Abstract
Community-acquired pneumonia affects approximately 4 million people in the United States, with 40,000 deaths per year. The incidence is increased about 35-fold in HIV-infected individuals, and this rate has decreased since the antiretroviral era has begun. Bacterial pneumonia has decreased from 5 to 20 cases per 100 person-years to less than 1 to 5 cases per 100 person-years in the era of antiretroviral therapy. HIV-1 infection impairs the function of neutrophils in the lung and infects CD4+ cells and alveolar macrophages. Opportunistic infections dramatically increase local HIV replication in the lung cells, especially alveolar macrophages and CD4+ cells. This enhanced replication increases viral mutations and provides opportunities for viral escape from latent reservoirs. Mortality is increased with more comorbidities in this highly susceptible population. Immunization with vaccines is recommended, especially pneumococcal vaccines, although the vaccine itself may stimulate viral replication. Recent studies show that the lower respiratory tract is a microbial reservoir in HIV-infected individuals rather than being a sterile environment, as originally thought. This may provide new opportunities for preventing opportunistic infections in HIV-infected subjects. Bacterial pneumonia presents an ongoing challenge in these high-risk individuals, particularly in studying the functions of the innate and acquired immune response.
Keywords: bacterial pneumonia, HIV antiretroviral therapy
Community-acquired pneumonia affects approximately 4 million people in the United States per year, resulting in more than 10 million physician contacts and more than 1 million hospital admissions with approximately 40,000 deaths per year (1). The widespread use of antiretroviral therapy (ART) has changed the spectrum of pulmonary disease in HIV-infected individuals, with an increase in noninfectious disease caused by immune reconstitution or facilitated by prolonged survival (i.e., chronic obstructive lung disease and neoplastic diseases) (2). Despite the significant advances in treatment, bacterial pneumonia continues to have a significant impact on the mortality rates of HIV-infected patients (Figure 1), especially in populations with poor access to health care services who frequently present with acute AIDS-related opportunistic infections (3).
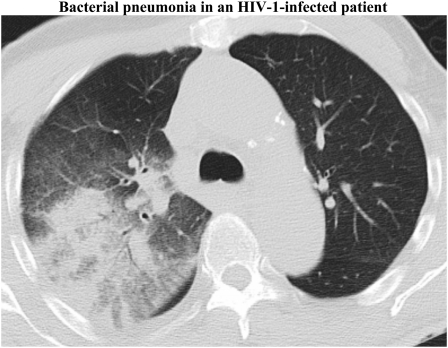
Figure 1.
Representative CT scan showing a right upper lobe infiltrate corresponding to a bacterial pneumonia in an HIV-infected patient.
The degree of immunosuppression and the abnormalities seen in pulmonary cells and function contribute to the observed increased incidence of opportunistic infections (4). However, a different pattern of pulmonary infectious disease has emerged with the more readily available use of ART. Although HIV-infected patients continue to have an increased risk of acquiring bacterial pneumonia compared with HIV-noninfected individuals, there is a declining incidence of opportunistic infections, such as Pneumocystis jiroveci pneumonia (PCP) and tuberculosis (TB).
The incidence of PCP initially declined after the introduction of trimethoprim-sulfasoxazole prophylaxis and declined further with the broad use of ART. In the United States, the incidence of PCP decreased 3.4% per year during 1992 to 1995 and declined 21.5% per year from 1996 to 1998. Similarly, in Europe, the incidence declined from 4.9 cases per 100 person-years before the introduction of ART to 0.3 cases per 100 person-years in 1998 (5). The impact of ART can be demonstrated by the fact that restoration of immune competence leads to long-term protection from PCP (6). Therefore, long-term primary and secondary prophylaxis can be safely discontinued if the CD4+ cell count is greater than 200 per mm3 (5, 7). In developing countries, TB continues to be the most common opportunistic disease and cause of death in patients with HIV infection. HIV is associated with significant increased risk of reactivation of latent TB and progression to active disease in recently acquired infections. In South Africa, the incidence of TB has grown 3-fold in the last decade, and 70% of the patients with TB are coinfected with HIV (8).
