review the progress in tissue engineering, encompassing biologic scaffolds and tissue culture systems as they apply to the lung. The session began with Dame Julia Polak, Imperial College, London (by videoconference), who provided a general overview of lung tissue engineering and highlighting the many challenges currently facing the field. It is not yet clear which progenitor-cell sources will be optimal for generating lung tissue. Candidates include primary cells (adult and fetal), embryonic stem cells (ESCs), adult stem cells from bone marrow and cord blood, circulating mesenchymal cells, niche-specific cells, and adipose-derived cells. Much effort is currently spent on how to enhance lineage-specific differentiation using chemical factors and factors of the microenvironment. There has been some success in driving murine ESCs to express Type II pneumocyte proteins (e.g., SP-C) via direct and indirect coculture methods. An early report showed that removal of retinoic acid from culture increases differentiation of ES cells, but the process was long (33 days) and the yield was low (2.8%) (557). Nevertheless, it is a step in defining factors that inhibit or stimulate the differentiation process. The use of pneumocyte cell extracts may decrease the culture time required and increase yield (558, 559). Wang and colleagues did show that the use of gene modulation by transfection with an SPC-promoter-neomycin transgene (for selection) resulted in an almost pure population of cells with lamellar bodies, SP-A, SP-B, SP-C, and other Type II pneumocyte markers (535). Recently, several laboratories have begun studies in an effort to engineer lung tissue ex vivo using 3D constructs. The material used to generate such constructs should be able to stimulate cell growth and differentiation, cell attachment, and deposition of extracellular matrix, as well as act as a template for tissue vascularization growth in 3D, be resorbable for implantation, and be insoluble for in vitro screening and toxicity studies. Another major challenge is that of scale up. For expansion, static two-dimensional cultures have many drawbacks, including lack of mixing and control options, and they require constant feeding, which typically results in low yield and poor quality of cells. In contrast, the advantages of 3D cultures is their simplicity, applicability to process integration, and automation, which leads to enhanced proliferation, thus providing higher yields in a shorter time. 3D cultivation methods better approximate the in vivo environment of cell to cell and cell to matrix interactions. The use of a bioreactor permits nutrient penetration, and integrated monitoring systems can assess viability and specific cell needs. Alginate beads (from brown seaweed), that are biocompatible and FDA approved, have been used for cell expansion and bone formation. Cell encapsulation has been used for mass production of embryoid bodies, maintenance of pluripotency of human ES cells, and engineering of bone tissues (560–563). The key features of stem cell bioprocessing are control, reproducibility, automation, validation, and safety. Preclinical testing requires animal models, demonstration of safety, engraftment, efficacy, growth control, and immunocompatibility. Demonstration of correction of a defect in vivo by a cell construct would be required so that clinical quality production can proceed toward clinical applications. Alternatively, it is possible that the cells could be used in organ-support devices to increase efficiency such as in an interventional lung assist device. A multidisciplinary approach is required to engineer grafts to replace part of a diseased organ, as was recently demonstrated by Macchiarini (484). Denuded trachea was used as the biologic scaffold onto which cultured tracheal epithelial cells and cartilage from bone marrow stem cells was seeded and placed in a custom bioreactor. With this engineered construct, 5 cm of the left bronchus was replaced with restoration of normal function up to 4 months. Current challenges that are a barrier to the translation of stem cell therapy for lung diseases are the poor understanding of lineage specific differentiation, low functionality of differentiated cells, the need for protocols with defined reagents and inefficient 3D growth in culture. Other issues to be considered if a product is developed include the cost and reimbursement, industrial scale production, transport and logistics such as storage and tracking of tissues. Stem cell cultures require a reliable method for cell expansion and differentiation to reduce variability and heterogeneity and to increase the affordability of the reagents. Regulatory protocols need to be implemented to address any donor-associated risks, contamination during bioprocessing, final product purity (including any genetic instability), efficacy, safety, and survival in vitro. Other regulatory challenges may include deciding on which in vivo models are appropriate to assess cell dose, microenvironment, tumor formation, and immunological issues. Dr. Polak discussed a proposal that was approved in May of 2007 for a single European Union-wide integrated and tailored regulatory framework for advanced therapies. With pharmaceutical companies and industry now starting to be involved in cell therapies, the basic question that needs to be addressed is whether the health care system is ready to incorporate cell-based therapies for lung disease and whether it will be limited to selected patients. The session ended with the conclusion that stem cell therapies for lung disease are reaching maturity, but many challenges have yet to be met, as outlined above.
Christine Finck, Connecticut Children's Medical Center, discussed work aimed at using stem cells to treat pulmonary hypoplasia of preterm infants. Treatments to date consist of mechanical ventilation, surfactant therapy, inhaled NO, extracorporeal membrane oxygenation (ECMO) ECMO, and VEGF administration with variable success. A hypoplastic lung has reduced lung weight, fewer generations of airways resulting in fewer alveoli, poor epithelial cell differentiation and surfactant deficiency, along with hypoplasia of the corresponding pulmonary arteries. Hence, this represents an ideal environment to introduce stem cell therapy, albeit with the advantages and challenges as described by Dr. Polak. Dr. Finck summarized what is known of how the lung is derived from definitive endoderm and how attempts to devise a protocol to recapitulate the critical induction events of the early embryo (e.g., Wnt, TGFb, Activin-A) have been used to induce pluripotent cells to become definitive endoderm (530, 564). In a comparison of seeding methods, it was shown that if ESCs are first allowed to become embryoid bodies (EBs) before seeding onto collagen IV coated plates, only cells that migrated away from the EBs expressed Gata4 whereas cells within the EBs retained expression of Oct4 (486). In contrast, ESCs plated as disaggregated cells on collagen-IV coated plates expressed Gata4 uniformly and no Oct4 (i.e., more directed differentiation). To further direct differentiation of derived endoderm, the Finck lab developed and optimized a 2-step protocol (537) in which ESCs are first incubated in media containing Activin-A and Wnt3a (6 days, single-cell suspension on collage-IV coated plates) as was similarly shown for derivation of hepatic endoderm (564, 565). Importantly for the lung, using 50 ng/ml Activin A with 1 ng/ml Wnt3a, which is important in migration of epiblast cells though the primitive streak in embryogenesis, gave rise to the highest number of cells expressing the definitive endoderm markers Sox17 and FoxA2 and lowered the expression of the visceral endoderm marker HNF4 (qRT-PCR). Increasing the dose of Activin above 50 ng/ml resulted in more cells but not a greater proportion of FoxA2+ cells. Cells expressing E-cadherin and cytokeratin were seen by immunofluorescent staining after a 5-day induction with optimal concentrations of Activin A and Wnt3a. Step 2 of the protocol involves the addition of FGF2, which is important in patterning the foregut endoderm (566). After 5 days in as little as 5 ng/ml FGF2, the cells began to express surfactant proteins A, B, and C, as well as CC10 (Clara cell secretory protein). Increasing FGF2 to 50 ng/ml resulted in increased FoxA2 and SPs and decreased expression of the pancreatic marker PDX-1. In these conditions, approximately 12% of the cells expressed SP-C, 48% expressed vimentin (i.e., mesenchymal), 37% expressed Sox17 (i.e., endodermal), and 1% expressed Pax6 (ectodermal). Lamellar body formation was also demonstrated. Future work will determine if the addition of FGF10 will enhance yield. Preliminary data was shown using the protocol in the context of 3D collagen and matrigel hydrogel with similar results. In other work to develop nonepithelial tissues of the lung, differentiating ESCs in hypoxic conditions (5% O2) for 5 days decreased the expression of endoderm markers (FoxA2, Bry) and increased expression of the endothelial marker Flk-1 (binds VEGF, as does Flt1). After 11 days, vessel-like networks were observed as well as increased frequency of PECAM+ cells. Using an explants model of pulmonary damage, premature mouse lungs (E18.5) were treated with Flt1 receptor to block VEGF resulting in a hypoplastic lung (decreased mean cord length and increased frequency of TUNEL+ cells). Undifferentiated and differentiated endodermal cells were added to the bottom of a transwell system containing hypoplastic lung at the air–liquid interface. When compared with undifferentiated ES cells, differentiated endodermal and distal airway cells promoted alveologenesis (increased cord length and frequency of CD31+ cells and decreased TUNEL+ cells), likely through production of VEGF. Intratracheal delivery of ES-derived distal airway cells into adult mice resulted in increased levels of VEGF in the brochoalveolar lavage (BAL) fluid. In other experiments relevant to treatment of premature babies in utero, it was demonstrated that cells injected into the tail vein of the dam resulted in cells migrating to the lungs of the pups. Future work will investigate the derivation of pure populations of differentiated cells, the use of scaffolds and treatment of premature mice (via intratracheal, tail vein, and intra-amniotic injections).
A vascularized approach to tissue-engineered lung was described by David Hoganson (Massachusetts General Hospital). Because organ shortage is the most critical problem in transplantation, lessons learned from stem-cell biology, tissue engineering, and regenerative medicine may be able to supply transplantable tissue or an organ replacement. At its basic level, successful engineering of tissue must result in delivery of oxygen and nutrients to the cells, which depends on surface area, concomitant with removal of waste and CO2. For a 3D organ, tissue engineering must match surface area to volume for effective mass transfer (surface area increases as r2 but volume increases as r3). The challenge of vascularization by tissue engineering is to mimic the complexity and microfluidics of the vasculature. This will vary depending on the application, for example, making a fully developed tissue-engineered adult lung versus a neonatal lung versus an ambulatory lung assist device. In recapitulating the basic structure of an alveolus, Dr. Hoganson described the concept of a lung assist device that would be composed of an alveolar chamber separated from a microvascular network of channels, lined with endothelial cells, by a respiratory membrane that would be covered with pneumocytes. Such a device would be a bridge to transplant for end-stage lung disease. It would be implanted in the upper chest with blood flow from the pulmonary artery and returning to the left atrium. Oxygen tubing would be hooked up to and from the device (through the skin). A prototype multilayered (more surface area) cellular device has been developed with “vascular” and “alveolar” chambers separated by a silicone respiratory membrane that is highly permeable to O2 and CO2. While anticoagulated sheep blood was circulated through the device at 37°C, O2 and CO2 transfer was observed to increase with an increasing rate of blood flow. However, in extrapolating the data for use as an adult lung assist device, it appears that 357 layers of chambers separated by a silicone-coated membrane would be required for 0.64 m2 of surface area at a flow rate of 4 ml/min per layer. It would remove 20% of CO2 generated at rest and would handle 1.4 L/min blood flow, which is approximately 25% of cardiac output. This would increase Po2 of that blood from 57 to 79 mm Hg. Further improvements to the design are needed to mimic a high density capillary system by maximizing the network while minimizing physiologic shear stress (otherwise platelets will get activated and obstruct the conduits). Biomimetic design principals were used, taking into account physiological vessel diameters and lengths (parent and daughter vessels) as well as bifurcation angles (that are smoothly rounded at the apex). This resulted in all channels having a 1:1 aspect ratio with the smallest channels having a diameter of 100 μm. Micro-machining using brass molds can be used to create exquisite 3D features (i.e., connections in 3 dimensions). In vitro testing with anticoagulated sheep blood demonstrated atrial pressures varying with flow rate almost identical to the computational fluid dynamics analysis. To achieve more physiological relevance, biomimetic vascular design must be translated to biomimetic scaffold material consisting of collagen-coated channels with diameters down to just 2 μm. To determine if gas exchange efficiently occurs across a collagen membrane, transwells were created with a thin collagen film as a respiratory membrane and pneumocytes and endothelial cells were juxtaposed to opposite sides of the membrane. Transwells were attached to gas exchange fixtures so that blood was allowed to flow on the endothelial side of the collagen membrane at a flow rate of 0.0625 ml/min while the pneumocyte side was exposed to 100% oxygen at a flow rate of 20 ml/min. The presence of pneumocytes and endothelial cells resulted in an increase in Po2 in the blood from 20 mm Hg (no cells, or endothelial cells only) to 40 mm Hg. No effect was seen on Pco2 using cells on the collagen membrane. When cultured for 14 days in the presence of pneumocytes and endothelial cells, the thickness of the collagen film decreased from approximately 45 μm to 31 μm. With further incubation, collagen thickness decreased to 8.6 μm, thus beginning to approximate physiological measurements. It is hoped that the collagen would be digested over time and replaced with natural extracellular matrix. Although these studies were conducted using cell lines, human endothelial cells have successfully been seeded onto collagen membranes but it is unknown whether specialized endothelial cells are required.
Ed Ingenito (Brigham and Women's Hospital) spoke about biologic scaffolds for stem and progenitor cell engraftment in the lung. Emphysema was chosen as a target disease, but the principles learned may be applicable to any structural lung disease. One must understand the progenitor cells to be used as well as the cells to be repaired. Other elements to incorporate are scaffold design, impedance matching, mechanical considerations, cell attachment and spreading, cell kinetics, and in vivo testing. Phenotypic modulation could be accomplished in several ways including use of growth factors and small molecules (pharmacologic), modulation of extra- cellular matrix (epigenetic), and use of RNAi or gene therapy (genetic). In an ovine model of elastase-induced bullous emphysema, autologous adult progenitor cells and endobronchial delivery were selected over other cell types and intravenous delivery to facilitate cell attachment, spreading, migration, and survival. At 4 weeks after site-specific injection of cells using a fibrin-based engraftment scaffold, the size of the emphysematous lesion was reduced by 40%. Progenitor cells isolated from biopsies of sheep lung demonstrated the presence of epithelial cells (pancytokeratin+, collagen IV−, and fibrillin−) and mesenchymal cells (vimentin+, collagen IV+, and fibrillin+). In culture (at passage 5), mesenchymal lung progenitors (MLPCs) express αSMA, vimentin, S100A4, and PDGFRα. They make extracellular matrix proteins such as collagen I and collagen IV, the adhesive glycoproteins fibronectin, vitronectin and laminin, as well as elastin components such as fibrillin-1 and tropoelastin. They also express growth factors such as VEGF-A, FGF2 and FGF10, important for lung growth and development. These cells have a doubling time of 36–48 hours and demonstrate stable genetics and phenotype. MLPCs express the integrins β1, β5, αV, and α5. Integrin characterization defines the extracellular matrix (ECM) attachment and spreading patterns important in both cell kinetics and cell phenotype. This needs to be taken into consideration when designing scaffolds with attachment ligands to maximize attachment and spreading. If there is a barrier to attachment, the injected cells will apoptose. The 3-part strategy to promote engraftment of progenitors is to 1) prime the target tissue (precondtioning), 2) develop an engraftment system, and 3) modulate the desired effect. The design specifications of preconditioning should be focal, easy to control, result in cell removal, be biocompatible, and be conducive to provisional matrix deposition. Various enzymatic systems were tested and tryspin was selected as it was found to be safe, simple to control, it stimulates cell cycling, is Good Manufacturing Practice (GMP) available, and inexpensive. Its omission was found to reduce engraftment in vivo. Other approaches that use detergents and thermal shock have not been developed. Several potential scaffold components with relevant properties were tested alone and in combination for their capacity to promote cell attachment and spreading in vitro in different medium conditions. It was found that col IV, fibronectin, and poly-l-lysine (PLL), each alone, promoted good attachment at 20 μg/ml. However, expression of the attachment ligands varied with growth conditions and cell cycle phase. For promotion of spreading (in vitro assay), combinations containing 20 μg/ml fibronectin, or col I or col IV worked best, although data was shown that these components alone worked almost as well (as did laminin). PLL alone did not promote spreading but did not adversely affect it in combination with the spread-promoting components (i.e., does not inhibit formation of focal adhesion complexes by integrins). As with attachment, spreading varied with growth and cell cycle conditions. There are also mechanical considerations that need to be considered in scaffold design. Hydrogels with mechanical properties different from lung tissue can cause damage and prevent engraftment. For example, 1.2% alginate is a physical hydrogel that supports cell growth but is very rigid and brittle, whereas 2% fibrinogen is chemical hydrogel that supports cell growth, and is soft and elastic with stress–strain properties similar to lung (at low physiologic stress). Substrates also influence the ability of progenitor cells to enter cell cycle after delivery. They also affect cell survival/apoptosis after degradation of the scaffold, which has implications for engraftment potential. For example, fibrin-mediated apoptosis can be blunted by integrin binding. In vivo testing of the role of site conditioning and scaffold composition in healthy sheep showed that sites that received an autologous MLPC graft (10–50 × 106 cells in each segmental site) delivered in fibrin-fibronectin-PLL hydrogel had no radiographic evidence of parenchymal inflammation or pleural or mediastinal pathology at 3 weeks after treatment, and the lungs appeared grossly normal. Histological examination of the injected sites demonstrated that cell retention (defined as number of fields with labeled cells) was substantially greater and was maintained longer after grafting was performed with hydrogel scaffold compared with grafting of cells in aqueous suspension. Transplanted cells were shown to express both collagen IV and fibrillin-1 at 3 weeks after injection, showing maintenance of the phenotype. In vivo testing in sheep with experimental elastase-induced emphysema showed that treatment with MLPCs in FFV hydrogel scaffold was associated with a reduction in bulla size and improved perfusion when compared with sham treated with scaffold alone. Furthermore, the MLPC treatment was associated with a retention of the infused cells at the treatment sites, increased tissue cellularity, increased extracellular matrix,, and increased tissue elastin at 4 weeks. Dr. Ingenito did say that there is an angiogenic response but it is not known if this is due to the cells infused or due to the scaffold. It was also stated that lung regeneration relies on host epithelial cells to re-epithelialize the basement membrane, but it is not known if mesenchymal to epithelial transition (MET) is occurring in this system. Future areas of research will include the role of MMPs, ADAMs, and s100A4 in cell migration; prevention of apoptosis; role of matricellular proteins (TN-C and TSP-1) and pre-incubation strategies to augment scaffold binding or obviate the need for scaffolds.