Despite the overall reduction in the incidence of pneumonia in developed countries after the introduction of ART, bacterial pneumonia continues to be an important comorbidity in HIV- infected patients (1, 9–11). Before the wide-spread use of ART, Streptococcus pneumoniae, the most common cause of pneumonia, was associated with a 100-fold increase in the rates of invasive pneumococcal disease during advanced HIV infection (4). In the original Pulmonary Complications of HIV Infection Study Group pre-ART, 1,130 HIV-infected adults were prospectively followed for 64 months, and 237 episodes of bacterial pneumonia (5.5 per 100 person-years) were observed, compared with six episodes among HIV-negative participants (0.9 per 100 person-years; P < 0.001). The rate of bacterial pneumonia increased with decreasing CD4+ cells (2.3, 6.8, and 10.8 episodes per 100 person-years in the strata with more than 500, 200–500, and <200 cells per mm3, respectively; P < 0.022 for each comparison). Injection drug users had a higher rate of bacterial pneumonia than did homosexual or bisexual men or female partners. In the stratum with the fewest CD4+ cells, cigarette smoking was associated with an increased rate of pneumonia. Mortality was four times higher among participants with an episode of bacterial pneumonia (4). In this cohort followed over 5 years, upper respiratory infections were the most common infection, occurring twice as frequently as in HIV-negative individuals, and acute bronchitis was the most frequent lower respiratory infection (13.7 vs. 7.4 episodes per 100 person-years; P < 0.0001) (12). In a retrospective review of 39,086 HIV-1–infected patients from the Adult and Adolescent Spectrum of HIV Disease Project, the incidence of pneumococcal disease was calculated to be 8.2 episodes per 1,000 person-years, where 81% of them were pneumonia (13). This incidence was independently associated with CD4+ cell count, history of pneumococcal disease, and AIDS-related opportunistic infections. Further analysis demonstrated that a decrease in the risk of pneumococcal disease was associated with antiretroviral treatment. PCP prophylaxis with trimethoprim-sulfamethoxazole was not associated with a decrease in pneumococcal disease (13). The effect of antiretroviral treatment was further evaluated by Sullivan and colleagues, who showed that in HIV-infected subjects with at least one CD4+ cell count of less than 200 cells/mm3, ART was significantly associated with a reduction in the incidence of bacterial pneumonia (10). Similarly, Hefferman and colleagues demonstrated that there was a 57% decline in invasive pneumococcal disease incidence among subjects with AIDS during the first 5 years after the introduction of ART, based on data obtained from the Active Bacterial Core Surveillance/Emerging Infections Program network (14). In a different study, the incidence of pneumococcal bacteremia had a 2.9-fold decrease after the institution of ART (11). Other risks factors associated with the development of pneumococcal bacteremia were CD4+ cell count, alcohol abuse, prior pneumonia, and smoking status. They also found high rates of antibiotic resistance in pneumococcal blood isolates (11). Despite this overall reduction in the incidence of pneumococcal disease with the introduction of ART, the risk of HIV-infected individuals developing invasive pneumococcal disease was 35 times higher than the estimated rate among similarly aged non–HIV-infected adults (1, 14, 15). Bacterial pneumonia in HIV-infected individuals still presents significant morbidity.