Viranuj Sueblinvong (Emory University) presented work on lung tissue engineering from MSCs. In vitro experiments that induce mechanical stretch in a uniaxial stretching system (1 Hz with 5–10% elongation) in basal medium promoted AT2 cell differentiation after 2 to 5 days. The stretch does not cause significant morphological changes of the MSCs after 2 or 5 days, but it does induce MSC proliferation at 2 days and expression of CCSP and CFTR mRNA and surface protein. SPC and Aquaporin-5 expression were not induced. Notably, stretch attenuated the expression of collagen type I and α-smooth muscle action (SMA) messenger (m)RNA. Using small airways growth medium (SAGM) in place of basal medium, increased levels of AQP-5, CCSP, CFTR and SP-C mRNA (5–15 cycles) and protein were achieved with stretch, and this effect was synergistic. Small airways growth media (SAGM) also augmented the effects of stretch on reducing collagen type I and α-SMA mRNA expression. Dr. Sueblinvong concluded that MSCs are an excellent source of cells for ex vivo lung tissue regeneration due to their differentiation potential and their ease of supply. Furthermore, mechanical stretch can be used to augment lung differentiation potential. Consideration must be given to the composition of the matrix/scaffold because ECM regulates lung development and repair and ECM has been shown to regulate MSC differentiation in vitro (324–328). When MSCs are cultured on fibronectin (10 μg/ml), they show more fibroblastic morphology and increased α-SMA and α-integrin receptors than those cultured on laminin (10 μg/ml). The importance of applying force to promote cell growth was also demonstrated in a report of the clinical transplantation of a tissue-engineered airway using the recipient's cells seeded onto donor bronchi (484). Future work is required to understand how mechanical stretch and surface matrices affect stem cell phenotypic aquisition of receptors that enable interaction with matrix components and other mechanoreceptors such as CFTR and ion channels.
The session ended with Charles Vacanti's (Harvard, Brigham and Women's) talk on the use of adult stem cells for the engineering of new pulmonary tissue. The two main components in tissue engineering are the cell delivery system, or the scaffold, and the appropriate cell type. The limitation of biologic material, such as decellularized lungs, is the batch-to-batch variability. In contrast, hydrogels provide consistency, are biodegradable, and result in a good distribution of cells when seeded. Reverse thermally sensitive polymers such as pluronics are liquid at room temperature (and can be injected in vivo in this form), and they gel upon reaching body temperature and can used to generate specific shapes. Cells suspended in this gel remain viable after implantation. The best type of cell to use for engineered transplantable tissue is likely one that avoids rejection. Therefore, adult stem cells, very-small-embryonic-like (vsel) cells, and pluripotent stem cells are good candidates. Dr. Vacanti's lab has described an adult stem cell named “SPORE-like cells” (i.e., sub population of retained embryonic-like stem cells). They are small cells (less than 7 microns in diameter) and are present in all tissues of the body. It is unknown if they are seeded from the bone marrow. After isolation from tissue, they initially express many embryonic stem cell markers (e.g., Oct4 and Sox9) and can be induced to differentiate into cells of all three germ layers in appropriate culture conditions. Initial sphere formation begins with a small aggregation of clusters. If not driven toward any particular cell type, the cells typically prefer to revert to the cell type of the tissue from which they were isolated. Dr. Vacanti described that the SPORE-like cells from the lung were procured predominantly from distal lung tissue, minced, exposed to enzyme, passed through a 40 μm filter followed by a long-drawn Pasteur pipette. Nonadherent cells form into aggregates and pneumospheres that differentiate into lung cells expressing SP-C, and CC10 when cultured in serum-containing medium. When put in vivo on hydrogel scaffolding, they appeared similar to distal airway tissue, but the cell patterning was atypical. Dr. Vacanti concluded that, in the short term, using decellularized lung may be a better scaffold to guide pneumospheres. Pneumospheres can also be driven to other cell types if cultured in appropriate conditions. It is unclear if SPORE-like cells are stem cells or if they exist/function in vivo (or are culture phenomena).
The discussion panel consisted of Drs. Jason Bates (UVM), Peter Lelkes (Drexel), and Thomas Gilbert (Pitt). The overall consensus was that there has been “too much engineering and not enough tissue.” Investigators have been relying on having a design and hoping that the cells being used follow the design principle and self-organize (after a little prodding to direct them into the right differentiation path). It was agreed that using 3D matrices is better than 2D culture dishes and that the normoxic microenvironments typically used are wrong because, in fact, for the embryonic environment, when the lung is developing, normoxia is hyperoxia (fetal hemoglobin conducts oxygen to tissues at much lower oxygen tension than postnatal). The discussion then moved to a question of “what is the research question?” From the Ph.D. perspective, the question is “what is the language of the cells?” In other words, it is necessary to understand the rules of how cells behave (which requires the interdisciplinary fields of math and computing). From the M.D. perspective, the question is “can pulmonary function be improved in patients with deteriorating lung function?” Ultimately, we may not need cells, because if we understand the factors involved in development and repair, then perhaps administering the appropriate factors or extracellular matrix material may suffice. The discussion then evolved to questions of whether the cells are smart (i.e., do they know what to do to provide a correct environment?), and whether one really needs to know how lung engineering works or just that it works (case in point: not much is known about how anesthesia works). How does one define the endpoint of what one wants the cells to be? Should it be by function or by expression of certain markers, and if so, how many markers? The session ended with an expression of the continuing need for an in vitro assay system as well as in vivo functional assays to evaluate stem/progenitor cells. It may be possible to use intravital microscopy to evaluate the function of infused cells. Since the last workshop meeting, advances have been made in the use of 3D in vitro models but not in the translation of these models for in vivo functional assays.
Session 4: MSC Modulation of Immune and Inflammatory Responses
The goal of the session 4 was to review the different studies that have identified how MSCs interact with cells of the innate and adaptive immune systems. Several groups have documented the anti-inflammatory effects of exogenously administered MSC in animal models of injury, including lung injury, asthma, and sepsis (31, 35, 42, 397–399, 401–411, 413–444). Discussed in this session were preclinical data for the therapeutic use of MSC in humans and the promises and challenges associated with exploring potential clinical protocols to investigate the safety and efficacy of MSC therapy for lung diseases (445).
Armand Keating, (University of Toronto) presented key issues in MSC studies: novel type of bone marrow–derived progenitor cells and their role on immune modulation. The studies of MSCs in tissue regeneration and in immunology have progressed at a remarkably rapid pace over the past 5 years, and with potentially important new data appearing weekly in the scientific literature, there has been little opportunity to either fully assess their contribution or adequately evaluate the direction of the field. In this presentation, two key areas were explored in a first attempt to provide some context: the potential importance of novel progenitors in tissue repair and the role and significance of MSCs in mediating immune responses. Studies with murine and human MSCs from different sources were discussed as well as how these populations might provide insights into the investigation of tissue repair. Investigations of MSCs in the modulation of immune responses were placed into context and evaluated on the basis of the in vitro assays, preclinical models used, the source of cells, the species differences, and the immune cells affected. All of these key issues have relevance in future cell therapies for lung diseases.
Ryang Hwa Lee, (Texas AandM Health Science Center) presented a novel work demonstrating that in mice, intravenous administration of human MSCs (hMSC) reduce myocardial infarction damage. The protective effect apparently is by a paracrine effect on the secretion of anti-inflammatory proteins by MSCs trapped in the lung in the absence of significant engraftment (218). Furthermore, the hMSCs in the lung can up-regulate the expression of multiple genes with a large increase in the anti-inflammatory protein TSG-6. TSG-6 is a multifunctional 35-kD–secreted protein associated with inflammation. TSG-6 is not constitutively expressed in normal adult tissues but is rapidly induced in many different cell types by inflammatory mediators such as IL-1β, TNF-α, endotoxin, and prostaglandin E2 (PGE2). Intravenous administration of hMSCs, but not hMSCs transduced with TSG-6 small interfering (si)RNA, decreased inflammatory responses, which in turn reduce infarct size and improve cardiac function. A similar effect in reducing inflammatory responses and infarct size is observed by IV administration of recombinant TSG-6. The results suggest improvements in animal models and patients after IV infusions of MSCs are at least in part explained by activation of MSCs to secrete TSG-6.
Conrad Liles, (University of Toronto/University Health Network; McLaughlin-Rotman Centre for Global Health, Toronto General Hospital) presented work on the use of MSC as a therapy for sepsis. Sepsis and its complications, including acute lung injury/acute respiratory distress syndrome and multiple organ dysfunction syndrome, are important causes of morbidity and mortality in critically ill patients, despite appropriate antimicrobial therapy. It has been previously demonstrated that administration of MSCs can ameliorate experimental LPS-induced pulmonary inflammation ALI in mice (230, 400–411). However, one of the main concerns regarding the use of MSC in patients with acute lung injury is if by down-regulating the immune response there is increased possibility of sepsis. Dr. Liles hypothesized that administration of MSCs would also reduce sepsis-associated inflammation and organ injury in a clinically relevant model of sepsis. To investigate this hypothesis, sepsis was induced in C57Bl/6J mice by cecal ligation and puncture (CLP), followed by an intravenous injection of MSCs or saline 6 hours later. MSC treatment significantly reduced systemic and pulmonary cytokine (IL-6, IL-1β, IL-10, KC, JE, and CCL5) production in mice with CLP-induced sepsis (415). Sepsis-related acute lung injury and organ dysfunction were prevented by MSC treatment. In addition, bacterial clearance was significantly improved by MSC treatment. Importantly, MSC treatment significantly reduced mortality in septic mice receiving appropriate antimicrobial therapy. Expression microarray analysis of the spleen revealed that MSC therapy affects a number of pathways implicated in sepsis-related morbidity and mortality. These findings, in conjunction with the results reported in a study by Nemeth and colleagues (413), demonstrate the feasibility and effectiveness of MSC administration for experimental sepsis and providing the basis for development of a potentially effective immunomodulatory strategy to decrease morbidity and mortality in clinical sepsis.
Michael A. Matthay, (University of California, San Francisco) presented stimulating work on the use of MSCs to reduce acute lung injury in mice and in the ex vivo perfused human lung. Matthay's research group has used in vitro, in vivo and ex vivo experimental models to test the therapeutic value of MSCs for the treatment of ALI (400, 445). A spirited discussion ensued as to whether MSCs should be considered for treatment of acute lung injury. According to Dr. Matthay, the evidence that supports the use of MSCs is founded on growing evidence suggesting profound immunonodulatory actions of MSCs in lung injury models. To demonstrate the efficacy of MSC in ALI, intratracheal delivery of allogeneic MSCs were used to improve the function of the alveolar epithelium, reduce inflammation, and improve survival in mouse models of ALI. In mice injured with intratracheal endotoxin (5 mg/kg), bone marrow–derived MSC (750,000 cells), given into the airspaces 4 hours after the endotoxin, reduced the severity of lung injury as measured by lung protein permeability, pulmonary edema, and lung histology. The therapeutic effect was associated with a decrease in proinflammatory cytokines and an increase in anti-inflammatory cytokines, including IL-10. Survival was also improved with MSC treatment (400). More recent work by this group has indicated that MSCs have therapeutic value in bacterial pneumonia in mice. By using a standard model of Escherichia. coli pneumonia in mice (instilled 106 bacteria) treated with MSC 4 hours after instilling live bacteria, bone marrow–derived MSCs reduce lung injury and improve survival. MSC treatment was associated with a reduction in the number of live bacteria in the lung compared with saline and fibroblast controls. In summary the results of MSC studies in mice demonstrated that treatment with MSC improves survival and reduces lung injury in the endotoxin model of ALI. This can be as a consequence of the down-regulation of proinflammatory response while increasing anti-inflammatory cytokines. At the same time, MSC reduces lung injury and improve survival following live E. coli lung injury, with an associated decrease in bacterial counts.
The most relevant results to translate these observations into clinic are derived from the ex vivo lung reperfusion model. Using a perfused (5% albumin in a physiologic solution plus fresh human blood) human lung preparation that was developed to study lung fluid balance under clinically relevant pathological conditions, the administration into the airspaces of either allogeneic human MSC or the medium from cultured human MSC reversed endotoxin-induced lung injury as measured by lung water, lung endothelial permeability, histology, and the rate of alveolar fluid clearance (445). Based on siRNA knockdown experiments, the beneficial effects were mediated in part by secretion of keratinocyte growth factor (KGF) by MSC, which reduced lung edema, normalized lung endothelial permeability, and restored the amiloride sensitive sodium and fluid transport capacity of the injured human alveolar epithelium. Mechanisms for how KGF may restore alveolar fluid clearance include up-regulation of sodium transporters ENaC and NaKATPase at a transcriptional level (446).
An important consideration is the development of more preclinical studies to determine mechanisms, efficacy, and safety of the use of MSC in ALI. It is also necessary to determine the most efficient route, dose, and timing of delivery, as well as to when and how many MSCs need to be inoculated. Finally, we should test MSC-conditioned medium or key products in the medium, such as IL-10 or KGF or the recently described TGS-6. All of these are important considerations that need to be evaluated before MSCs can be use in humans as cell therapy for any type of lung disease.
Daniel J. Weiss (University of Vermont College of Medicine) presented the use of MSCs in immunomodulation of allergic airways inflammation. This work is based on previous in vitro observations that MSCs can inhibit proliferation and activities of a wide range of immune cells, notably CD4 T lymphocytes. These properties have served as a basis for recent successful clinical trials of autologous and allogeneic mouse MSCs for immune and inflammatory diseases such as graft versus host disease, Crohn's disease, and most recently, chronic obstructive pulmonary disease. However, the mechanisms by which the MSCs suppress inflammatory-based diseases in vivo are poorly understood, and it is not clear whether effects observed in vitro parallel those occurring in vivo. Dr. Weiss reasoned that asthma, a CD4 T lymphocyte-mediated disease, might be an amenable target for therapeutic MSC administration and might also provide an opportunity to elucidate mechanisms of MSC actions in vivo. His group found that systemic administration of either syngeneic or allogeneic MSCs during antigen sensitization in a mouse model of ovalbumin-induced allergic airways inflammation inhibited both airways hyperreactivity as well as Th2-mediated eosinophilic lung inflammation through an IFNγ-dependent but T regulatory cell, Th17, and TRL9-independent mechanism (432). They further found that CMFDA Cell Tracker Dye–labeled MSCs migrate to mediastinal lymph nodes and spleen, sites of antigen-specific CD4 T lymphocyte activation in this model. Contrary to existing in vitro data, MSC administration did not affect antigen-specific CD4 proliferation but rather promoted a Th1 phenotype in vivo by increasing antigen-specific Th1 immunoglobulins in serum. Dr. Weiss and colleagues therefore speculated that the mechanism by which MSCs decrease allergic airways inflammation is, at least in part, by influencing the CD4 phenotype at sites of CD4 T lymphocyte activation in addition to direct actions in lung. In addition, MSC administration has no obvious effect on the generation of T regulatory cells. Dr. Weiss concluded that systemic MSC administration during antigen-sensitization does not inhibit antigen-specific CD4 T cell proliferation, interfere with antigen presentation by dendritic cells, effect T regulatory cell function to inhibit proliferation of clonal antigen-specific T cells, or have an obvious effect on Th17 CD4 T cells. What they do is shift CD4 T cell phenotype from Th2 to Th1, and IFNγ is necessary for the ameliorating effects of MSCs on allergic airways inflammation.
Session 5: EPCs and Clinical Trials in Lung Diseases
This session addressed the methods used to define endothelial progenitor populations and the current limitations of these approaches. Participants discussed the contributions of specific populations of endothelial, hematopoietic, and endogenous lung progenitor cells to postnatal vasculogenesis, and reviewed the potential therapeutic applications of specific cell types in lung diseases, including PAH, ALI, and ARDS.
The session began with Mervin Yoder (Indiana University) who defined different circulating populations of proangiogenic cells and discussed approaches to modulate the ability of endothelial colony forming cells (ECFCs) to generate vascular networks in vivo, focusing on modulation of vessel formation by 3D collagen matrices. He began with an overview of different types of circulating angiogenic cells, emphasizing the difficulty in identifying true EPCs and the need for complementary approaches to establish progenitor potential of whichever population is being assessed given that there is no specific surface marker with which to identify true EPCs (Figure 3) (19, 40, 41, 282). Circulating EPCs are typically defined as adherent cells that capture and ingest acetylated low-density lipoprotein (Ac-LDL) and bind the lectin Ulex europaeus I (UEA-1) after 4 to 9 days of in vitro culture in defined endothelial cell culture medium. However, uptake of Ac-LDL is not specific for endothelial cells and UEA-1 does not distinguish between endothelial cells (EC), progenitors, and hematopoietic cells, necessitating the use of additional properties to define specific cell types. The adherent population of mononuclear cells can be divided into early outgrowth cells (also known as CFU-Hill) and late outgrowth cells (known as ECFC) depending on the method used for culture. Dr. Yoder reviewed the evidence supporting a true progenitor role for a population of cells described as ECFC. ECFC are defined functionally by their ability to undergo clonal proliferation and replate into secondary endothelial colonies, to form tubes in vitro and new vessels in vivo, and are contained within the CD34+CD45- population. ECFCs derived from different sources vary in growth potential, with cord blood–derived ECFC displaying greater proliferative potential than ECFC from adult peripheral blood. Similarly, rat pulmonary microvascular endothelial cells (PMVECs) display a higher proliferative potential following serum stimulation than do pulmonary arterial endothelial cells, suggesting enrichment for ECFC. In vivo transplantation experiments demonstrate that ECFC cultured in collagen/fibronectin gels for 24 hours and then implanted subcutaneously in NOD/SCID mice demonstrate vessel formation after 2 to 4 weeks with strong CD31 staining. Biophysical properties of the gels are modulated by collagen concentration, which in turn dictates the morphology of ECFC-derived structures in vivo, with increased matrix collagen concentration resulting in increased average and total vessel area. Dr. Yoder then discussed newer strategies for isolation of ECFC from cord blood based on surface phenotype and suggested that modifications in approach to flow cytometry could allow identification of populations not previously detected. In particular, bi-exponential display may facilitate detection of previously undetected populations. This approach was used to characterize CD34+CD45− cells in cord blood, which was shown to be highly enriched for ECFC, and this population also expresses CD31, CD146 and CD105. CD34+CD45− ECFC form blood vessels in vivo in collagen gels. Women with breast cancer appear to mobilize live CD34+CD45− ECFC and apoptotic CD34+CD45− circulating endothelial cells in their peripheral bloodstream after treatment with sunitinib and paclitaxel.