In a cohort comparing HIV infection in women, the rate of bacterial pneumonia among 9,885 HIV-infected women was 8.5 cases per 100 person-years, compared with 0.7 cases per 100 person-years in 425 HIV-noninfected women (P < 0.001) (9). Among women who had used TMP-SMX for 12 months, each month of ART decreased bacterial pneumonia risk by 8%. Increments of 50 CD4+ cells per mm3 decreased the risk, and smoking doubled the risk. Overall, bacterial pneumonia increased mortality risk (hazard ratio, 5.02; 95% confidence interval, 2.12–11.87). When protease inhibitors were added to the ART regimen, bacterial pneumonia at the Johns Hopkins HIV Clinics dropped from 22.7 episodes per 100 person-years in 1993 to 9.1 episodes per 100 person-years in 1997 (P < 0.05) (10). Chronic obstructive pulmonary disease and asthma were not risk factors for community-acquired pneumonia in a public hospital setting, but prior pneumonia was a risk factor among HIV-infected patients. The same bacterial organisms causing community-acquired pneumonia were found in HIV-infected individuals as in those who were HIV negative, with a small increase in Pseudomonas aeruginosa and mycobacteria. Initial disease severity appeared to be an imperfect predictor of specific microbial etiologies. Initial empiric antibiotic therapy recommendations were still optimal against Streptococcus pneumoniae. Hefferman and colleagues used time–trend analysis of a population-based active surveillance system from 1995 to 2000 and showed that the invasive pneumococcal disease incidence in persons with HIV infection was half that of the pre-ART era but was still 35 times higher than that in similarly aged, non-HIV–infected adults (14). Compared with the pre-ART era, HIV-infected patients had higher comorbidities (42 vs. 26%; P = 0.04), fewer recurrences of bacteremia (4 vs. 15%; P = 0.04), and a higher 30-day mortality rate (26 vs. 8%; P = 0.04) (11). Multivariate analysis showed comorbidity (odds ratio [OR], 3.36), alcohol abuse (OR, 5.28) and current smoking (OR, 5.19) as risks factors, while use of ART and pneumococcal vaccine were protective factors.
The impact of pneumonia on HIV progression and outcome should also be considered. The median duration of survival among 150 individuals after initial bacterial pneumonia was 24 months, compared with 37 months for 299 HIV-infected control subjects matched by CD4+ cells in the Pulmonary Complications of HIV Cohort Study (16). Similar results were found in PCP and TB: Whalen and colleagues compared the survival and incidence rate of opportunistic infections in 106 HIV-infected patients with active TB versus 106 HIV-infected persons without TB and with a similar level of immunosuppression (17). Their results showed an increase in the incidence rate of new AIDS-defining opportunistic infections and a shorter overall survival rate, suggesting that TB may be a cofactor that accelerates the clinical course of HIV infection (17). The observed reduction in the long-term survival after bacterial pneumonia compared with HIV-infected persons without an episode of bacterial pneumonia may be influenced by different factors, including increased viral replication and viral burden, increased inflammation, and changes in immune status. Alternatively, pneumonia may be a marker of more severe immunosuppression than what is reflected by analyzing the CD4+ count only (16).
The degree of immunosuppression has been associated with increased risk of pneumonia. The incidence of bacterial pneumonia increases as CD4+ numbers decline and is consistently higher in individuals with less than 200 CD4+ cells. Furthermore, in a recent follow-up of the SMART study group, the increased risk of opportunistic disease correlated with the viral load and was independent of the CD4+ cell count (18). Multiple risk factors contribute to this increased risk of pneumonia, including intravenous drug use, smoking of illicit drugs, and alcoholism. Tobacco smoking, which is highly prevalent in HIV-infected populations, is also independently associated with an increased risk of bacterial pneumonia (19). Different mechanisms have been described for this association, including a selective increase in the susceptibility of the alveolar macrophage to HIV infection and impairment of phagocytic function (20).
LUNG CELLS IN BACTERIAL PNEUMONIA
During bacterial pneumonia, neutrophils migrate out of the pulmonary capillaries and into the air spaces. After phagocytosis, neutrophils kill ingested microbes with reactive oxygen species (e.g., hypochlorite, superoxide, hydrogen peroxide), antimicrobial proteins (e.g., bacterial permeability-increasing protein and lactoferrin), and degradative enzymes (e.g., elastase). Neutrophil extracellular traps ensnare and kill extracellular bacteria with a chromatin meshwork. As a consequence of the observed dysfunction of not only CD4+ cells but also B lymphocytes, neutrophils and alveolar macrophages, there is an impairment of the bacterial clearance, with an increased risk of pneumonia. Antimicrobial opsonic and bacteriostatic proteins include complement, natural antibodies, C-reactive protein, and pentraxin 3. Intracellular signaling pathways triggered by pattern recognition receptors converge on transcription factors such as NF-κB (Rel A or p65) to transcribe chemokines and cytokines necessary for the inflammatory response.