In the second part of the talk, Dr. Yoder discussed the role of cells referred to as circulating progenitor cells (CPCs) that have been reported to serve as a biomarker for cancer progression. Analysis of CPCs with advanced flow cytometry techniques demonstrated that these cells have variable expression of AC133 and are able to undergo multilineage engraftment in NOD/SCID mice but are unable to give rise to tube-like structures in vitro and blood vessels in NOD/SCID mice in vivo. Tail vein injection of CPC increases melanoma growth. Women with breast cancer have a higher ratio of proangiogenic to nonangiogenic CPC and this ratio decreases with treatment. Thus, CD31+CD34+CD45+AC133+ CPC are enriched in hematopoetic stem/progenitor activity, are proangiogenic and promote tumor growth, and are enriched in the bloodstream of patients with breast cancer. In contrast to the CD34+CD45− cells described above, these tumor promoting cells appear not to be vascular progenitors but to be capable of promoting vascular growth. Together, these findings suggest that there are circulating cells that can either promote vasculogenesis or can actually, themselves, participate in vasculogenesis. The former are likely of hematopoietic origin, whereas the latter are true ECFCs and are suggested to be derived from the endothelium.
The second topic in this session addressed the contribution of bone marrow–derived precursors and tissue-resident endothelial progenitors to vascular remodeling in PAH and was presented by Serpil Erzurum (Cleveland Clinic Foundation). Dr. Erzurum reviewed the histopathological features of PAH, noting abnormalities that indicate endothelial injury and dysfunction including the presence of thrombotic lesions, plexogenic lesions with aneurysm formation, platelet aggregates and fibrotic lesions, as well as increased endothelial cell proliferation reflected in an increase in Ki67 staining. In contrast to the ECFC described above by Dr. Yoder, the cell under consideration is a bone marrow–derived cell identified by the expression of CD34 and CD133. These cells are normally present in the circulation at low levels and give rise to daughter endothelial-like cells or colony forming units of endothelial cells (CFU-EC). Dr Erzurum addressed two central questions, namely, whether bone marrow–derived EPCs that are CD45+CD34+CD133+ are increased in the circulation of patients with PAH, and whether these numbers are related to disease severity and remodeling. FACS data from her laboratory (567) demonstrate an increase in CD133+CD34+ progenitors in patients with idiopathic pulmonary arterial hypertension (IPAH), which correlates directly with increases in pulmonary artery pressure. Similar to their work, a study by Toshner and colleagues (245) demonstrated an increase in CD133+/CD34+/VEGFR2+ cells in PAH and an increase in CD133+ cells in PAH vascular lesions in vivo consistent with dysfunction of endothelial progenitors in PAH. Work from Dr Erzurum's laboratory also showed an increase in CFU-EC derived from circulating proangiogenic precursors in IPAH, which was related to the percentage of CD133+CD34+ cells. Dr. Erzurum then described the role of isolated primary lung endothelial cells in vessel formation in IPAH and demonstrated increased affinity of CFU-EC for mature endothelial cells in a tube formation assay. Following in vivo innoculation of PAH CFU-EC in Matrigel plugs subcutaneously into NOD/SCID mice, there was increased vessel formation with migration into the surrounding tissue. Dr. Erzurum described a xenograft model of human CD133+ endothelial cells isolated from IPAH explant lungs. PAH CD133+ cells have higher engraftment than controls, increased myelopoiesis, and a trend for higher megakaryopoieis and increased morbidity. In contrast, animals injected with CD133− cells had no unfavorable events. Strikingly, infusion of PAH cells led to cardiac and lung endothelial injury and in situ thrombosis. Plasma von Willebrand Factor and nitric oxide levels indicate endothelial cell activation/injury as well as greater vascular network formation in lungs of mice engrafted with PAH CD133+ cells. These findings suggest an association between circulating human progenitors and lung vascular remodeling and that PAH CD133+ cells foster pulmonary angiogenic remodeling in NOD/SCID mice associated with in situ thrombus formation. Increased von Willebrand Factor further suggests endothelial cell injury and platelet activation as underlying mechanisms and that dysfunctional proangiogenic cells promote vascular injury and remodeling in PAH. Questions that remain to be answered include: what determines whether pro-angiogenic cells are beneficial to recovery from vascular injury or are they contributory to remodeling? Is it chronicity that determines the response? Does underlying lung health select for the type of EPC produced in the bone marrow and direct their function? And, lastly, is there a benefit to therapy that inhibits EPC for regression of vascular remodeling/inflammation?
Asrar Malik (University of Illinois at Chicago) then discussed work from his laboratory on the derivation of mesodermal cells from human embryonic stem (ES) cells and their protective effect in a septic shock model, and also addressed the contribution of bone marrow–derived progenitor cells (BMPCs) in the regulation of endothelial barrier function in mice as defined by microvascular permeability alterations at the level of adherens junctions. The hypothesis underlying the first part of the talk was that coexpression of vascular endothelial growth factor receptor (VEGFR) and ACE defines emerging endothelial and vascular lineages during differentiation of human embryonic stem cells. For these studies, ES cells were differentiated to embryoid bodies (EB) in suspension cultures. FACS on Day 7 allowed identification of MSC (KDR−, ACE−), HSC (ACE+, KDR−), and EPC (KDR+, ACE+). Subculture of dissociated EB cells on Day 7 revealed morphologically identifiable hematopoietic colonies and also EPC colonies. The hematopoietic cells were CD43+ and VE-cadherin−, whereas ACE+/KDR+ cells were VE-cadherin+. The KDR+/ACE+ cells formed tubes/lumens in Matrigel (BD Biosciences, Bedford, MA). Transplantation studies demonstrated improved survival following lipopolysaccharide (LPS) with administration of hES-derived EB cells (both KDR+/ACE+ and KDR−/ACE+), whereas there was no advantage after transplantation of hES-derived adherent cells or of conditioned medium from EBs. Lung IL-10 levels were greatest in LPS+ Day 7 EB-derived cells. In the second part of the talk, Dr. Malik discussed effects of BMPC on accumulation of extravascular lung water after LPS. BMPC reduced extravascular lung leak after LPS. There appeared to be integration of progenitor cells, suggesting that leak was prevented by barrier re-annealing. Future studies will be directed at characterizing surface markers of mesoderm derived precursors, lineage tracing and characterization, evaluating effects of growth factor signals, and evaluating functional studies in vitro and in vivo. BMPCs characterized as Sca1+, CD133+, CD31−, VE-cadherin low and CD14+. BMPCs express TLR4, which is LPS sensing and taken up into the lung at 3 days post-LPS challenge. BMPCs induce activation of RhoGTPAses in endothelial cells involving Rac1 and Cdc42 and required paracrine release of sphingosine-1-phosphatase suggesting cellular cross-talk as the basis for progenitor cell mediated barrier protection.
Judith Shizuru (University of California, Los Angeles) next described an approach that led to the enrichment of a population of lung-derived cells that engraft and are able to generate alveolar endothelium. She first described the strategy traditionally used to isolate and characterize hematopoietic stem cells (HSC), which was based on their ability to repopulate the bone marrow of lethally irradiated mice. This approach was adapted to isolate and transplant a population of marked lung cells using irradiation injury to create niche space in the lung. To obtain candidate lung-derived progenitor cells, lungs from juvenile mice (5 wk of age) that constitutively express green fluorescent protein (GFP) were dissociated and sorted into distinct populations that were based on positive selection for surface markers expressed on stem cells in other tissues, namely, Sca-1, Thy-1, and Notch-1, and negative selection for markers of mature lung cells (e.g., CD45, T1α, and Maclura pomifera lectin). Marked lung subfractions were intravenously cotransplanted together with nonlabeled purified HSC into lethally irradiated adult recipient mice. Whole bone marrow was used as control. Of five fractions sorted on the basis of surface expression or lack of expression of CD45, Sca1, and Notch, only the population marked by CD45−, Sca1+, and Notch-1−/lo exhibited engraftment and expansion as GFP+ clusters in recipient lungs. At 24 hours postinfusion, the CD45− fraction could be visualized in the lung and persisted up to 35 days. The greatest frequency of these cells was obtained from young mice 4 weeks of age. The clusters demonstrated the morphology and surface marker expression of capillary endothelial cells and showed increased expansion between 4 and 8 weeks. Compared with bone marrow–derived CD45− cells, the population was enriched for cells expressing markers of EPCs (CD34+, CD133+, and KDR+). Thus, a candidate lung population of cells has been identified that express stem cell and EPC surface markers and are capable of expansion and engraftment as clusters of contiguous cells in radiation-injured recipient lungs in vivo. Further questions to be addressed include whether these cell clusters are clonal, whether the cells contribute meaningfully to repair after lung injury, whether there is a finite amount of niche space for these cells, where these cells reside in normal lung, and finally, what are the dynamics of the orchestrated regeneration of endothelial and epithelial components. In addition, whether the cell clusters are in continuity with capillaries will need to be determined.
In the final presentation of this session, Duncan Stewart (Ottawa Health Research Institute, University of Ottawa) reviewed preclinical studies assessing cell-based therapies for PAH and ALI/ARDS. The rationale for administration of bone marrow–derived EPCs is based on evidence that EC apoptosis is the initiating lesion in PAH that subsequently leads to degeneration or obliteration of precapillary arterioles and the possibility that administration of EPCs may contribute to repair and regeneration of pulmonary vessels. Studies of culture-derived EPCs in the monocrotaline (MCT) model of PAH demonstrated engraftment of these endothelial-like cells into distal precapillary arterioles 1 week post-MCT and prevention of development of pulmonary hypertension at 21 days when cells are delivered 3 days after MCT. Delaying cell therapy until 3 weeks postinjury still had an effect and prevented further progression. Delivery of EPCs that were genetically engineered to overexpress endothelial nitric oxide synthase (eNOS) reversed established PAH. Based on these preclinical findings, an early phase clinical trial (pulmonary hypertension and cell therapy trial, or PHACeT) has been initiated to test efficacy and safety of eNOS transfected autologous EPCs in patients with refractory PAH. The study was designed as a safety study with up to 18 patients and the primary endpoint was tolerability of cell transplantation in patients with PAH refractory to all standard therapies. Autologous early growth EPCs transfected with eNOS were delivered by Swan Ganz catheter to the right ventricle. Dose ranging studies were performed over 3 days with cells given in divided doses in an overlapping protocol. Exclusion criteria included hemodynamic instability or hospitalization for worsening right heart failure in the previous 3 months. Preliminary data thus far indicate a trend toward lower total pulmonary vascular resistance at 3 days with at an intermediate dose. Seven more patients are needed to complete the trial, with an anticipated time line of 7 to 12 months until completion. The last part of the talk focused on MSC therapy for ARDS. Advantages of MSCs include ease of growth and transfection, immunomodulatory activity, and the potential for use of allogeneic cells. Preclinical studies were performed in an LPS model in mice using MSCs or MSCs transduced with angiopoietin-1 30 minutes after administration of LPS. There was a dramatic improvement in lung histology, release of interferon-γ, TNF-α, IL-6 and IL-1β. Similar studies were undertaken in a cecal ligation model. Cells were administered 6 hours after surgery with killing at 48 hours. There was a significant reduction in circulating cytokines, improvement in multiorgan dysfunction, and an improvement in survival. It is suggested that the MSCs may reprogram monocytes/macrophages to increase IL-10 through release of prostaglandin E2. In ALI and ARDS, endothelial activation is one of the first events in the pathological chain leading to increased air–blood permeability, leukocyte adhesion, and alveolar filling.
A subsequent discussion on the pros and cons of moving toward further clinical trials was led by Drs. Zea Borok (University of Southern California), Kenneth Brigham (Emory University), Polly Parsons (University of Vermont), Stephen Rennard (University of Nebraska), and Steven Shapiro (University of Pittsburgh). There are currently two active clinical trials of stem/progenitor cells in lung diseases, one each for pulmonary hypertension and for COPD. There is also one clinical investigation of progenitor cells in lung cancer occurring in the United States and Canada. These are listed in clinicaltrials.gov (Table 5). The discussion began with the question of whether a single cell type will be the answer for lung regeneration. There was some discussion regarding which cell type is best and that it may be different for different diseases. It was suggested that perhaps multiple cell types and a more orchestrated approach will be needed. The question was raised as to whether we are ready for clinical trials, and considerable discussion focused on the need for empiricism in clinical medicine. It was felt that the field as a whole had advanced from initial discussions 4 years ago regarding whether cells had engrafted to now evaluating other effects of cell-based therapy, including immune effects. It was felt by some panel members that overall there was a need to proceed with human studies and that animal studies were insufficient. There was strong sentiment regarding the need for good databases and sample/tissue banks to generate as much information as possible from ongoing clinical trials. It was felt that a registry should be established to include small trials and that all trials should store blood and other samples for later analysis. There was concern that many commercially run trials are not collecting sufficient samples or patient information and are not making the data publicly available. The final discussion focused on the ultimate goal of cell-based therapy being to alter the structure of the lung with a need for good functional/physiologic correlates. There was no uniform agreement on the best end-point to evaluate structure. The session ended with the recognition that since clinical trials have started, there is a major need to define mechanistic and clinical end-points to learn as much as we can from ongoing trials. Overall, there was a consensus that lung diseases still present major challenges, but there is increasing, although not uniform, support to cautiously move ahead with additional carefully designed trials and investigations. This is a shift from previous conferences. Salient points from the pro-con discussion are summarized in Table 6.
TABLE 5.
CLINICAL INVESTIGATIONS AND TRIALS OF STEM/PROGENITOR CELLS IN LUNG DISEASES LISTED IN CLINICALTRIALS.GOV
| Pulmonary Hypertension | |
|---|---|
| (1) Safety and Efficacy Study of Transplantation of EPCs to Treat Idiopathic Pulmonary Arterial Hypertension. (completed, Wang et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:566-1571). NCT00257413. Completed.The First Affiliated Hospital, College of Medicine, Zhejiang University, China PI: Junzhu Chen, M.D. |
Objectives: The goal of this study was to investigate the feasibility, safety, and initial clinical outcome of intravenous infusion of autologous endothelial progenitor cells (EPCs) in patients with idiopathic pulmonary arterial hypertension (IPAH). Background: Experimental data suggest that transplantation of EPCs attenuates monocrotaline-induced pulmonary hypertension in rats and dogs. In addition, clinical studies suggest that autologous progenitor cell transplantation is feasible and safe in patients with ischemic diseases. Methods: We conducted a prospective, randomized trial comparing the effects of EPC transplantation plus conventional therapy with those of conventional therapy alone in patients with IPAH. The primary end point was change in the 6-minute-walk distance using a standardized protocol. The secondary end points were changes in hemodynamic variables as assessed by right heart catheterization. Results: After 12 weeks of follow-up, the mean distance walked in 6 minutes increased by 48.2 m in the cell infusion group (from 263 ± 42 m to 312 ± 34 m), and an increase of 5.7 m occurred in the conventional therapy group (from 264 ± 42 m to 270 ± 44 m). The mean difference between the two groups was 42.5 m (95% confidence interval 28.7 to 56.3 m, P < 0.001). The patients in the cell infusion group also had significant improvement in mean pulmonary artery pressure, pulmonary vascular resistance, and cardiac output. There were no severe adverse events with cell infusion. Conclusions: This preliminary study showed that intravenous infusion of autologous EPCs seemed to be feasible and safe, and might have beneficial effects on exercise capacity and pulmonary hemodynamics in patients with IPAH. |
| (2) Pulmonary Hypertension: Assessment of Cell Therapy (PHACeT). NCT00469027. Recruiting.PIs: Michael Ward, M.D.. (St. Michael's Hospital, Toronto, ON, Canada); David Langleben, M.D., Sir Mortimer B. Davis (Jewish General Hospital, Montreal, PQ, Canada) | The primary objective is to establish the safety of autologous progenitor cell-based gene therapy of heNOS in patients with severe pulmonary arterial hypertension (PAH) refractory to conventional treatment. Northern Therapeutics. This is a two-center, phase I clinical trial. A total of 18 patients will be studied using an open-label, dose-escalating protocol; three patients will be entered into each of the five dosing panels. An additional three patients will be entered into the final dose panel to establish safety at the maximum tolerated dose. |
| (3) Endothelial Progenitor Cells and Pulmonary Idiopathic Arterial Hypertension. NCT00551408. Active, not recruiting. Unidad de Investigacion Clinica en Medicina SC, Monterrey, Mexico PI: Carlos J. Sanchez Diaz, M.D. | Current evidence established that endothelial progenitor cells (EPC) participate in several models of vascular disease as acute coronary syndromes, stroke, diabetes, peripheral artery disease, etc. However EPC in the setting of PAH is less well established. The target of this study is to demonstrate if the number of EPC is increased in a Mexican population of patients with PAH. |
| COPD | |
| (1) A Phase II, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of PROCHYMAL (Ex Vivo Cultured Adult Human Mesenchymal Stem Cells Intravenous Infusion for the Treatment of Subjects With Moderate to Severe Chronic Obstructive Pulmonary Disease [COPD]).NCT00683722. Active, not recruiting.Osiris Therapeutics, Columbia, MD. | COPD is currently the fourth leading cause of death in the United States. It is clear that there is a significant unmet medical need for safe and effective therapies to treat moderate to severe COPD. This patient population has a high mortality rate and requires frequent hospitalizations due to disease-related exacerbations. Based on severity distribution estimates, approximately 70% of all current COPD patients have either moderate or severe COPD. |
| COPD has no known cure, thus current therapeutic intervention is aimed at providing relief of symptoms. Oxygen therapy is the only treatment that has been shown to improve survival. Smoking cessation has been shown to slow the rate of FEV1 decline and COPD progression. In general patients are treated with bronchodilators and inhaled corticosteroids, but again, these measures do not provide any significant benefit regarding disease progression or prognosis. The characteristics and biologic activity of PROCHYMAL, along with a good safety profile in human trials to date, suggest that PROCHYMAL may be a good candidate for addressing this unmet medical need. | |
| (2) Unicentric Study Protocol of Cell Therapy in Chronic Obstructive Pulmonary Disease. NCT01110252. Active, not recruiting.Aboratório de Genética Humana e Terapia Celular, Unesp–Assis Instituto de Molestias Cardiovasculares, Sao Paulo, Brazil.PI: João T. Ribeiro-Paes, Ph.D., M.D. | The main feature of the pulmonary emphysema, included in range of the chronic obstructive pulmonary disease (COPD), is the airflow obstruction resulting from the destruction of the alveolar walls distal to the terminal bronchiole, without significant pulmonary fibrosis. The existing clinical approaches has contributed to the enlargement and amelioration of the emphysema patients life quality, although no effective or curative treatment has been achieved. The surgical treatment, on the other hand, involves complex procedures and, in the specific case of lung transplantation, a lack of donors. |
| Considering these aspects, several experimental models have been proposed aiming to increase knowledge about the pathophysiological processes and enable new clinical approaches to the pulmonary emphysema. The cell therapy, briefly described as the use of cells in disease treatment, presents itself as a promising therapeutic approach with great potential applicability in degenerative pulmonary diseases. In this way, it is intended in this project, the proposition of a protocol to evaluate the safety of cell therapy with pool of mononuclear cells from bone marrow in patients with clinical and laboratory diagnosis of pulmonary emphysema in advanced stage (stage IV dyspnea). | |
| Lung Cancer | |
| (1) Microarray Analysis of Gene Expression and Identification of Progenitor Cells in Lung Carcinoma. NCT00568906. Recruiting. Stanford. PI: Glenn D. Rosen. | This study will investigate gene expression profiles in normal human lung tissue, lung carcinoma, and metastatic tumor to the lung. The expression of up to 20,000 genes in a given lung tissue sample will be examined by cDNA microarray analysis and compared to normal lung tissue. In addition, we hope to identify a particular subset of lung cancer cells with an enhanced capacity for proliferation and self-renewal, analogous to the stem cells recently identified for certain types of leukemia, breast cancer, and brain tumors. |
| (2) Detection of Circulating Endothelial Progenitor Cells (EPCs) in Peripheral Blood From Non-Small Cell Lung Cancer Patients. NCT00826683. Recruiting.University Hospital, Limoges, Belgium. PI: Boris Melloni, M.D. | The aim of this study is to study blood circulating levels bone-marrow-derived progenitor cells (EPCS). In a first phase, EPCs will be detected in healthy nonsmokers volunteers to validate flow cytometry method (n = 25). In addition, EPC will be characterized by primary cultures to analyze EPC-specific markers. In a second phase, EPCs will detect in peripheral blood from 50 patients with chronic obstructive pulmonary disease (COPD) and 50 patients with non-small cell lung cancers (NSCLC). Primary cultures will be made to confirm EPCS isolation. |
| Miscellaneous | |
| (1) Phase-1 Study of Autologous Bone Marrow Cell Intrabronchial Instillation for Patients with Silicosis. NCT01239862. Recruiting. Federal University of Rio de Janeiro, Brazil. PI: Marcelo Marcos Morales, M.D., Ph.D. | This study will perfume the safety (Phase I) study of 10 patients with silicosis treated with intrabronchial instillation of autologous bone marrow derived mononuclear cells (BMDMC, 2 × 107) through bronchoscopy. The inclusion criteria is: age between 18 and 50, chronic and accelerated silicosis, characterized with a fibrotic increase in the last two years, FEV1 < 60% and > 40%, FVC > 60% and SaO2 > 90%, while the exclusion criteria were: smoking, active tuberculosis or other infections, cancer, auto-immune disorders, hematological, hepatic or cardiac diseases, and pregnancy. All patients will be subjected to clinical examination, answered questionnaires of quality of life (SGRQ and SF36) and dyspnea score (Borg), performed high resolution CT of thorax, pulmonary function tests with DlCO and 6-minute-walk test and lung perfusion scintigraphy before and 7, 30, 60, 180, and 360 days after treatment. |
TABLE 6.