Alveolar macrophages secrete inflammatory cytokines, including IL-8, which are chemotactic for neutrophils, and key inflammatory cytokines, including IL-1β, IL-6, and TNF-α. Sepsis induces IL-1 receptor–associated kinase in alveolar macrophages, which inhibits pattern recognition and cytokine receptors that activate NF-κB, compromising or toning down host defense. IL-17 activates lung epithelial cells to express chemokines and colony-stimulating factors and is essential for neutrophil-mediated host defense during Klebsiella pneumonia (21).
The host defense against pneumococcal pneumonia depends largely on alveolar macrophages and opsonization of specific IgG to pneumococcal capsular polysaccharide and complement, followed by phagocytic killing. Takahashi and colleagues described a decrease in serum opsonic activity against S. pneumoniae, despite the observed increased levels of type-specific IgG among HIV-infected patients (22). Furthermore, there was a significant correlation between HIV loads and peripheral blood CD4+ lymphocyte counts with serum opsonic activity against the type 3 and type 9 strains of S. pneumoniae among asymptomatic HIV-infected persons. Therefore, they postulated that HIV-infected individuals who are susceptible to invasive pneumococcal infection may have specific functional abnormalities of type-specific IgG.
B-cell perturbations include hypergammaglobulinemia, loss of memory B-cell type, and diminished antibody responses to vaccination. These B-cell abnormalities are linked with the inability of HIV-infected individuals to form effective antibody responses against opportunistic pathogens. Titanji and colleagues evaluated the effects of HIV infection on the maintenance of serologic memory and showed a low percentage of memory B cells that correlated with the CD4+ cell count in persons with chronic HIV (23). Furthermore, there was a depletion of antigen-specific memory B cells, with a dramatic reduction in antibodies to S. pneumoniae that may lead to a decline of serologic immune response. The increase in CD4+ cell counts and the decrease in viral load achieved by ART did not seem to improve the serologic memory response.
The infiltration of CD8+ cytotoxic lymphocytes may represent an immune response to HIV-infected cells and appears to correlate with high viral load burden within the lung (24). Increasing HIV replication during acute infection leads to a general activation of CD4+ and CD8+ cells, including HIV-specific CD4+ cells. Activated CD4+ cells are likely to be successfully infected with HIV; therefore, it is likely that the cells necessary to orchestrate a robust immune response are targeted for destruction during acute infection (25). Although CD4+ lymphocytes are the primary targets for HIV, other CD4+ cells, such as macrophages and dendritic cells, can function as a reservoir or transmit infection (26–28). We have previously demonstrated that even after 6 months of ART, a significant amount of integrated provirus can be identified in cells obtained by bronchoalveolar lavage (29).
In the case of HIV infection, NF-κB binds to the HIV long terminal repeat (LTR), which contains two tandem repeat NF-κB binding sites just upstream of the transcription start site. LPS enhances HIV expression in chronically infected monocyte/macrophages by activating NF-κB. The HIV-1 LTR also has binding elements for the C/EBPβ gene (also called NF-IL6, LAP/LIP, and NF-M). This gene has no introns, but two different proteins are produced from the same mRNA (30). The stimulatory isoform is approximately 30 to 37 kD (LAP for liver activation protein), whereas the small isoform, approximately 16 to 20 kD (described in rat liver and also called LIP for liver-induced inhibitory protein), functions as an inhibitory transcription factor. The inhibitory 16-kD C/EBPβ isoform is a dominant negative transcription factor, inhibiting transcription if present at 20% the level of the stimulatory isoform (31, 32). This dominant negative regulator is repressed during TB, leading to high levels of HIV-1 replication in the radiographically involved lung segments (33). Bacterial pneumonia presents with neutrophil-predominant inflammation. Hoshino and colleagues have shown, in a model of TB pneumonia, that contact between PMNs and macrophages leads to loss of inhibitory 16-kD C/EBPβ, enhancement of HIV-1 LTR transcription and increased HIV-1 replication (34). He and his colleagues have also shown that lymphocytes, in contact with alveolar macrophages, reduce inhibitory C/EBPβ, activate NF-κB, and enhance HIV replication. Using a 0.4-mm insert to separate lymphocytes from macrophages in vitro and allowing soluble factors to pass through, they showed that contact (down-regulation of inhibitory ∼16 kD C/EBPβ) and soluble factors activate NF-κB and are necessary for maximal HIV-1 5′ LTR transcription (35).
LUNG MICROBIOME IN HIV
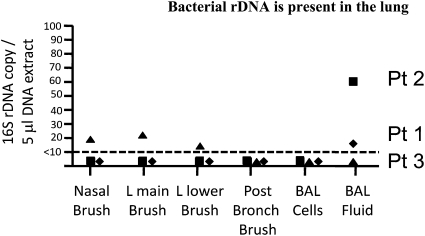
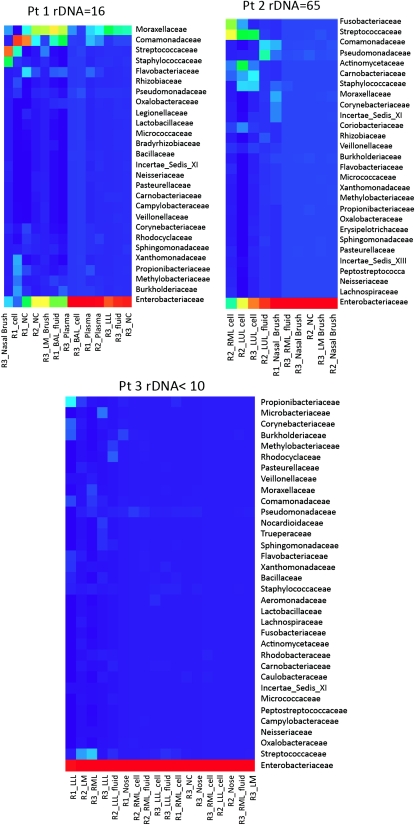
In studying bacterial–HIV interaction, we found evidence that HIV-infected individuals with no respiratory symptoms and normal chest radiographs had bacterial rDNA in their lungs. Fiberoptic bronchoscopy on patients with HIV infection was performed, and the lung microbiome was analyzed with 16S rRNA sequences to evaluate patterns of prokaryotic diversity. The range of bacterial colonization of the lower respiratory tract varied in different locations (Figure 2). The patient with the highest bacterial burden was an HIV-infected cigarette smoker, and cigarette smoke impairs mucociliary clearance. Smokers are at greater risk for bacterial pneumonia and for progression from LTBI to active TB (36). We calculated an rDNA concentration of up to 700 bacteria per ml of alveolar lining fluid. The sequence analysis of three HIV-infected patients without lung infiltrates resulted in 345,148 reads on 30 samples using three independent primer pairs (8,217 ± 2,026 sequences per sample) (Figure 3). The heat map of bacterial families found in the lung based on taxonomic classification of sequences using the RDP classifier demonstrated a complex community of bacteria colonizing the lung of these patients. Enterobacteriaceae predominated, although there were few samples collected. In patients with higher bacterial burden, there was a different distribution, with Staphylococcaceae, Streptococcaceae, Moraxellaceae, and Comamonadaceae displacing the Enterobacteriaceae. A low mycobacterial signal was seen in the BAL cell extract of patient 1 but only with the R1 primer set. From the BAL cell extract of patient 2, 17 high-quality sequences (average read lengths 403 bp) of the 1,194 rDNA sequences classified to the level of family mapped to mycobacteria (although all with high >0.95 confidence), as shown in the Actimomycetaceae row of the heat map. This supports the existence of a bacterial reservoir in the lung, with the possibility of expansion in case further immunosuppression were to occur.
Figure 2.