SUMMARY OF CONSIDERATIONS FOR CURRENT AND FUTURE CLINICAL TRIALS OF STEM/PROGENITOR CELLS IN LUNG DISEASES
| Pro/Prospects |
| 1. There is a considerable safety record with the clinical use of adult stem and progenitor cells. A long history of clinical trials with stem cells for hematopoietic cells has justified the risk versus benefit evaluations for many diseases. Approximately 80 clinical trials with MSCs and other cells from bone marrow are currently listed in www.clinicaltrials.gov and no significant adverse effects have been reported to date. |
| 2. Promising results have been obtained with administration of MSCs for several non-pulmonary diseases, even though definitive data have not yet been published. |
| 3. Promising results on both safety and efficacy have recently been published on use of endothelial precursor cells in patients with primary pulmonary hypertension. Most recently, interim results from the current trial of allogeneic MSCs for COPD demonstrates safety and suggests efficacy. |
| 4. Numerous recent reports indicate that MSCs and similar cells can repair injury to lung and other tissues in animal models by being activated to secrete therapeutic factors and without long-term engraftment of the cells. Therefore, the probability of long-term adverse effects from engraftment of the cells is greatly decreased. |
| 5. If the therapeutic factors can be identified, administration of the factors may provide an alternative strategy for the therapy of some lung diseases. However, MSCs and similar cells demonstrate a remarkable ability to engage in cross-talk with injured tissues and produce therapeutic benefits both by secretion of soluble factors and by cell-to-cell contacts that may not be duplicated by administration of soluble factors. Therefore, optimal therapy for lung diseases may involve administration of soluble factors for some conditions, cells for others, and a combination of both for still other conditions. |
| 6. Impressive data have recently been obtained demonstrating that MSCs or other cells such as alveolar cells, or conditioned medium from the cells, are both safe and effective in animal models for lung disease including, acute lung injury, asthma, COPD, bronchopulmonary dysplasia, and others. Most recently, human MSCs have been demonstrated to have comparable effects in endotoxin-injured isolated human lung preparations. These data appear to provide a firm basis for developing clinical trials in patients. |
| 7. Rapid progress continues to be made with a number of creative approaches in identifying progenitor and stem-like cells in the lung and in defining the hierarchy of differentiation of such cells both during embryonic development and in response to injury. New therapeutic strategies are likely to arise by further research on these topics that may identify factors for lung diseases that enhance propagation and differentiation of such stem-like cells. |
| 8. Results in animal models have indicated that some cells from bone marrow and other sources can engraft and differentiate into to multiple types of cells in the lung. However, early reports were marred by artifacts and the degree of engraftment is usually low. Whether there are specific cells in bone marrow that engraft in lung is not well understood. Therefore, there is no obvious current clinical strategy for therapies requiring long-term engraftment in lung of cells from bone marrow. |
| 9. In contrast, progress has recently been made in devising artificial lung constructs that may provide temporary lung assist devices for patients awaiting lung transplant much as left ventricular assist devises have proven useful for patients awaiting cardiac transplants. |
| 10. In parallel, rapid progress is being made in devising ex vivo three-dimensional and other culture systems for generating functional lung tissue that might conceivably be implanted into diseased lungs. As such, reconstructing the damaged structure of the lung in patients with chronic lung diseases remains a specific but still distant goal for current clinical research. |
| 11. Clinical trials with cell therapies in lung diseases have lagged behind similar therapies for other diseases in part because these diseases present difficult disease targets. However, representatives of several patient oriented respiratory disease foundations asked that we not allow “perfect to become the enemy of good.” |
| Con/Precautions |
| 1. Clinical trials of cell and related therapies in lung diseases must proceed with a keen awareness that every known therapeutic measure in patients has the potential to produce adverse effects. Any trial of a new therapy must therefore be based on careful evaluation of the potential benefits and potential risks to patients as far as these can be discerned. |
| 2. Among the potential adverse effects of cell therapies currently under consideration is the creation of tumors and malignancies in patients. The risk is present with any cell type that can be propagated in culture. However, the risks vary greatly by the biological properties of the cells and the conditions under which they are cultured. On the basis of this evidence, there is clear difference in the probability of risks between administration of cells like human MSCs and similar cells that regularly undergo senescence as they are expanded, and those associated with administration of immortalized cells like ES and iPS cells that do not undergo senescence in culture. |
| 3. Cell therapies probably pose other risks that are still undefined. Among the potential risks: After intravenous infusion, many cells are trapped in the lung and therefore pose the risk of lethal pulmonary emboli. Cells that repair tissues may enhance the growth of undetected cancers, as has been demonstrated for MSCs in some animal models. Local administration of a bolus of cells may prompt the cells to aggregate and differentiate into bone and other inappropriate structures. |
Definiton of abbreviations: COPD = chronic obstructive pulmonary disease; ES = ; iPS = ; MSCs = mesenchymal stem cells.
Session 6: Recommendations—Summation, and Future Directions
This session included a presentation from Diane Krause (Yale) on the current status of potential engraftment of bone marrow–derived cells as lung epithelium. This concept remains controversial, although several papers suggest that less-explored or less-understood bone marrow–derived cell populations may indeed engraft, although still at low level, as airway or alveolar epithelium. A subsequent presentation from Jeffrey Kahn (University of Minnesota) further considered the ethical and policy issues regarding the use of stem cells and moving toward further clinical trials and cell therapy investigations in lung diseases. Following this, James Kiley PhD, Chief of the Lung Biology and Disease Branch of the NHLBI, presented the NHLBI perspective on stem cell and cell therapy approaches for lung diseases. Available resources from the NHLBI and the NIH were described including the NIH Production Assistance for Cellular Therapies (PACT) program, at http://www.pactgroup.net. Additional resources are listed in the conference recommendations. Also included in this session were presentations from Daniel Rose, M.D.; John Walsh, M.D. (University of Florida); and Amy Farber, Ph.D., CEOs, respectively, of the Pulmonary Fibrosis Foundation, Alpha One Foundation, and the LAM Treatment Alliance, on the importance of stem cell research to these particular patient populations and the role of nonprofit research organizations in supporting this and other novel areas of research. Zea Borok, M.D. (University of Southern California) gave a comparable presentation on behalf of the American Thoracic Society.
SUMMARY AND CONCLUSIONS
A continuing accumulation of data in both animal models and in clinical trials suggests that cell- based therapies and novel bioengineering approaches may be potential therapeutic strategies for lung repair and remodeling after injury. In parallel, further understanding of the role of endogenous lung progenitor cells will provide further insight into mechanisms of lung development and repair after injury and may also provide novel therapeutic strategies. Remarkable progress has been made in these areas since the last conference 2 years ago. It is hoped that the workshop recommendations (Table 2) will spark new research that will provide further understanding of the mechanisms of repair of lung injury and a sound scientific basis for therapeutic use of stem and cell therapies in lung diseases.
Organizing Committee:
Zea Borok, M.D., University of Southern California
Carla Kim, Ph.D., Boston Children's Hospital
Luis Ortiz, M.D., University of Pittsburgh
Angela Panoskaltsis-Mortari, Ph.D., University of Minnesota
Darwin J. Prockop, M.D., Ph.D., Tulane University
Jeffrey Spees, Ph.D., University of Vermont
Benjamin T. Suratt, M.D., University of Vermont
Daniel J. Weiss, M.D., Ph.D., University of Vermont
A list of participants, executive summaries of speaker presentations, and poster abstracts are included in the accompanying online supplement.
Supplementary Material
Acknowledgments
The organizers are indebted to the staff of the University of Vermont Continuing Medical Education and University of Vermont College of Medicine Communications Offices, notably Terry Caron, Natalie Remillard, Jennifer Nachbur, and Carole Whitaker for organizational support and to Carla Kim, Bethany Moore, Susan Reynolds, Barry Stripp, Viranuj Sueblinvong, and Mervin Yoder for editing and conceptual support for the final conference report.
Conference was supported by: Alpha-1 Foundation, American Thoracic Society, Emory Center for Respiratory Health, LAM Treatment Alliance, National Heart, Lung, and Blood Institute, R13 Conference Grant (HL097533), Pulmonary Fibrosis Foundation, University of Vermont Department of Medicine, and the Vermont Lung Center.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: D.J.W. was a consultant for GlaxoSmithKline, is on the Advisory Board for the ALA, and received clinical trials support from Osiris Therapeutics Inc. I.B. owns a patent through the Australian Stem Cell Centre. Z.B., C.K., A.P.-M., S.R., and M.R. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.S. received lecture fees from AstraZeneca. D.W. and D.J.P. do not have a financial relationship with a commercial entity that has an interest in the conference.
References
- 1.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 2006;3:193–207. [DOI] [PubMed] [Google Scholar]
- 2.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltis-Mortari A, Prockop DJ. Stem Cells and Cell Therapy Approaches for Lung Diseases. Conference Report. Proc Am Thorac Soc 2008;5:637–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JK, Hogan BLM, Randell SH, Stripp B., Weiss DJ. Human embryonic stem cell research: An Official ATS Research Policy Statement. Am J Respir Crit Care Med 2006;173:1–3.16368789 [Google Scholar]
- 4.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007;87:858–870. [DOI] [PubMed] [Google Scholar]
- 5.Flotte TR, Ng P, Dylla DE, McCray PB Jr, Wang G, Kolls JK, Hu J. Viral vector-mediated and cell-based therapies for treatment of cystic fibrosis. Mol Ther 2007;15:229–241. [DOI] [PubMed] [Google Scholar]
- 6.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 2007;24:819–841. [DOI] [PubMed] [Google Scholar]
- 7.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med 2007;175:547–553. [DOI] [PubMed] [Google Scholar]
- 8.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol 2007;82:449–456. [DOI] [PubMed] [Google Scholar]
- 9.Kim CB. Advancing the field of lung stem cell biology. Front Biosci 2007;12:3117–3124. [DOI] [PubMed] [Google Scholar]
- 10.Kim CF. Paving the road for lung stem cell biology: bronchioalveolar stem cells and other putative distal lung stem cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L1092–L1098. [DOI] [PubMed] [Google Scholar]
- 11.Kotton DN, Fine A. Lung stem cells. Cell Tissue Res 2008;331:145–156. [DOI] [PubMed] [Google Scholar]
- 12.Loebinger MR, Janes SM. Stem cells for lung disease. Chest 2007;132:279–285. [DOI] [PubMed] [Google Scholar]
- 13.Olsson F, Denham M, Cole TJ, Hooper SB, Mollard R. Deriving respiratory cell types from stem cells. Curr Stem Cell Res Ther 2007;2:197–208. [DOI] [PubMed] [Google Scholar]
- 14.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 2007;25:2896–2902. [DOI] [PubMed] [Google Scholar]
- 15.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 2007;82:241–243. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood 2007;109:3147–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sueblinvong V, Suratt BT, Weiss DJ. Novel therapies for the treatment of cystic fibrosis: new developments in gene and stem cell therapy. Clin Chest Med 2007;28:361–379. [DOI] [PubMed] [Google Scholar]
- 18.Weiss DJ. Stem cells and cell therapies for cystic fibrosis and other lung diseases. Pulm Pharmacol Ther 2008;21:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28:1584–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause DS. Bone marrow-derived lung epithelial cells. Proc Am Thorac Soc 2008;5:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc 2008;5:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Engelhardt JF. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc 2008;5:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loebinger MR, Sage EK, Janes SM. Mesenchymal stem cells as vectors for lung disease. Proc Am Thorac Soc 2008;5:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy J, Summer R, Fine A. Stem cells in airway smooth muscle: state of the art. Proc Am Thorac Soc 2008;5:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlins EL. Lung epithelial progenitor cells: lessons from development. Proc Am Thorac Soc 2008;5:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawlins EL, Okubo T, Que J, Xue Y, Clark C, Luo X, Hogan BL. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb Symp Quant Biol 2008;73:291–295. [DOI] [PubMed] [Google Scholar]
- 27.Rippon HJ, Lane S, Qin M, Ismail NS, Wilson MR, Takata M, Bishop AE. Embryonic stem cells as a source of pulmonary epithelium in vitro and in vivo. Proc Am Thorac Soc 2008;5:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc 2008;5:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008;5:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varanou A, Page CP, Minger SL. Human embryonic stem cells and lung regeneration. Br J Pharmacol 2008;155:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abman SH, Matthay MA. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia: delivering the secretome. Am J Respir Crit Care Med 2009;180:1039–1041. [DOI] [PubMed] [Google Scholar]
- 32.Alison MR, Lebrenne AC, Islam S. Stem cells and lung cancer: future therapeutic targets? Expert Opin Biol Ther 2009;9:1127–1141. [DOI] [PubMed] [Google Scholar]
- 33.Blaisdell CJ, Gail DB, Nabel EG. National Heart, Lung, and Blood Institute perspective: lung progenitor and stem cells–gaps in knowledge and future opportunities. Stem Cells 2009;27:2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Matsumoto K, Stripp BR. Bronchiolar progenitor cells. Proc Am Thorac Soc 2009;6:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther 2009;9:1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 2009;17:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder JC, Teisanu RM, Stripp BR. Endogenous lung stem cells and contribution to disease. J Pathol 2009;217:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sueblinvong V, Weiss DJ. Cell therapy approaches for lung diseases: current status. Curr Opin Pharmacol 2009;9:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss DJ. Intersection of gene therapy and progenitor cell biology in the lung. Mol Ther 2009;17:214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoder MC, Ingram DA. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim Biophys Acta 2009;1796:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol 2009;16:269–273. [DOI] [PubMed] [Google Scholar]
- 42.Bonfield TL, Caplan AI. Adult mesenchymal stem cells: an innovative therapeutic for lung diseases. Discov Med 2010;9:337–345. [PubMed] [Google Scholar]
- 43.Critser PJ, Yoder MC. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Curr Opin Organ Transplant 2010;15:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J 2010;35:418–425. [DOI] [PubMed] [Google Scholar]
- 45.Panoskaltsis-Mortari A, Weiss DJ. Breathing New Life Into Lung Transplantation Therapy. Mol Ther 2010;18:1581–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol 2010;42:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss DJ, Finck CM. Embryonic stem cells and repair of lung injury. Mol Ther 2010;18:460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams DA, Keating A. Enhancing research in regenerative medicine. 2010. Blood 2010;116:866–867. [DOI] [PubMed] [Google Scholar]
- 49.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoyt RF Jr, McNelly NA, Sorokin SP. Dynamics of neuroepithelial body (NEB) formation in developing hamster lung: light microscopic autoradiography after 3H-thymidine labeling in vivo. Anat Rec 1990;227:340–350. [DOI] [PubMed] [Google Scholar]
- 51.Johnson NF, Hotchkiss JA, Harkema JR, Henderson RF. Proliferative responses of rat nasal epithelia to ozone. Toxicol Appl Pharmacol 1990;103:143–155. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Engelhardt JF. Progress toward generating a ferret model of cystic fibrosis by somatic cell nuclear transfer. Reprod Biol Endocrinol 2003;1:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harkema JR, Plopper CG, Hyde DM, St George JA, Wilson DW, Dungworth DL. Response of the macaque nasal epithelium to ambient levels of ozone: a morphologic and morphometric study of the transitional and respiratory epithelium. Am J Pathol 1987;128:29–44. [PMC free article] [PubMed] [Google Scholar]
- 54.Plopper CG, Smiley-Jewell SM, Miller LA, Fanucchi MV, Evans MJ, Buckpitt AR, Avdalovic M, Gershwin LJ, Joad JP, Kajekar R, et al. Asthma/allergic airways disease: does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol Pathol 2007;35:97–110. [DOI] [PubMed] [Google Scholar]
- 55.Khoor A, Gray ME, Singh G, Stahlman MT. Ontogeny of Clara cell-specific protein and its mRNA: their association with neuroepithelial bodies in human fetal lung and in bronchopulmonary dysplasia. J Histochem Cytochem 1996;44:1429–1438. [DOI] [PubMed] [Google Scholar]
- 56.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]
- 57.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol 2004;286:L643–L649. [DOI] [PubMed] [Google Scholar]
- 58.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA 2006;104:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 2002;99:10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cell a facultative progenitor cell pool. Am J Pathol 2010;177:362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA 2009;106:9286–9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 67.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 2009;27:623–633. [DOI] [PubMed] [Google Scholar]
- 68.Engelhardt JF, Allen ED, Wilson JM. Reconstitution of tracheal grafts with a genetically modified epithelium. Proc Natl Acad Sci USA 1991;88:11192–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995;121:2031–2046. [DOI] [PubMed] [Google Scholar]
- 70.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662–670. [DOI] [PubMed] [Google Scholar]
- 71.Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC, Randell SH. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol 2004;286:L631–L642. [DOI] [PubMed] [Google Scholar]
- 72.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 1976;35:246–257. [PubMed] [Google Scholar]
- 73.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest 1978;38:648–653. [PubMed] [Google Scholar]