Quantitative PCR with molecular beacons of nasal brushing, endobronchial brushing, and bronchoalveolar lavage (BAL). Universal primers for bacterial 16S rDNA. The location of brushing is shown on the x axis. The brushes were placed in lysozyme solution and incubated at 65°C for 30 minutes followed by the addition of sodium dodecyl sulfate and proteinase K. The BAL was kept on ice and spun at low speed, followed by separation of fluid from the cell pellet. Aliquots of BAL fluid (200 μl) were then incubated in sodium dodecyl sulfate and proteinase K for 12 hours at 65°C. DNA was extracted from 100,000 BAL cells. In all samples, 2.5% of a 200 μl extract were used in the quantitative PCR reactions.
Figure 3.
Bacterial rDNA sequence analysis and amount from lung cells and BAL fluid of three patients with HIV from the longitudinal cohort. All had normal BAL cell differentials and chest radiographs.
HIV-infected patients have an improvement in morbidity and mortality with early initiation of ART, even during an acute opportunistic infection. This benefit can be detected as early as 6 months after initiation of therapy (37). Furthermore, there is growing evidence of the benefits of early initiation of ART in subjects coinfected with TB (38). In a recent study done by Karim and colleagues, there was a 56% reduction in mortality when ART was initiated during TB therapy as compared with delaying it until completion of TB therapy (38). This was further supported by a metaanalysis involving 6,934 patients at five hospitals in Madrid that showed a significant 63% improvement in survival among patients who began antiretroviral therapy while they were receiving TB therapy (39). ART leads to a decrease in viral load and an increase in CD4+ counts. Nevertheless, the pace at which central memory CD4+ and effector CD4+ T cells reconstitute during ART differs, as shown by Wilkinson and colleagues in a group of patients who were highly susceptible to developing active TB (40). Evaluation of phenotypic characterization of PPD-stimulated lymphocytes showed that CD4+ cells expressing the costimulatory molecule CD27 and lacking CD45RA (markers of central memory T cells) expanded by a median of 29%. In contrast, CD4+ CCR5+ effector cells proportionally declined during 48 weeks of ART. These findings suggest that the immune reconstitution achieved by ART is predominantly driven by the consequent expansion of central memory cells. This immune reconstitution determines an inflammatory state where there is a 35-fold increase in PPD-specific, IFN-γ–producing T cells, increased T-cell activation markers, and increased Th1 cytokines (IFN-γ, IL-12, IP-10, and Mig) as well as other proinflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10, and MCP-1) (41). This increased production of IFN-γ and IL-10 may contribute to the induction of inhibitory C/EBPβ in macrophages that has been shown to further repress HIV-1 transcription (42, 43).
The increased morbidity and mortality of HIV-infected patients with bacterial pneumonia, associated with altered mucosal and cellular immunity in the lung, suggest that this disorder may still take a toll in the era of ART. Because pulmonary infection induces cellular recruitment and activation in the lung, leading to enhanced HIV-1 replication, the influence of bacterial pneumonia on HIV progression should also be considered.
This work was supported by National Institutes of Health grants R01HL090316-1 and K24 AI080298A.
Author Disclosure: L.N.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.A.M. received grant support from the NIH and the DOE. A.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.N.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.B. was a consultant for Boehringer Ingelheim (BI), Morria Biopharmaceuticals, Almirall/Forest, ALKAbella, and Hoffman la Roche. He was on the Board or Advisory Board for AstraZeneca (AZ), Merck, BI, GlaxoSmithKline (GSK), Nycomed, Novartis, Schering Plough, Pfizer, and Sanofi Aventis . He received lecture fees from AZ, BI, GSK, Nycomed, Pfizer, and TEVA and he received grant support from AZ, BI, GSK, Nycomed, Merck, the NIH, Aeras, Eumedic Ltd, Fogerty, and the National Research Foundation.