- 74.Evans MJ, Dekker NP, Cabral-Anderson LJ, Freeman G. Quantitation of damage to the alveolar epithelium by means of type 2 cell proliferation. Am Rev Respir Dis 1978;118:787–790. [DOI] [PubMed] [Google Scholar]
- 75.Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol 1986;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- 76.Dupuit F, Gaillard D, Hinnrasky J, Mongodin E, de Bentzmann S, Copreni E, Puchelle E. Differentiated and functional human airway epithelium regeneration in tracheal xenografts. Am J Physiol Lung Cell Mol Physiol 2000;278:L165–L176. [DOI] [PubMed] [Google Scholar]
- 77.Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E, Peault B. Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells 2005;23:992–1001. [DOI] [PubMed] [Google Scholar]
- 78.Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 2007;25:139–148. [DOI] [PubMed] [Google Scholar]
- 79.Nolen-Walston RD, Kim CF, Mazan MR, Ingenito EP, Gruntman AM, Tsai L, Boston R, Woolfenden AE, Jacks T, Hoffman AM. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol 2008;294:L1158–L1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 2008;26:1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol 2009;40:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teisanu RM, Lagasse E, Whitesides JF, Stripp BR. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells 2009;27:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA 2010;107:1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian J, Mahmood R, Hnasko R, Tian J, Mahmood R, Hnasko R, Locker J. Loss of Nkx2.8 deregulates progenitor cells in the large airways and leads to dysplasia. Cancer Res 2006;66:10399–10407. [DOI] [PubMed] [Google Scholar]
- 86.Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D, Clurman BE, Dyer MA, Roberts JM. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev 2007;21:1731–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pei XH, Bai F, Smith MD, Xiong Y. p18Ink4c collaborates with Men1 to constrain lung stem cell expansion and suppress non-small-cell lung cancers. Cancer Res 2007;67:3162–3170. [DOI] [PubMed] [Google Scholar]
- 88.Sicinski P, Zacharek S, Kim C. Duality of p27Kip1 function in tumorigenesis. Genes Dev 2007;21:1703–1706. [DOI] [PubMed] [Google Scholar]
- 89.Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, Pasparakis M, Nebreda AR. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet 2007;39:750–758. [DOI] [PubMed] [Google Scholar]
- 90.Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest 2007;117:2929–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dovey J, Zacharek S, Kim CF, Lees JA. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci 2008;105:11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, Iwanaga K, Raso MG, Wislez M, Hanna AE, Wieder ED, Molldrem JJ, Wistuba II, Powis G, Demayo FJ, et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS ONE 2008;3:e2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, Fields AP. Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis. Cancer Res 2009;69:7603–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiozzo C, De Langhe S, Yu M, Londhe VA, Carraro G, Li M, Li C, Xing Y, Anderson S, Borok Z, et al. Deletion of Pten expands lung epithelial progenitor pools and confers resistance to airway injury. Am J Respir Crit Care Med 2009;180:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003;422:313–317. [DOI] [PubMed] [Google Scholar]
- 96.Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol 2009;41:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Driskell RR, Goodheart M, Neff T, Liu X, Luo M, Moothart C, Sigmund CD, Hosokawa R, Chai Y, Engelhardt JF. Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev Biol 2007;305:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell 2007;128:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hollande E, Cantet S, Ratovo G, Daste G, Bremont F, Fanjul M. Growth of putative progenitors of type II pneumocytes in culture of human cystic fibrosis alveoli. Biol Cell 2004;96:429–441. [DOI] [PubMed] [Google Scholar]
- 100.Pan J, Bear C, Farragher S, Cutz E, Yeger H. Cystic fibrosis transmembrane conductance regulator modulates neurosecretory function in pulmonary neuroendocrine cell-related tumor cell line models. Am J Respir Cell Mol Biol 2002;27:553–560. [DOI] [PubMed] [Google Scholar]
- 101.Yeger H, Pan J, Fu XW, Bear C, Cutz E. Expression of CFTR and Cl(-) conductances in cells of pulmonary neuroepithelial bodies. Am J Physiol Lung Cell Mol Physiol 2001;281:L713–L721. [DOI] [PubMed] [Google Scholar]
- 102.Pan J, Luk C, Kent G, Cutz E, Yeger H. Pulmonary neuroendocrine cells, airway innervation, and smooth muscle are altered in Cftr null mice. Am J Respir Cell Mol Biol 2006;35:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reddy R, Buckley S, Doerken M, Barsky L, Weinberg K, Anderson KD, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L658–L667. [DOI] [PubMed] [Google Scholar]
- 104.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem 2010;25:55–62. [DOI] [PubMed] [Google Scholar]
- 105.Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, Driscoll B. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol 2009;296:L57–L70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ling TY, Kuo MD, Li CL, Yu AL, Huang YH, Wu TJ, Lin YC, Chen SH, Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci USA 2006;103:9530–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia 2007;21:860–867. [DOI] [PubMed] [Google Scholar]
- 108.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol 2008;38:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X, Luo M, Guo C, Yan Z, Wang Y, Lei-Butters DC, Engelhardt JF. Analysis of adeno-associated virus progenitor cell transduction in mouse lung. Mol Ther 2008;17:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mishra S, Wang X, Smiley N, Xia P, Hong CM, Senadheera D, Bui KC, Lutzko C. Genetic modification of airway progenitors following lentiviral gene delivery to the amniotic fluid of murine fetuses. Am J Respir Cell Mol Biol 2010;44:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cortiella J, Nichols JE, Kojima K, Bonassar LJ, Dargon P, Roy AK, Vacant MP, Niles JA, Vacanti CA. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng 2006;12:1213–1225. [DOI] [PubMed] [Google Scholar]
- 112.Ingenito E, Tsai L, Murthy S, Tyagi S, Mazan M, Hoffman A. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant (In press). [DOI] [PubMed]
- 113.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol 2007;37:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hegab AE, Kubo H, Fujino N, Suzuki T, He M, Kato H, Yamaya M. Isolation and characterization of murine multipotent lung stem cells. Stem Cells Dev 2009;19:523–536. [DOI] [PubMed] [Google Scholar]
- 115.Bentley JK, Popova AP, Bozyk PD, Linn MJ, Baek AE, Lei J, Goldsmith AM, Hershenson MB. Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype. Respir Res 2010;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 2008;10:140–151. [DOI] [PubMed] [Google Scholar]
- 117.Jakob M, Hemeda H, Janeschik S, Bootz F, Rotter N, Lang S, Brandau S. Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells. Stem Cells Dev 2010;19:635–644. [DOI] [PubMed] [Google Scholar]
- 118.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 2007;175:1158–1164. [DOI] [PubMed] [Google Scholar]
- 119.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 2007;117:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 2008;181:4389–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karoubi G, Cortes-Dericks L, Breyer I, Schmid RA, Dutly AE. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest 2009;89:1100–1114. [DOI] [PubMed] [Google Scholar]
- 122.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 2007;36:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 2006;34:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu R, Harper R, Kao CY, Thai P, Wu D, Chen Y, Chang MMJ. New insights into airway mucous cell differentiation. J Organ Dysfunct 2006;2:30–36. [Google Scholar]
- 125.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 2006;3:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol 2002;283:L510–L517. [DOI] [PubMed] [Google Scholar]
- 127.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 2007;293:L525–L534. [DOI] [PubMed] [Google Scholar]
- 129.Willis BC, Borok Z. Epithelial-mesenchymal transition: potential role in obliterative bronchiolitis? 2009. Thorax 2009;64:742–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111. [DOI] [PubMed] [Google Scholar]
- 131.Morrison SJ. Cancer stem cells. Clin Adv Hematol Oncol 2005;3:171–172. [PubMed] [Google Scholar]
- 132.Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther 2009;17:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moreira AL, Gonen M, Rekhtman N, Downey RJ. Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol 2010;23:889–895. [DOI] [PubMed] [Google Scholar]
- 134.Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell 2010;7:279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008;15:504–514. [DOI] [PubMed] [Google Scholar]
- 136.Seo DC, Sung JM, Cho HJ, Yi H, Seo KH, Choi IS, Kim DK, Kim JS, El-Aty AA, Shin HC. Gene expression profiling of cancer stem cell in human lung adenocarcinoma A549 cells. Mol Cancer 2007;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res 2007;67:4827–4833. [DOI] [PubMed] [Google Scholar]
- 138.Tirino V, Camerlingo R, Franco R, Malanga D, La Rocca A, Viglietto G, Rocco G, Pirozzi G. The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Eur J Cardio-Thorac Surg 2009;36:446–453. [DOI] [PubMed] [Google Scholar]
- 139.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA 2009;106:16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sabisz M, Skladanowski A. Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models. Cell Cycle 2009;8:3208–3217. [DOI] [PubMed] [Google Scholar]
- 141.Levina V, Marrangoni A, Wang T, Parikh S, Su Y, Herberman R, Lokshin A, Gorelik E. Elimination of human lung cancer stem cells through targeting of the stem cell factor-c-kit autocrine signaling loop. Cancer Res 2010;70:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer 2010;126:950–958. [DOI] [PubMed] [Google Scholar]
- 143.Vroling L, Lind JS, de Haas RR, Verheul HM, van Hinsbergh VW, Broxterman HJ, Smit EF. CD133+ circulating haematopoietic progenitor cells predict for response to sorafenib plus erlotinib in non-small cell lung cancer patients. Br J Cancer 2010;102:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett 2005;222:1–10. [DOI] [PubMed] [Google Scholar]
- 145.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 2004;64:8492–8495. [DOI] [PubMed] [Google Scholar]
- 146.Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Woll E, Kahler CM. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol 2004;57:965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science 2004;306:1568–1571. [DOI] [PubMed] [Google Scholar]
- 148.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 2004;96:1593–1603. [DOI] [PubMed] [Google Scholar]
- 149.Aghi M, Chiocca EA. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther 2005;12:994–1005. [DOI] [PubMed] [Google Scholar]
- 150.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res 2006;66:9054–9064. [DOI] [PubMed] [Google Scholar]
- 151.Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C, Noel D. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation 2006;82:1060–1066. [DOI] [PubMed] [Google Scholar]
- 152.Avital I, Moreira AL, Klimstra DS, Leversha M, Papadopoulos EB, Brennan M, Downey RJ. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells 2007;25:2903–2909. [DOI] [PubMed] [Google Scholar]
- 153.Cogle CR, Theise ND, Fu D, Ucar D, Lee S, Guthrie SM, Lonergan J, Rybka W, Krause DS, Scott EW. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells 2007;25:1881–1887. [DOI] [PubMed] [Google Scholar]
- 154.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 155.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 2007;21:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oh HK, Ha JM, Eunju O, Lee BH, Lee SK, Shim BS, Hong YK, Joe YA. Tumor angiogenesis promoted by ex vivo differentiated endothelial progenitor cells is effectively inhibited by an angiogenesis inhibitor, TK1–2. Cancer Res 2007;67:4851–4859. [DOI] [PubMed] [Google Scholar]
- 157.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 2008;319:195–198. [DOI] [PubMed] [Google Scholar]
- 158.Madlambayan GJ, Butler JM, Hosaka K, Jorgensen M, Fu D, Guthrie SM, Shenoy AK, Brank A, Russell KJ, Otero J, et al. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood 2009;114:4310–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer 2009;125:1771–1777. [DOI] [PubMed] [Google Scholar]
- 160.Arap W, Pasqualini R. Engineered embryonic endothelial progenitor cells as therapeutic Trojan horses. Cancer Cell 2004;5:406–408. [DOI] [PubMed] [Google Scholar]
- 161.Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax 2010;65:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, Vlastos GA, Chan G, Fischer KD, Rattat D, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell 2004;5:477–488. [DOI] [PubMed] [Google Scholar]
- 163.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol 2007;86:8–16. [DOI] [PubMed] [Google Scholar]
- 164.Kanehira M, Xin H, Hoshino K, Maemondo M, Mizuguchi H, Hayakawa T, Matsumoto K, Nakamura T, Nukiwa T, Saijo Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther 2007;14:894–903. [DOI] [PubMed] [Google Scholar]
- 165.Miletic H, Fischer Y, Litwak S, Giroglou T, Waerzeggers Y, Winkeler A, Li H, Himmelreich U, Lange C, Stenzel W, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther 2007;15:1373–1381. [DOI] [PubMed] [Google Scholar]
- 166.Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther 2007;14:828–835. [DOI] [PubMed] [Google Scholar]
- 167.Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, Rocconi RP, Numnum TM, Everts M, Chow LT, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat 2007;105:157–167. [DOI] [PubMed] [Google Scholar]
- 168.Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T, Saijo Y. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells 2007;25:1618–1626. [DOI] [PubMed] [Google Scholar]
- 169.Zhang X, Zhao P, Kennedy C, Chen K, Wiegand J, Washington G, Marrero L, Cui Y. Treatment of pulmonary metastatic tumors in mice using lentiviral vector-engineered stem cells. Cancer Gene Ther 2008;15:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, Janes SM. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res 2009;69:8862–8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res 2009;69:4134–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. [DOI] [PubMed] [Google Scholar]
- 173.Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, Wu JC, Chen X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells 2009;27:1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett 2009;281:32–41. [DOI] [PubMed] [Google Scholar]
- 175.Akay I, Oxmann D, Helfenstein A, Mentlein R, Schünke M, Hassenpflug J, Kurz B. Tumor risk by tissue engineering: cartilaginous differentiation of mesenchymal stem cells reduces tumor growth. Osteoarthritis Cartilage 2010;18:389–396. [DOI] [PubMed] [Google Scholar]
- 176.Bak XY, Yang J, Wang S. Baculovirus-transduced bone marrow mesenchymal stem cells for systemic cancer therapy. Cancer Gene Ther 2010;17:721–729. [DOI] [PubMed] [Google Scholar]
- 177.Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J, Marini F, et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer 2010;70:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mueller LP, Luetzkendorf J, Widder M, Nerger K, Caysa H, Mueller T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther 29 October 2010; 10.1038/cgt.2010.68. [DOI] [PubMed]
- 179.Romieu-Mourez R, Francois M, Abate A, Boivin MN, Birman E, Bailey D, Bramson JL, Forner K, Young YK, Medin JA, et al. Mesenchymal stromal cells expressing ErbB-2/neu elicit protective antibreast tumor immunity in vivo, which is paradoxically suppressed by IFN-{gamma} and tumor necrosis factor-{alpha} priming. Cancer Res 2010;70:7742–7747. [DOI] [PubMed] [Google Scholar]
- 180.Rejman J, Colombo C, Conese M. Engraftment of bone marrow-derived stem cells to the lung in a model of acute respiratory infection by Pseudomonas aeruginosa. Mol Ther J Am Soc Gene Ther 2009;17:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT, Weiss DJ. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther 2005;12:680–686. [DOI] [PubMed] [Google Scholar]
- 182.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002;30:1333–1338. [DOI] [PubMed] [Google Scholar]
- 183.Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells 2006;24:1986–1992. [DOI] [PubMed] [Google Scholar]
- 184.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells 2007;25:2593–2600. [DOI] [PubMed] [Google Scholar]
- 187.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2007;177:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Macpherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W, Forrester L, Dorin J. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci 2005;118:2441–2450. [DOI] [PubMed] [Google Scholar]
- 189.MacPherson H, Keir PA, Edwards CJ, Webb S, Dorin JR. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res 2006;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 2006;173:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yan C, Lian X, Dai Y, Wang X, Qu P, White A, Qin Y, Du H. Gene delivery by the hSP-B promoter to lung alveolar type II epithelial cells in LAL-knockout mice through bone marrow mesenchymal stem cells. Gene Ther 2007;14:1461–1470. [DOI] [PubMed] [Google Scholar]
- 192.Serikov VB, Popov B, Mikhailov VM, Gupta N, Matthay MA. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290:1033–1045. [DOI] [PubMed] [Google Scholar]
- 193.Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, Keshavjee S, Hu J, Waddell TK. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol 2007;293:L740–L752. [DOI] [PubMed] [Google Scholar]
- 194.Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC, Egan ME, Krause DS. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci USA 2006;103:2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells 2006;24:2299–2308. [DOI] [PubMed] [Google Scholar]
- 196.Spees JL, Pociask DA, Sullivan DE, Whitney MJ, Lasky JA, Prockop DJ, Brody AR. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Raoul W, Wagner-Ballon O, Saber G, Hulin A, Marcos E, Giraudier S, Vainchenker W, Adnot S, Eddahibi S, Maitre B. Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res 2007;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J 2008;22:1226–1236. [DOI] [PubMed] [Google Scholar]
- 199.Duchesneau P, Wong AP, Waddell TK. Optimization of targeted cell replacement therapy: a new approach for lung disease. Mol Ther J Am Soc Gene Ther. 2010;18:1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Levis J, Loi R, Butnor KJ, Vacek P, Steele C, Mossman BT, Weiss DJ. Decreased asbestos-induced lung inflammation and fibrosis after radiation and bone marrow transplant. Am J Respir Cell Mol Biol 2008;38:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest 2009;119:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leblond AL, Naud P, Forest V, et al. Developing cell therapy techniques for respiratory disease: intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther 2009;20:1329–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Moodley Y, Ilancheran S, Samuel C, Vaghjiani V, Atienza D, Williams ED, Jenkin G, Wallace E, Trounson A, Manuelpillai U. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med 2010;182:643–651. [DOI] [PubMed] [Google Scholar]
- 204.Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 2008;26:2902–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, Togashi I, Suzuki J, Satake M, Nakamizo H, Tanaka M, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res 2010;106:1613–1623. [DOI] [PubMed] [Google Scholar]
- 206.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, Strieter RM. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol 2006;176:1916–1927. [DOI] [PubMed] [Google Scholar]
- 207.Berger MJ, Adams SD, Tigges BM, Sprague SL, Wang XJ, Collins DP, McKenna DH. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy 2006;8:480–487. [DOI] [PubMed] [Google Scholar]
- 208.Kuang PP, Lucey E, Rishikof DC, Humphries DE, Bronsnick D, Goldstein RH. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol 2005;33:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med 2007;175:1014–1026. [DOI] [PubMed] [Google Scholar]
- 210.Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2007;176:1261–1268. [DOI] [PubMed] [Google Scholar]
- 211.Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell–derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther 2010;18:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 2003;100:2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004;305:90–93. [DOI] [PubMed] [Google Scholar]
- 214.Herzog EL, Van Arnam J, Hu B, Zhang J, Chen Q, Haberman AM, Krause DS. Lung-specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J 2007;21:2592–2601. [DOI] [PubMed] [Google Scholar]
- 215.Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol 2007;35:1466–1475. [DOI] [PubMed] [Google Scholar]
- 216.Kahler CM, Wechselberger J, Hilbe W, Gschwendtner A, Colleselli D, Niederegger H, Boneberg EM, Spizzo G, Wendel A, Gunsilius E, et al. Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir Res 2007;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Suzuki H, Hogg JC, van Eeden SF. Sequestration and homing of bone marrow-derived lineage negative progenitor cells in the lung during pneumococcal pneumonia. Respir Res 2008;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009;5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:213–224. [DOI] [PubMed] [Google Scholar]
- 220.Tolar J, O'Shaughnessy MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell S, Riddle M, McIvor RS, Yant SR, Kay MA, Krause D, et al. Host factors that impact the biodistribution and persistence of multipotent adult progenitor cells. Blood 2006;107:4182–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Viswanathan A, Painter RG, Lanson NA Jr, Wang G. Functional expression of N-formyl peptide receptors in human bone marrow-derived mesenchymal stem cells. Stem Cells 2007;25:1263–1269. [DOI] [PubMed] [Google Scholar]
- 222.Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, Karibe A, Minegishi N, Suzuki N, Yamamoto M, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 2006;113:1442–1450. [DOI] [PubMed] [Google Scholar]
- 223.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 2006;24:1254–1264. [DOI] [PubMed] [Google Scholar]
- 224.Miller JD, Lankford SM, Adler KB, Brody AR. Mesenchymal stem cells require MARCKS protein for directed chemotaxis in vitro. Am J Respir Cell Mol Biol 2010;43:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Barrilleaux BL, Phinney DG, Fischer-Valuck BW, Russell KC, Wang G, Prockop DJ, O'Connor KC. Small-molecule antagonist of macrophage migration inhibitory factor enhances migratory response of mesenchymal stem cells to bronchial epithelial cells. Tissue Eng Part A 2009;15:2335–2346. [DOI] [PubMed] [Google Scholar]
- 226.Fischer-Valuck BW, Barrilleaux BL, Phinney DG, Russell KC, Prockop DJ, O'Connor KC. Migratory response of mesenchymal stem cells to macrophage migration inhibitory factor and its antagonist as a function of colony-forming efficiency. Biotechnol Lett 2010;32:19–27. [DOI] [PubMed] [Google Scholar]
- 227.Barrilleaux BL, Fischer-Valuck BW, Gilliam JK, Phinney DG, O'Connor KC. Activation of CD74 inhibits migration of human mesenchymal stem cells. In Vitro Cell Dev Biol Anim 2010;46:566–572. [DOI] [PubMed] [Google Scholar]
- 228.Popov BV, Serikov VB, Petrov NS, Izusova TV, Gupta N, Matthay MA. Lung epithelial cells induce endodermal differentiation in mouse mesenchymal bone marrow stem cells by paracrine mechanism. Tissue Eng 2007;13:2441–2450. [DOI] [PubMed] [Google Scholar]
- 229.Field-Corbett C, O'Dea S. Soluble signals from mechanically disrupted lung tissue induce lung-related gene expression in bone marrow-derived cells in vitro. Stem Cells Dev 2007;16:231–242. [DOI] [PubMed] [Google Scholar]
- 230.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L131–L141. [DOI] [PubMed] [Google Scholar]
- 231.Van Vranken BE, Romanska HM, Polak JM, Rippon HJ, Shannon JM, Bishop AE. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Eng 2005;11:1177–1187. [DOI] [PubMed] [Google Scholar]
- 232.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847–856. [DOI] [PubMed] [Google Scholar]
- 233.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, Puente N, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 2007;25:2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 235.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 2003;23:1185–1189. [DOI] [PubMed] [Google Scholar]
- 236.Asosingh K, Erzurum SC. Angioplasticity in asthma. Biochem Soc Trans 2009;37:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol 2007;178:6482–6494. [DOI] [PubMed] [Google Scholar]
- 238.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854–860. [DOI] [PubMed] [Google Scholar]
- 239.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax 2005;60:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 2006;24:1806–1813. [DOI] [PubMed] [Google Scholar]
- 241.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 2006;27:529–541. [DOI] [PubMed] [Google Scholar]
- 242.Fadini GP, Schiavon M, Rea F, Avogaro A, Agostini C. Depletion of endothelial progenitor cells may link pulmonary fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2007;176:724–725, author reply 725. [DOI] [PubMed] [Google Scholar]
- 243.Fadini GP, Schiavon M, Avogaro A, Agostini C. The emerging role of endothelial progenitor cells in pulmonary hypertension and diffuse lung diseases. Sarcoidosis Vasc Diffuse Lung Dis 2007;24:85–93. [PubMed] [Google Scholar]
- 244.Ward MR, Stewart DJ, Kutryk MJ. Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc Interv 2007;70:983–998. [DOI] [PubMed] [Google Scholar]
- 245.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 2009;180:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sun CK, Lee FY, Sheu JJ, Yuen CM, Chua S, Chung SY, Chai HT, Chen YT, Kao YH, Chang LT, et al. Early combined treatment with cilostazol and bone marrow-derived endothelial progenitor cells markedly attenuates pulmonary arterial hypertension in rats. J Pharmacol Exp Ther 2009;330:718–726. [DOI] [PubMed] [Google Scholar]
- 247.Xia L, Fu GS, Yang JX, Zhang FR, Wang XX. Endothelial progenitor cells may inhibit apoptosis of pulmonary microvascular endothelial cells: new insights into cell therapy for pulmonary arterial hypertension. Cytotherapy 2009;11:492–502. [DOI] [PubMed] [Google Scholar]
- 248.Yao W, Firth AL, Sacks RS, Ogawa A, Auger WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA, et al. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2009;296:L870–L878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Re Junhui Z. Xingxiang W. Guosheng F. Yunpeng S. Furong Z. Junzhu C. duced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med 2008;102:1073–1079. [DOI] [PubMed] [Google Scholar]
- 250.Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 2008;117:3020–3030. [DOI] [PubMed] [Google Scholar]
- 251.Marsboom G, Pokreisz P, Gheysens O, Vermeersch P, Gillijns H, Pellens M, Liu X, Collen D, Janssens S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 2008;26:1017–1026. [DOI] [PubMed] [Google Scholar]
- 252.Yip HK, Chang LT, Sun CK, Sheu JJ, Chiang CH, Youssef AA, Lee FY, Wu CJ, Fu M. Autologous transplantation of bone marrow-derived endothelial progenitor cells attenuates monocrotaline-induced pulmonary arterial hypertension in rats. Crit Care Med 2008;36:873–880. [DOI] [PubMed] [Google Scholar]
- 253.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res 2006;66:7341–7347. [DOI] [PubMed] [Google Scholar]
- 254.Pircher A, Kahler CM, Skvortsov S, Dlaska M, Kawaguchi G, Schmid T, Gunsilius E, Hilbe W. Increased numbers of endothelial progenitor cells in peripheral blood and tumor specimens in non-small cell lung cancer: a methodological challenge and an ongoing debate on the clinical relevance. Oncol Rep 2008;19:345–352. [PubMed] [Google Scholar]
- 255.Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, Ferrari G, Bonetti E, Chiesa G, de Silvestri A, et al. Circulating endothelial progenitor cells in pre-term infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2009;180:540–546. [DOI] [PubMed] [Google Scholar]
- 256.Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea Am J Respir Crit Care Med 2010;182(1):92–97. [DOI] [PMC free article] [PubMed]
- 257.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005;112:1618–1627. [DOI] [PubMed] [Google Scholar]
- 259.Smits PA, Kleppe LS, Witt TA, Mueske CS, Vile RG, Simari RD. Distribution of circulation-derived endothelial progenitors following systemic delivery. Endothelium 2007;14:1–5. [DOI] [PubMed] [Google Scholar]
- 260.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007;292:L1073–L1084. [DOI] [PubMed] [Google Scholar]
- 261.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK. Differentiation in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form long lasting vessels. Blood 2008;111:1302–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med 2009;180:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol 2010;298:L315–L323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 2007;116:2818–2829. [DOI] [PubMed] [Google Scholar]
- 265.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol 2003;42:2073–2080. [DOI] [PubMed] [Google Scholar]
- 266.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J Clin Invest 2001;108:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Henrich D, Seebach C, Wilhelm K, Marzi I. High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. J Surg Res 2007;142:13–19. [DOI] [PubMed] [Google Scholar]
- 268.Simcock JW, Penington AJ, Morrison WA, Thompson EW, Mitchell GM. Endothelial Precursor Cells Home to a Vascularized Tissue Engineering Chamber by Application of the Angiogenic Chemokine CXCL12. Tissue Engineering Part. Am Arch 2009;15:655–664. [DOI] [PubMed] [Google Scholar]
- 269.Smythe J, Fox A, Fisher N, Frith E, Harris AL, Watt SM. Measuring Angiogenic Cytokines, Circulating Endothelial Cells, and Endothelial Progenitor Cells in Peripheral Blood and Cord Blood: VEGF and CXCL12 Correlate with the Number of Circulating Endothelial Progenitor Cells in Peripheral Blood. Tissue Eng Part C Methods 2008;14:59–67. [DOI] [PubMed] [Google Scholar]
- 270.Maeng YS, Choi HJ, Kwon JY, Park Y-W, Choi KS, Min JK, Kim YH, Suh PG, Kang KS, Won MH, et al. Endothelial progenitor cell homing: prominent role of the IGF2–IGF2R-PLCβ2 axis. Blood 2009;113:233–243. [DOI] [PubMed] [Google Scholar]
- 271.Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J, Strausz J, Ostoros G, Klepetko W, Ankersmit HJ, et al. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin Cancer Res 2009;15:1741–1746. [DOI] [PubMed] [Google Scholar]
- 272.Suriano R, Chaudhuri D, Johnson RS, Lambers E, Ashok BT, Kishore R, Tiwari RK. 17β-Estradiol Mobilizes Bone Marrow–Derived Endothelial Progenitor Cells to Tumors. Cancer Res 2008;68:6038–6042. [DOI] [PubMed] [Google Scholar]
- 273.Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, Hino J, Harada-Shiba M, Okumura H, Tabata Y, et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 2003;108:889–895. [DOI] [PubMed] [Google Scholar]
- 274.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng 2004;10:771–779. [DOI] [PubMed] [Google Scholar]
- 275.Zhao Q, Liu Z, Wang Z, Yang C, Liu J, Lu J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg 2007;84:544–552. [DOI] [PubMed] [Google Scholar]
- 276.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96:442–450. [DOI] [PubMed] [Google Scholar]
- 277.Lam CF, Liu YC, Hsu JK, Yeh PA, Su TY, Huang CC, Lin MW, Wu PC, Chang PJ, Tsai YC. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology 2008;108:392–401. [DOI] [PubMed] [Google Scholar]
- 278.Ormiston ML, Deng Y, Stewart DJ, Courtman DW. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol Biol 2010;43:546–554. [DOI] [PubMed] [Google Scholar]
- 279.Kitaichi YH, Urata T, Kurobe M, Kanbara H, Motoki T, Kitagawa T. Syngeneic bone marrow mononuclear cells improve pulmonary arterial hypertension through vascular endothelial growth factor upregulation. Ann Thorac Surg 2009;88:418–424. [DOI] [PubMed] [Google Scholar]
- 280.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a p54. Pilot randomized controlled trial. J Am Coll Cardiol 2007;49:1566–1571. [DOI] [PubMed] [Google Scholar]
- 281.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant 2008;12:650–655. [DOI] [PubMed] [Google Scholar]
- 282.Critser PJ. Yoder MC. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Current Opinion in Organ Transplantation. 2010. 15(1):68–72, 2010 Feb. [DOI] [PMC free article] [PubMed]
- 283.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 284.Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep 2000;2:501–505. [DOI] [PubMed] [Google Scholar]
- 285.Keeley EC, Mehrad B, Strieter RM. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol 2010;42:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun 2007;353:104–108. [DOI] [PubMed] [Google Scholar]
- 288.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Stenmark KR. Circulating mononuclear cells with a dual, macrophage-fibroblast phenotype contribute robustly to hypoxia-induced pulmonary adventitial remodeling. Chest 2005;128:583S–584S. [DOI] [PubMed] [Google Scholar]
- 289.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 290.Nihlberg K, Larsen K, Hultgardh-Nilsson A, Malmstrom A, Bjermer L, Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res 2006;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Brocker V, Langer F, Fellous TG, Mengel M, Brittan M, Bredt M, Milde S, Welte T, Eder M, Haverich A, et al. Fibroblasts of recipient origin contribute to bronchiolitis obliterans in human lung transplants. Am J Respir Crit Care Med 2006;173:1276–1282. [DOI] [PubMed] [Google Scholar]
- 292.Andersson-Sjöland A, Erjefält JS, Bjermer L, Eriksson L, Westergren-Thorsson G. Fibrocytes are associated with vascular and parenchymal remodelling in patients with obliterative bronchiolitis. Respir Res 2009;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588–594. [DOI] [PubMed] [Google Scholar]
- 294.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, Matsushima K, Mukaida N. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol 2007;170:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sato M, Hirayama S, Lara-Guerra H, Anraku M, Waddell TK, Liu M, Keshavjee S. MMP-dependent migration of extrapulmonary myofibroblast progenitors contributing to posttransplant airway fibrosis in the lung. Am J Transplant 2009;9:1027–1036. [DOI] [PubMed] [Google Scholar]
- 298.Larsen K, Macleod D, Nihlberg K, Gurcan E, Bjermer L, Marko-Varga G, Westergren-Thorsson G. Specific haptoglobin expression in bronchoalveolar lavage during differentiation of circulating fibroblast progenitor cells in mild asthma. J Proteome Res 2006;5:1479–1483. [DOI] [PubMed] [Google Scholar]
- 299.Vannella KM, McMillan TR, Charbeneau RP, Wilke CA, Thomas PE, Toews GB, Peters-Golden M, Moore BB. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J Immunol 2007;179:7883–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ishii G, Ito TK, Aoyagi K, Fujimoto H, Chiba H, Hasebe T, Fujii S, Nagai K, Sasaki H, Ochiai A. Presence of human circulating progenitor cells for cancer stromal fibroblasts in the blood of lung cancer patients. Stem Cells 2007;25:1469–1477. [DOI] [PubMed] [Google Scholar]
- 301.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976;4:267–274. [PubMed] [Google Scholar]
- 302.Caplan AI. Osteogenesis imperfecta, rehabilitation medicine, fundamental research and mesenchymal stem cells. Connect Tissue Res 1995;31:S9–S14. [DOI] [PubMed] [Google Scholar]
- 303.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 304.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004;103:1669–1675. [DOI] [PubMed] [Google Scholar]
- 305.Lee MW, Choi J, Yang MS, Moon YJ, Park JS, Kim HC, Kim YJ. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Commun 2004;320:273–278. [DOI] [PubMed] [Google Scholar]
- 306.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004;22:625–634. [DOI] [PubMed] [Google Scholar]
- 307.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 2004;22:1330–1337. [DOI] [PubMed] [Google Scholar]
- 308.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 2004;22:649–658. [DOI] [PubMed] [Google Scholar]
- 309.Li D, Wang GY, Dong BH, Zhang YC, Wang YX, Sun BC. Biological characteristics of human placental mesenchymal stem cells and their proliferative response to various cytokines. Cells Tissues Organs 2007;186:169–179. [DOI] [PubMed] [Google Scholar]
- 310.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–129. [DOI] [PubMed] [Google Scholar]
- 311.Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006;24:2582–2591. [DOI] [PubMed] [Google Scholar]
- 312.Ingenito EP, Sen E, Tsai LW, Murthy S, Hoffman A. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J Tissue Eng Regen Med 2010;4:259–272. [DOI] [PubMed] [Google Scholar]
- 313.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003;174:101–109. [DOI] [PubMed] [Google Scholar]
- 314.Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 2004;22:1263–1278. [DOI] [PubMed] [Google Scholar]
- 315.Phinney DG, Hill K, Michelson C, DuTreil M, Hughes C, Humphries S, Wilkinson R, Baddoo M, Bayly E. Biological activities encoded by the murine mesenchymal stem cell transcriptome provide a basis for their developmental potential and broad therapeutic efficacy. Stem Cells 2006;24:186–198. [DOI] [PubMed] [Google Scholar]
- 316.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 317.Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells 2006;24:679–685. [DOI] [PubMed] [Google Scholar]
- 318.Tsai MS, Hwang SM, Chen KD, Lee YS, Hsu LW, Chang YJ, Wang CN, Peng HH, Chang YL, Chao AS, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells 2007;25:2511–2523. [DOI] [PubMed] [Google Scholar]
- 319.Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, Vukicevic S, et al. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res 2007;48:132–140. [DOI] [PubMed] [Google Scholar]
- 320.Keyser KA, Beagles KE, Kiem HP. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant 2007;16:555–562. [DOI] [PubMed] [Google Scholar]
- 321.Kunisaki SM, Fuchs JR, Steigman SA, Fauza DO. A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells. Tissue Eng 2007;13:2633–2644. [DOI] [PubMed] [Google Scholar]
- 322.Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, Jansen JH, Kögler G, Figdor CG, Torensma R, et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev 2010;19:481–490. [DOI] [PubMed] [Google Scholar]
- 323.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 2005;85:962–971. [DOI] [PubMed] [Google Scholar]
- 324.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–689. [DOI] [PubMed] [Google Scholar]
- 325.Uygun BE, Stojsih SE, Matthew HWT. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng Part A 2009;15:3499–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pek S, Wan ACA, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 2010;31:385–391. [DOI] [PubMed] [Google Scholar]
- 327.Lindner U, Kramer J, Behrends J, Driller B, Wendler NO, Boehrnsen F, Rohwedel J, Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy 2010;12:992–1005. [DOI] [PubMed] [Google Scholar]
- 328.Lisignoli G, Cristino S, Piacentini A, Cavallo C, Caplan AI, Facchini A. Hyaluronan-based polymer scaffold modulates the expression of inflammatory and degradative factors in mesenchymal stem cells: Involvement of CD44 and CD54. J Cell Physiol 2006;207:364–373. [DOI] [PubMed] [Google Scholar]