References
- 1.Park DR, Sherbin VL, Goodman MS, Pacifico AD, Rubenfeld GD, Polissar NL, Root RK. The etiology of community-acquired pneumonia at an urban public hospital: influence of human immunodeficiency virus infection and initial severity of illness. J Infect Dis 2001;184:268–277. [DOI] [PubMed] [Google Scholar]
- 2.Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS 2006;20:1095–1107. [DOI] [PubMed] [Google Scholar]
- 3.Schwarcz S, Hsu L, Dilley JW, Loeb L, Nelson K, Boyd S. Late diagnosis of HIV infection: trends, prevalence, and characteristics of persons whose HIV diagnosis occurred within 12 months of developing AIDS. J Acquir Immune Defic Syndr 2006;43:491–494. [DOI] [PubMed] [Google Scholar]
- 4.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, Markowitz N, Rosen MJ, Mangura BT, Hopewell PC. Pulmonary Complications of HIV Infection Study Group. Bacterial pneumonia in persons infected with the human immunodeficiency virus. N Engl J Med 1995;333:845–851. [DOI] [PubMed] [Google Scholar]
- 5.Weverling GJ, Mocroft A, Ledergerber B, Kirk O, Gonzales-Lahoz J, d'Arminio Monforte A, Proenca R, Phillips AN, Lundgren JD, Reiss P. Eurosida Study Group. Discontinuation of pneumocystis carinii pneumonia prophylaxis after start of highly active antiretroviral therapy in HIV-1 infection. Lancet 1999;353:1293–1298. [DOI] [PubMed] [Google Scholar]
- 6.Lopez Bernaldo de Quiros JC, Miro JM, Pena JM, Podzamczer D, Alberdi JC, Martinez E, Cosin J, Claramonte X, Gonzalez J, Domingo P, et al. A randomized trial of the discontinuation of primary and secondary prophylaxis against pneumocystis carinii pneumonia after highly active antiretroviral therapy in patients with HIV infection. N Engl J Med 2001;344:159–167. [DOI] [PubMed] [Google Scholar]
- 7.Ledergerber B, Mocroft A, Reiss P, Furrer H, Kirk O, Bickel M, Uberti-Foppa C, Pradier C, D'Arminio Monforte A, Schneider MM, et al. Discontinuation of secondary prophylaxis against pneumocystis carinii pneumonia in patients with HIV infection who have a response to antiretroviral therapy. N Engl J Med 2001;344:168–174. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Global tuberculosis control: a short update to the 2009 report. World Health Organization; 2009.
- 9.Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, Rompalo AM, Moskaleva G, Schuman P, Schoenbaum EE. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (her) study. Clin Infect Dis 2006;43:90–98. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan JH, Moore RD, Keruly JC, Chaisson RE. Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am J Respir Crit Care Med 2000;162:64–67. [DOI] [PubMed] [Google Scholar]
- 11.Grau I, Pallares R, Tubau F, Schulze MH, Llopis F, Podzamczer D, Linares J, Gudiol F. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med 2005;165:1533–1540. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JM, Hansen NI, Lavange L, Glassroth J, Browdy BL, Rosen MJ, Kvale PA, Mangura BT, Reichman LB, Hopewell PC. Pulmonary Complications of HIV Infection Study Group. Respiratory disease trends in the pulmonary complications of HIV infection study cohort. Am J Respir Crit Care Med 1997;155:72–80. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis 2001;32:794–800. [DOI] [PubMed] [Google Scholar]
- 14.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 2005;191:2038–2045. [DOI] [PubMed] [Google Scholar]
- 15.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 16.Osmond DH, Chin DP, Glassroth J, Kvale PA, Wallace JM, Rosen MJ, Reichman LB, Poole WK, Hopewell PC. Pulmonary Complications of HIV Study Group. Impact of bacterial pneumonia and pneumocystis carinii pneumonia on human immunodeficiency virus disease progression. Clin Infect Dis 1999;29:536–543. [DOI] [PubMed] [Google Scholar]
- 17.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med 1995;151:129–135. [DOI] [PubMed] [Google Scholar]
- 18.Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, Neaton JD, Neuhaus J, Phillips AN. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the smart study: role of CD4+ cell counts and HIV RNA levels during follow-up. J Infect Dis 2008;197:1145–1155. [DOI] [PubMed] [Google Scholar]