- 329.Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 2006;207:331–339. [DOI] [PubMed] [Google Scholar]
- 330.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 2010;16:159–168. [DOI] [PubMed] [Google Scholar]
- 331.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 2008;29:74–82. [DOI] [PubMed] [Google Scholar]
- 332.Leroux L, Descamps B, Tojais NF, Séguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamazière JD, et al. Hypoxia Preconditioned Mesenchymal Stem Cells Improve Vascular and Skeletal Muscle Fiber Regeneration After Ischemia Through a Wnt4-dependent Pathway. Mol Ther 2010;18:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Volkmer E, Kallukalam BC, Maertz J, Otto S, Drosse I, Polzer H, Bocker W, Stengele M, Docheva D, Mutschler W, et al. Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Eng Part A 2010;16:153–164. [DOI] [PubMed] [Google Scholar]
- 334.Zhao F, Veldhuis JJ, Duan Y, Yang Y, Christoforou N, Ma T, Leong KW. Low oxygen tension and synthetic nanogratings improve the uniformity and stemness of human mesenchymal stem cell layer. Mol Ther 2010;18:1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE 2007;2:e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med 2010;5:713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kurpinski K, Chu J, Wang D, Li S. Proteomic profiling of mesenchymal stem cell responses to mechanical strain and TGF-beta1. Cell Mol Bioeng 2009;2:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Bassaneze V, Barauna VG, Lavini-Ramos C, Kalil J, Schettert IT, Miyakawa AA, Krieger JE. Shear stress induces nitric oxide-mediated vascular endothelial growth factor production in human adipose tissue mesenchymal stem cells. Stem Cells Dev 2010;19:371–378. [DOI] [PubMed] [Google Scholar]
- 339.Lee IC, Wang JH, Lee YT, Young TH. The differentiation of mesenchymal stem cells by mechanical stress or/and co-culture system. Biochem Biophys Res Commun 2007;352:147–152. [DOI] [PubMed] [Google Scholar]
- 340.Stolzing A, Scutt A. Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free Radic Biol Med 2006;41:326–338. [DOI] [PubMed] [Google Scholar]
- 341.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol 1996;166:585–592. [DOI] [PubMed] [Google Scholar]
- 342.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE 2005;2005:pe37. [DOI] [PubMed] [Google Scholar]
- 343.Crop MJ, Baan CC, Korevaar SS, Ijzermans JNM, Pescatori M, Stubbs AP, Van Jacken WFJ, Dahlke MH, Eggenhofer W. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol 2010;162:474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, Prockop DJ. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood 2006;107:2153–2161. [DOI] [PubMed] [Google Scholar]
- 345.Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 2006;198:54–64. [DOI] [PubMed] [Google Scholar]
- 346.Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle 2007;6:2884–2889. [DOI] [PubMed] [Google Scholar]
- 347.Keating A. Mesenchymal stromal cells. Curr Opin Hematol 2006;13:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res 2006;312:2169–2179. [DOI] [PubMed] [Google Scholar]
- 349.Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens 2007;69:1–9. [DOI] [PubMed] [Google Scholar]
- 350.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110:3499–3506. [DOI] [PubMed] [Google Scholar]
- 351.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells.]. Cytokine Growth Factor Rev 2009;20:419–427. [DOI] [PubMed] [Google Scholar]
- 352.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant 2010;19:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther 2010;21:1641–1655. [DOI] [PubMed] [Google Scholar]
- 354.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–3843. [DOI] [PubMed] [Google Scholar]
- 355.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42–48. [DOI] [PubMed] [Google Scholar]
- 356.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722–3729. [DOI] [PubMed] [Google Scholar]
- 357.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 2005;12:47–57. [DOI] [PubMed] [Google Scholar]
- 358.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 359.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringden O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 2004;60:307–315. [DOI] [PubMed] [Google Scholar]
- 360.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res 2005;305:33–41. [DOI] [PubMed] [Google Scholar]
- 361.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003;76:1208–1213. [DOI] [PubMed] [Google Scholar]
- 362.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005;105:2214–2219. [DOI] [PubMed] [Google Scholar]
- 363.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004;103:4619–4621. [DOI] [PubMed] [Google Scholar]
- 364.Deng W, Han Q, Liao L, You S, Deng H, Zhao RC. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol 2005;24:458–463. [DOI] [PubMed] [Google Scholar]
- 365.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008;111:1327–1333. [DOI] [PubMed] [Google Scholar]
- 366.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007;110:3691–3694. [DOI] [PubMed] [Google Scholar]
- 367.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 2006;177:2080–2087. [DOI] [PubMed] [Google Scholar]
- 368.Nasef A, Mathieu N, Chapel A, Frick J, Francois S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 2007;84:231–237. [DOI] [PubMed] [Google Scholar]
- 369.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006;108:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 2009;27:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lanz TV, Opitz CA, Ho PP, Agrawal A, Lutz C, Weller M, Mellor AL, Steinman L, Wick W, Platten M. Mouse Mesenchymal Stem Cells Suppress Antigen-Specific TH Cell Immunity Independent of Indoleamine 2,3-Dioxygenase 1 (IDO1). Stem Cells Dev 2009;19:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Guo Z, Zheng C, Chen Z, Gu D, Du W, Ge J, Han Z, Yang R. Fetal bone marrow-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol 2009;39:2840–2849. [DOI] [PubMed] [Google Scholar]
- 373.Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev 2010; 10.1089/scd.2009.0418 (in press) [DOI] [PMC free article] [PubMed]
- 374.Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng 2006;12:2263–2273. [DOI] [PubMed] [Google Scholar]
- 375.Najar M, Raicevic G, Boufker HI, Fayyad-Kazan H, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. Adipose-tissue-derived and Wharton's jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng Part A 2010;16:3537–3546. [DOI] [PubMed] [Google Scholar]
- 376.Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol 2007;179:1549–1558. [DOI] [PubMed] [Google Scholar]
- 377.DelaRosa O, Lombardo E, Beraza A, Mancheno-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, Garcia L, Abad JL, et al. Requirement of IFN-g-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A 2009;15:2795–2806. [DOI] [PubMed] [Google Scholar]
- 378.Hemeda H, Jakob M, Ludwig A, Giebel B, Lang S, Brandau S. Interferon-g and tumor necrosis factor-a differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev 2010;19:693–706. [DOI] [PubMed] [Google Scholar]
- 379.François M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood 2009;114:2632–2638. [DOI] [PubMed] [Google Scholar]
- 380.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 2010;5:e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon B, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells 2010;28:1856–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 2009;37:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Huang W, La Russa V, Alzoubi A, Schwarzenberger P. Interleukin-17A: a T-cell-derived growth factor for murine and human mesenchymal stem cells. Stem Cells 2006;24:1512–1518. [DOI] [PubMed] [Google Scholar]
- 384.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 2007;109:1422–1432. [DOI] [PubMed] [Google Scholar]
- 385.Raicevic G. Inflammation modifies the pattern and the function of TLR expressed by human mesenchymal stromal cells. J Immunol 2001;166:1471–1481. [DOI] [PubMed] [Google Scholar]
- 386.Romieu-Mourez R, François M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol 2009;182:7963–7973. [DOI] [PubMed] [Google Scholar]
- 387.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010;5:e10088. (Electronic Resource). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Vilalta M, Dégano IR, Bagó J, Aguilar E, Gambhir SS, Rubio N, Blanco J. Human adipose tissue-derived mesenchymal stromal cells as vehicles for tumor bystander effect: a model based on bioluminescence imaging. Gene Ther 2009;16:547–557. [DOI] [PubMed] [Google Scholar]
- 389.Park SH, Doh J, Park SI, Lim JY, Kim SM, Youn JI, Jin HT, Seo SH, Song MY, Sung SY, et al. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene Ther 2010;17:1052–1061. [DOI] [PubMed] [Google Scholar]
- 390.Ahmed AU, Rolle CE, Tyler MA, Han Y, Sengupta S, Wainwright DA, Balyasnikova IV, Ulasov IV, Lesniak MS. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther 2010;18:1846–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol 2007;211:27–35. [DOI] [PubMed] [Google Scholar]
- 392.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. [DOI] [PubMed] [Google Scholar]
- 393.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363:1439–1441. [DOI] [PubMed] [Google Scholar]
- 394.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006;81:1390–1397. [DOI] [PubMed] [Google Scholar]
- 395.Fang B, Song YP, Liao LM, Han Q, Zhao RC. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant 2006;38:389–390. [DOI] [PubMed] [Google Scholar]
- 396.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Matthay MA, Taylor Thompson B, Read EJ, McKenna DH Jr, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 2010;138:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med 2009;51:5–16. [PubMed] [Google Scholar]
- 399.Brody AR, Salazar KD, Lankford SM. Mesenchymal stem cells modulate lung injury. Proc Am Thorac Soc 2010;7:130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855–1863. [DOI] [PubMed] [Google Scholar]
- 401.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007;4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol 2008;214:472–481. [DOI] [PubMed] [Google Scholar]
- 405.Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, Qi HW. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc 2008;40:1700–1705. [DOI] [PubMed] [Google Scholar]
- 406.Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 2009;175:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Germano D, Blyszczuk P, Valaperti A, Kania G, Dirnhofer S, Landmesser U, Lüscher TF, Hunziker L, Zulewski H, Eriksson U, et al. Prominin-1/CD133+ lung epithelial progenitors protect from bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:939–949. [DOI] [PubMed] [Google Scholar]
- 408.Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, Bonnet D, Janes SM. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE 2009;4:e8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J 2009;34:740–748. [DOI] [PubMed] [Google Scholar]
- 410.Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, Lombardi G, Albertini A, Wengler GS, Parolini O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant 2010;18:405–422. [DOI] [PubMed] [Google Scholar]
- 411.Lee S, Jang A, Kim Y, Cha J, Kim T, Jung S, Park S, Lee Y, Won J, Kim Y, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res 2010;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res 2010;156:188–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009;58:929–939. [DOI] [PubMed] [Google Scholar]
- 415.Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 2010;182:1047–1057. [DOI] [PubMed] [Google Scholar]
- 416.Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant 2010;19:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 2010;18:1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 2007;292:H1120–H1128. [DOI] [PubMed] [Google Scholar]
- 419.Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;297:H1606–H1616. [DOI] [PubMed] [Google Scholar]
- 420.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 2006;114:I181–I185. [DOI] [PubMed] [Google Scholar]
- 421.Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P, Salgar SK. Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther 2010;21:713–727. [DOI] [PubMed] [Google Scholar]
- 422.Yang Z, Sharma AK, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol 2009;40:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009;180:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 2009;180:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant 2009;18:869–886. [DOI] [PubMed] [Google Scholar]
- 426.Grove DA, Xu J, Joodi R, Torres-Gonzales E, Neujahr D, Mora AL, Rojas M. Attenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantation. J Heart-Lung Transpl 2010;30:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Cho KS, Park HK, Park HY, Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, Roh HJ. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells 2009;27:259–265. [DOI] [PubMed] [Google Scholar]
- 428.Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS, Park SK, Roh HJ. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev 2010;19:1811–1818. [DOI] [PubMed] [Google Scholar]
- 429.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA 2010;107:5652–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Bonfield TL, Koloze MF, Lennon DP, Zuchowski B, Yang SE, Caplan AL. Human Mesenchymal Stem Cells Suppress Chronic Airway Inflammation in the Murine Ovalbumin Asthma Model. Am J Physiol Lung Cell Mol Physiol 2010;299:L760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bonfield TL, Nolan MT, Koloze, Lennon DP, Caplan AI. Defining human mesenchymal stem cell efficacy in vivo. J Inflamm (Lond) 2010;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, Leclair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow derived mesenchymal stromal cells inhibit th2-mediated allergic airways inflammation in mice. Stem Cells 2011. May 4. doi: 10.1002/stem.656 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 433.Jang HJ, Cho KS, Park HY, Roh HJ. Adipose tissue-derived stem cells for cell therapy of airway allergic diseases in mouse. Acta Histochemica 2010;113:501–507. [DOI] [PubMed] [Google Scholar]
- 434.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252. [DOI] [PubMed] [Google Scholar]
- 435.Shigemura N, Okumura M, Mizuno S, Imanishi Y, Nakamura T, Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant 2006;6:2592–2600. [DOI] [PubMed] [Google Scholar]
- 436.Shigemura N, Okumura M, Mizuno S, Imanishi Y, Matsuyama A, Shiono H, Nakamura T, Sawa Y. Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema. Am J Respir Crit Care Med 2006;174:1199–1205. [DOI] [PubMed] [Google Scholar]
- 437.Yuhgetsu H, Ohno Y, Funaguchi N, Asai T, Sawada M, Takemura G, Minatoguchi S, Fujiwara H, Fujiwara T. Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits. Exp Lung Res 2006;32:413–426. [DOI] [PubMed] [Google Scholar]
- 438.Adachi Y, Oyaizu H, Taketani S, Minamino K, Yamaguchi K, Shultz LD, Iwasaki M, Tomita M, Suzuki Y, Nakano K. Treatment and transfer of emphysema by a new bone marrow transplantation method from normal mice to Tsk mice and vice versa. Stem Cells 2006;24:2071–2077. [DOI] [PubMed] [Google Scholar]
- 439.Zhen G, Liu H, Gu N, Zhang H, Xu Y, Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci 2008;13:3415–3422. [DOI] [PubMed] [Google Scholar]
- 440.Schweitzer K, Johnstone BH, Garrison J, Rush N, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M, Fisher AJ, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med (In press) [DOI] [PMC free article] [PubMed]
- 441.Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther 2010;19:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, Zhang Z. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 2010;12:605–614. [DOI] [PubMed] [Google Scholar]
- 443.Carreras A, Almendros I, Montserrat JM, Navajas D, Farre R. Mesenchymal stem cells reduce inflammation in a rat model of obstructive sleep apnea. Respir Physiol Neurobiol 2010;172:210–212. [DOI] [PubMed] [Google Scholar]
- 444.Carreras A, Rojas M, Tsapikouni T, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early activation of mesenchymal stem cells and enhancement of endothelial wound healing. Respir Res 2010;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 2009;106:16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 2010;285:26211–26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. 2010; Stem Cells 28:2229-2238. [DOI] [PMC free article] [PubMed]
- 448.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 2007;179:1595–1604. [DOI] [PubMed] [Google Scholar]
- 449.Dwyer RM, Kerin MJ. Mesenchymal stem cells and cancer: tumor-specific delivery vehicles or therapeutic targets? Hum Gene Ther 2010;21:1506–1512. [DOI] [PubMed] [Google Scholar]
- 450.Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VI. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther 2010;21:1513–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001;97:1227–1231. [DOI] [PubMed] [Google Scholar]
- 452.Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther 2004;9:747–756. [DOI] [PubMed] [Google Scholar]
- 453.Gregory CA, Reyes E, Whitney MJ, Spees JL. Enhanced engraftment of mesenchymal stem cells in a cutaneous wound model by culture in allogenic species-specific serum and administration in fibrin constructs. Stem Cells 2006;24:2232–2243. [DOI] [PubMed] [Google Scholar]
- 454.Muller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A, Viebahn S, Gieseke F, Langer H, Gawaz MP, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy 2006;8:437–444. [DOI] [PubMed] [Google Scholar]
- 455.Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, Del Fante C, Novara F, de Silvestri A, Amendola G, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol 2007;211:121–130. [DOI] [PubMed] [Google Scholar]
- 456.Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells 2007;25:1586–1594. [DOI] [PubMed] [Google Scholar]
- 457.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007;25:371–379. [DOI] [PubMed] [Google Scholar]
- 458.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc 2007;39:573–576. [DOI] [PubMed] [Google Scholar]
- 459.Rameshwar P. Casting doubt on the safety of “off-the-shelf” mesenchymal stem cells for cell therapy. Mol Ther J Am Soc Gene Ther 2009;17:216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 2010;12:576–578. [DOI] [PubMed] [Google Scholar]
- 461.Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res 2009;69:1255–1258. [DOI] [PubMed] [Google Scholar]
- 462.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007;67:9142–9149. [DOI] [PubMed] [Google Scholar]
- 463.Qin Y, Ji H, Wu Y, Liu H. Chromosomal instability of murine adipose tissue-derived mesenchymal stem cells in long-term culture and development of cloned embryos. Cloning Stem Cells 2009;11:445–452. [DOI] [PubMed] [Google Scholar]
- 464.Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, et al.; Société Française de Greffe de Moelle et Thérapie Cellulaire. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010;115:1549–1553. [DOI] [PubMed] [Google Scholar]
- 465.Rubin H. Multistage carcinogenesis in cell culture. Dev Biol (Basel). 2001;106:61–66; discussion 67, 143–160. [PubMed] [Google Scholar]
- 466.Rubio D, Garcia S, Paz MF, De la Cueva T, Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J, Bernad A. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS ONE 2008;3:e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res 2005;65:3035–3039. [DOI] [PubMed] [Google Scholar]
- 468.Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 2009;69:5331–5339. [DOI] [PubMed] [Google Scholar]
- 469.Torsvik A, Røsland GV, Svendsen A, Molven A, Immervoll H, McCormack E, Lønning PE, Primon M, Sobala E, Tonn JC, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track - letter. Cancer Res 2010;70:6393–6396. [DOI] [PubMed] [Google Scholar]
- 470.http://investor.osiris.com/releasedetail.cfm?ReleaseID=311598.
- 471.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI,Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 2008;14:213–221. [DOI] [PubMed] [Google Scholar]
- 472.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010;16:814–820. [DOI] [PMC free article] [PubMed]
- 473.Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, Fauza DO. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg 2007;42:974–979, discussion 979–980. [DOI] [PubMed] [Google Scholar]
- 474.Kunisaki SM, Jennings RW, Fauza DO. Fetal cartilage engineering from amniotic mesenchymal progenitor cells. Stem Cells Dev 2006;15:245–253. [DOI] [PubMed] [Google Scholar]
- 475.Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg 2006;41:675–682, discussion 675–682. [DOI] [PubMed] [Google Scholar]
- 476.Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP, Wilson JM, Fauza DO. Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg 2006;41:34–39, discussion 34–39. [DOI] [PubMed] [Google Scholar]
- 477.Fuchs JR, Hannouche D, Terada S, Zand S, Vacanti JP, Fauza DO. Cartilage engineering from ovine umbilical cord blood mesenchymal progenitor cells. Stem Cells 2005;23:958–964. [DOI] [PubMed] [Google Scholar]
- 478.Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW, Wilson JM, Fauza DO. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 2004;39:834–838, discussion 834–838. [DOI] [PubMed] [Google Scholar]
- 479.Kojima K, Ignotz RA, Kushibiki T, Tinsley KW, Tabata Y, Vacanti CA. Tissue-engineered trachea from sheep marrow stromal cells with transforming growth factor beta2 released from biodegradable microspheres in a nude rat recipient. J Thorac Cardiovasc Surg 2004;128:147–153. [DOI] [PubMed] [Google Scholar]
- 480.Omori K, Nakamura T, Kanemaru S, Magrufov A, Yamashita M, Shimizu Y. In situ tissue engineering of the cricoid and trachea in a canine model. Ann Otol Rhinol Laryngol 2008;117:609–613. [DOI] [PubMed] [Google Scholar]
- 481.Urita Y, Komuro H, Chen G, Shinya M, Saihara R, Kaneko M. Evaluation of diaphragmatic hernia repair using PLGA mesh-collagen sponge hybrid scaffold: an experimental study in a rat model. Pediatr Surg Int 2008;24:1041–1045. [DOI] [PubMed] [Google Scholar]
- 482.Omori K, Tada Y, Suzuki T, Nomoto Y, Matsuzuka T, Kobayashi K, Nakamura T, Kanemaru S, Yamashita M, Asato R. Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann Otol Rhinol Laryngol 2008;117:673–678. [DOI] [PubMed] [Google Scholar]
- 483.Gilbert TW, Gilbert S, Madden M, Reynolds SD, Badylak SF. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg 2008;86:967–974. [DOI] [PubMed] [Google Scholar]
- 484.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008;372:2023–2030. [DOI] [PubMed] [Google Scholar]
- 485.Mondrinos MJ, Koutzaki S, Lelkes PI, Finck CM. A tissue-engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol 2007;293:L639–L650. [DOI] [PubMed] [Google Scholar]
- 486.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI, Finck CM. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng 2006;12:717–728. [DOI] [PubMed] [Google Scholar]
- 487.Lin YM, Boccaccini AR, Polak JM, Bishop AE, Maquet V. Biocompatibility of poly-DL-lactic acid (PDLLA) for lung tissue engineering. J Biomater Appl 2006;21:109–118. [DOI] [PubMed] [Google Scholar]
- 488.Choe MM, Sporn PH, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol 2006;35:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Liu M, Xu J, Souza P, Tanswell B, Tanswell AK, Post M. The effect of mechanical strain on fetal rat lung cell proliferation: comparison of two- and three-dimensional culture systems. In Vitro Cell Dev Biol Anim 1995;31:858–866. [DOI] [PubMed] [Google Scholar]
- 490.Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol 1999;277:L667–L683. [DOI] [PubMed] [Google Scholar]
- 491.Liu M, Post M. Invited review: mechanochemical signal transduction in the fetal lung. J Appl Physiol 2000;89:2078–2084. [DOI] [PubMed] [Google Scholar]
- 492.Andrade CF, Wong AP, Waddell TK, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol 2007;292:L510–L518. [DOI] [PubMed] [Google Scholar]
- 493.Hoganson DM, Pryor HI II, Vacanti JP. Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr Res 2008;63:520–526. [DOI] [PubMed] [Google Scholar]
- 494.Mondrinos MJ, Koutzaki SH, Honesto M, Poblete M, Crisanti C, Lelkes PI, Finck CM. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng Part A 2008;14:361–368. [DOI] [PubMed] [Google Scholar]
- 495.Antunes MB, Woodworth BJ, Bhargave G, Xiong G, Aguilar JL, Ratner AJ, Kreindler JL, Rubenstein RC, Cohen NA. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques 2007;43:195–204. [DOI] [PubMed] [Google Scholar]
- 496.Lwebuga-Mukasa JS, Ingbar DH, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro: a novel system for type II pneumocyte culture. Exp Cell Res 1986;162:423–435. [DOI] [PubMed] [Google Scholar]
- 497.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 2010;16:2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Cortiella, J., Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 2010;16:2565–2580. [DOI] [PubMed] [Google Scholar]
- 499.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science 2010;329:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010;16:927–933. [DOI] [PubMed] [Google Scholar]
- 501.Ingenito EP, Tsai L, Murthy S, Tyagi S, Mazan M, Hoffman A. Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant 2011 [Epub ahead of print]. [DOI] [PubMed]
- 502.Daly A, Goodwin M, Sueblinvong V, Panoskaltsis-Mortari A, Weiss DJ. MSCs spontaneously express pro-SPC when cultured in intact de-cellularized mouse lungs [abstract]. Am J Respir Crit Care Med 2010;181:A4920. [Google Scholar]
- 503.Tenney RM, Discher DE. Stem cells, microenvironment mechanics, and growth factor activation. Curr Opin Cell Biol 2009;21:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009;324:1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Discher D, Dong C, Fredberg JJ, Guilak F, Ingber D, Janmey P, Kamm RD, Schmid-Schönbein GW, Weinbaum S. Biomechanics: cell research and applications for the next decade. Ann Biomed Eng 2009;37:847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol 1996;23:727. [PubMed] [Google Scholar]
- 507.Inanlou MR, Baguma-Nibasheka M, Kablar B. The role of fetal breathing-like movements in lung organogenesis. Histol Histopathol 2005;20:1261. [DOI] [PubMed] [Google Scholar]
- 508.Blott M, Greenough A, Nicolaides KH. Fetal breathing movements in pregnancies complicated by premature membrane rupture in the second trimester. Early Hum Dev 1990;21:41. [DOI] [PubMed] [Google Scholar]
- 509.Harding R. Fetal pulmonary development: the role of respiratory movements. Equine Vet J Suppl 1997; 24:32–39. [DOI] [PubMed] [Google Scholar]
- 510.Liu M, Skinner SJ, Xu J, Han RN, Tanswell AK, Post M. Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol Lung Cell Mol Physiol 1992;263:L376. [DOI] [PubMed] [Google Scholar]
- 511.Nakamura T, Liu M, Mourgeon E, Slutsky A, Post M. Mechanical strain and dexamethasone selectively increase surfactant protein C and tropoelastin gene expression. Am J Physiol Lung Cell Mol Physiol 2000;278:L974. [DOI] [PubMed] [Google Scholar]
- 512.Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, Rubin LP. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol 2001;91:589. [DOI] [PubMed] [Google Scholar]
- 513.Sanchez-Esteban J, Tsai SW, Sang J, Qin J, Torday JS, Rubin LP. Effects of mechanical forces on lung-specific gene expression. Am J Med Sci 1998;316:200. [PubMed] [Google Scholar]
- 514.Gutierrez JA, Gonzalez RF, Dobbs LG. Mechanical distension modulates pulmonary alveolar epithelial phenotypic expression in vitro. Am J Physiol Lung Cell Mol Physiol 1998;274:L196. [DOI] [PubMed] [Google Scholar]
- 515.Pugin J. Molecular mechanisms of lung cell activation induced by cyclic stretch. Crit Care Med 2003;31:S200–S206. [DOI] [PubMed] [Google Scholar]
- 516.Copland IB, Post M. Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J Cell Physiol 2007;210:133–143. [DOI] [PubMed] [Google Scholar]
- 517.Ning QM, Wang XR. Activations of mitogen-activated protein kinase and nuclear factor-kappaB by mechanical stretch result in ventilation-induced lung injury. Med Hypotheses 2007;68:356–360. [DOI] [PubMed] [Google Scholar]
- 518.Zhang P, Baxter J, Vinod K, Tulenko TN, Di Muzio PJ. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev 2009;18:1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ahsan T, Nerem RM. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng Part A 2010;16:3547–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Kisiday JD, Frisbie DD, McIlwraith CW, Grodzinsky AJ. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A 2009;15:2817–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sueblinvong V, Larson ER, Spees JL, Weiss DJ. Three-dimensional culture systems can induce lung epithelial cell marker expression by mesenchymal stem cells in vitro. Proc Am Thorac Soc 2008;177:A720. [Google Scholar]
- 522.Barrila J, Radtke AL, Crabbé A, Sarker SF, Herbst-Kralovetz MM, Ott CM, Nickerson CA. Organotypic 3D cell culture models: using the rotating wall vessel to study host–pathogen interactions. Nat Rev Microbiol 2010;8:791–801. [DOI] [PubMed] [Google Scholar]
- 523.Fridley KM, Fernandez I, Li MA, Kettlewell RB, Roy K. Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A 2010;16:3285–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Skardal Al, Sarker SF, Crabbé A, Nickerson CA, Prestwich GD. The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. 2010;31:8426–8435. [DOI] [PubMed]
- 525.Carterson AJ, Honer zu Bentrup K, Ott CM, Clarke MS, Pierson DL, Vanderburg CR, Buchanan KL, Nickerson CA, Schurr MJ. A549 lung epithelial cells grown as 3-D aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect Immun 2005;73:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Crabbe A, De Boever P, Van Houdt R, Moors H, Mergeay M, Cornleius P. Use of the rotating wall vessel technology to study the effect of shear stress on growth behavior of Pseudomonas aeruginosa PA01. Environ Microbiol 2008;10:2098–2110. [DOI] [PubMed] [Google Scholar]
- 527.Rothen-Rutishauser BM, Kiama SG, Gehr PA. Three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am J Respir Cell Mol Biol 2005;32:281–289. [DOI] [PubMed] [Google Scholar]
- 528.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. et al. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Samadikuchaksaraei A, Bishop AE. Derivation and characterization of alveolar epithelial cells from murine embryonic stem cells in vitro. Methods Mol Biol 2006;330:233–248. [DOI] [PubMed] [Google Scholar]
- 530.Rippon HJ, Polak JM, Qin M, Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells 2006;24:1389–1398. [DOI] [PubMed] [Google Scholar]
- 531.Denham M, Cole TJ, Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol 2006;290:L1210–L1215. [DOI] [PubMed] [Google Scholar]
- 532.Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, Bishop AE. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng 2006;12:867–875. [DOI] [PubMed] [Google Scholar]
- 533.Van Vranken BE, Rippon HJ, Samadikuchaksaraei A, Trounson AO, Bishop AE. The differentiation of distal lung epithelium from embryonic stem cells. In: Current protocols in stem cell biology. 1 Jul 2007. DOI:10.1002/9780470151808.sc01g01s2. [DOI] [PubMed]
- 534.Denham M, Conley BJ, Olsson F, Gulluyan L, Cole TJ, Mollard R. A murine respiratory-inducing niche displays variable efficiency across human and mouse embryonic stem cell species. Am J Physiol Lung Cell Mol Physiol 2007;292:L1241–L1247. [DOI] [PubMed] [Google Scholar]
- 535.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 2007;104:4449–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Winkler ME, Mauritz C, Groos S, Kispert A, Menke S, Hoffmann A, Gruh I, Schwanke K, Haverich A, Martin U. Serum-free differentiation of murine embryonic stem cells into alveolar type II epithelial cells. Cloning Stem Cells 2008;10:49–64. [DOI] [PubMed] [Google Scholar]
- 537.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A 2009;15:3351–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol 2005;32:87–92. [DOI] [PubMed] [Google Scholar]
- 539.Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res 2009;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Hewitt KJ, Shamis Y, Carlson MW, Aberdam E, Aberdam D, Garlick JA. Three-dimensional epithelial tissues generated from human embryonic stem cells. Tissue Eng Part A 2009;15:3417–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Lin YM, Zhang A, Rippon HJ, Bismarck A, Bishop AE. Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A 2010;16:1515–1526. [DOI] [PubMed] [Google Scholar]
- 542.Wang Y, Wong LB, Mao H. Induction of ciliated cells from avian embryonic stem cells using three-dimensional matrix. Tissue Eng Part C Methods 2010;16:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Pickering SJ, Minger SL, Patel M, Taylor H, Black C, Burns CJ, Ekonomou A, Braude PR. Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis. Reprod Biomed Online 2005;10:390–397. [DOI] [PubMed] [Google Scholar]
- 544.Mateizel I, De Temmerman N, Ullmann U, Cauffman G, Sermon K, Van de Velde H, De Rycke M, Degreef E, Devroey P, Liebaers I, et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod 2006;21:503–511. [DOI] [PubMed] [Google Scholar]
- 545.Deleu S, Gonzalez-Merino E, Gaspard N, Nguyen TM, Vanderhaeghen P, Lagneaux L, Toungouz M, Englert Y, Devreker F. Human cystic fibrosis embryonic stem cell lines derived on placental mesenchymal stromal cells. Reprod Biomed Online 2009;18:704–716. [DOI] [PubMed] [Google Scholar]
- 546.American Thoracic Society. ATS statement on embryonic stem cell research [electronic mail]. Message to ATS members. 2010. Sep 9.
- 547.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 548.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 549.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. [DOI] [PubMed] [Google Scholar]
- 550.Sommer CA, Sommer AG, Longmire TA, Christodoulou C, Thomas DD, Gostissa M, Alt FW, Murphy GJ, Kotton DN, Mostoslavsky G. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells 2010;28:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 2009;27:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells 2010;28:1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Jones DA, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 2008;9:11–21. [DOI] [PubMed] [Google Scholar]
- 554.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest 2009;119:2538–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R, Woolfenden AE, Kirsch DG, Wong KK, Kim CF. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell 2010;7:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development 2009;136:509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Rippon HJ, Ali NN, Polak JM, Bishop AE. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells 2004;6:49–56. [DOI] [PubMed] [Google Scholar]
- 558.Hakelien AM, Landsverk HB, Robl JM, Skalhegg BS, Collas P. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat Biotechnol 2002;20:460–466. [DOI] [PubMed] [Google Scholar]
- 559.Qin M, Tai G, Collas P, Polak JM, Bishop AE. Cell extract-derived differentiation of embryonic stem cells. Stem Cells 2005;23:712–718. [DOI] [PubMed] [Google Scholar]
- 560.Tan J, Gemeinhart RA, Ma M, Saltzman WM. Improved cell adhesion and proliferation on synthetic phosphonic acid-containing hydrogels. Biomaterials 2005;26:3663–3671. [DOI] [PubMed] [Google Scholar]
- 561.Randle WL, Cha JM, Hwang YS, Chan KL, Kazarian SG, Polak JM, Mantalaris A. Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells. Tissue Eng 2007;13:2957–2970. [DOI] [PubMed] [Google Scholar]
- 562.Hwang NS, Varghese S, Elisseeff J. Cartilage tissue engineering: Directed differentiation of embryonic stem cells in three-dimensional hydrogel culture. Methods Mol Biol 2007;407:351–373. [DOI] [PubMed] [Google Scholar]
- 563.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA 2007;104:11298–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA 2006;103:16806–16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA 2008;105:12301–12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 2005;132:35–47. [DOI] [PubMed] [Google Scholar]
- 567.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 2008;172:615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.