- 19.Gordin FM, Roediger MP, Girard PM, Lundgren JD, Miro JM, Palfreeman A, Rodriguez-Barradas MC, Wolff MJ, Easterbrook PJ, Clezy K, et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med 2008;178:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elssner A, Carter JE, Yunger TM, Wewers MD. HIV-1 infection does not impair human alveolar macrophage phagocytic function unless combined with cigarette smoking. Chest 2004;125:1071–1076. [DOI] [PubMed] [Google Scholar]
- 21.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008;358:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi H, Oishi K, Yoshimine H, Kumatori A, Moji K, Watanabe K, Nalwoga H, Tugume SB, Kebba A, Mugerwa R, et al. Decreased serum opsonic activity against streptococcus pneumoniae in human immunodeficiency virus-infected Ugandan adults. Clin Infect Dis 2003;37:1534–1540. [DOI] [PubMed] [Google Scholar]
- 23.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006;108:1580–1587. [DOI] [PubMed] [Google Scholar]
- 24.Twigg HL, Soliman DM, Day RB, Knox KS, Anderson RJ, Wilkes DS, Schnizlein-Bick CT. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med 1999;159:1439–1444. [DOI] [PubMed] [Google Scholar]
- 25.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002;417:95–98. [DOI] [PubMed] [Google Scholar]
- 26.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science 1997;276:1857–1861. [DOI] [PubMed] [Google Scholar]
- 27.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-sign-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 2002;16:135–144. [DOI] [PubMed] [Google Scholar]
- 28.Beck JM. The immunocompromised host: HIV infection. Proc Am Thorac Soc 2005;2:423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twigg HL, Weiden M, Valentine F, Schnizlein-Bick CT, Bassett R, Zheng L, Wheat J, Day RB, Rominger H, Collman RG, et al. Effect of highly active antiretroviral therapy on viral burden in the lungs of HIV-infected subjects. J Infect Dis 2008;197:109–116. [DOI] [PubMed] [Google Scholar]
- 30.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 1991;67:569–579. [DOI] [PubMed] [Google Scholar]
- 31.Henderson AJ, Calame KL. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4(+) T cells. Proc Natl Acad Sci USA 1997;94:8714–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda Y, Rogers L, Nakata K, Zhao BY, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M. Type 1 interferon induces inhibitory 16-kd CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med 1998;188:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse DB, Shioda T, Rom WN, Weiden M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med 2002;195:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino Y, Hoshino S, Gold JA, Raju B, Prabhakar S, Pine R, Rom WN, Nakata K, Weiden M. Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J Infect Dis 2007;195:1303–1310. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino Y, Tse DB, Rochford G, Prabhakar S, Hoshino S, Chitkara N, Kuwabara K, Ching E, Raju B, Gold JA, et al. Mycobacterium tuberculosis-induced CXCR4 and chemokine expression leads to preferential X4 HIV-1 replication in human macrophages. J Immunol 2004;172:6251–6258. [DOI] [PubMed] [Google Scholar]
- 36.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 2009;180:475–480. [DOI] [PubMed] [Google Scholar]
- 37.Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, Hogg E, Komarow L. Early antiretroviral therapy reduces aids progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE 2009;4:e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010;362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velasco M, Castilla V, Sanz J, Gaspar G, Condes E, Barros C, Cervero M, Torres R, Guijarro C. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr 2009;50:148–152. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson KA, Seldon R, Meintjes G, Rangaka MX, Hanekom WA, Maartens G, Wilkinson RJ. Dissection of regenerating T-cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. Am J Respir Crit Care Med 2009;180:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006;20:F1–F7. [DOI] [PubMed] [Google Scholar]
- 42.Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol 2000;165:2028–2039. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka N, Hoshino Y, Gold J, Hoshino S, Martiniuk F, Kurata T, Pine R, Levy D, Rom WN, Weiden M. Interleukin-10 induces inhibitory C/EBP beta through Stat-3 and represses HIV-1 transcription in macrophages. Am J Respir Cell Mol Biol 2005;33:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]