Abstract
Infection with HIV increases the risk for lung diseases, including noninfectious pulmonary hypertension (PH). HIV-associated PH (HIV-PH) is an important lung disease in HIV-infected persons who live longer with antiretrovirals. The early stages of HIV-PH may be overlooked by healthcare providers due to nonspecific symptoms, including progressive dyspnea and nonproductive cough. HIV-PH may be detected via chest radiographs, CT scans, or electrocardiograms, but Doppler echocardiography is the most useful screening test to identify candidates for right heart catheterization. HIV-PH has a poor prognosis with high mortality; improved biomarkers to identify earlier stages of PH would benefit clinical care. The HIV-PH mechanism remains unknown, but HIV proteins such as Tat and Nef may play a role. HIV-1 Nef is a broad-spectrum adaptor protein that may affect HIV-infected and uninfected pulmonary vascular cells. Studies in macaques suggest that Nef is important in HIV-PH pathogenesis because monkeys infected with a chimeric simian immunodeficiency virus (SIV) expressing HIV-nef (SHIVnef) alleles, but not monkeys infected with the native SIV, develop pulmonary vascular remodeling. Four consistent amino acid mutations arose spontaneously in Nef passaged in the monkeys. To translate these findings to humans, one research endeavor of the Lung HIV Study focuses on the identification of HIV nef mutations in HIV-infected individuals with PH compared with HIV-infected normotensive patients. We present some of the preliminary evidence. Ongoing longitudinal studies will establish the connection between Nef mutations and the propensity for HIV-PH.
Keywords: pulmonary hypertension, HIV proteins, HIV-1 Nef, pathogenesis, Lung HIV study
According to data from the Joint United Nations Program on HIV/AIDS, there were over 33 million people living with HIV at the end of 2009 (1). HIV-associated lung complications are frequent causes of illness and death in industrialized nations, where the greatly improved prognosis of HIV-infected patients due to combination antiretroviral therapy (ART) has turned HIV into a chronic disease. As growing numbers of HIV-infected persons are surviving longer, they are developing comorbid diseases that significantly affect mortality (2–4).
HIV infection confers an increased risk for a variety of infectious lung diseases, including bacterial pneumonia, tuberculosis, and Pneumocystis pneumonia. Our understanding of the global epidemiology of these diseases in the antiretroviral era is limited. HIV infection also appears to increase the risk for noninfectious pulmonary conditions, including chronic obstructive pulmonary disease (5, 6), lung cancer (7), and pulmonary hypertension (PH) (8). The mechanisms for the observed increases in these noninfectious conditions are not well understood. In addition, the long-term consequences of HIV infection and HIV-associated pulmonary conditions on overall lung health are unknown. Despite the many years of the AIDS epidemic, we are still trying to understand the causal factors that account for the increased frequency of noninfectious complications of HIV infection and the role of the lung as an end-organ target.
HIV infection is an established risk factor for PH. The prevalence of PH is several-fold higher in HIV-infected individuals compared with the general population (0.5 vs. 0.0015%, respectively, in conservative estimates). It remains controversial whether such prevalence has remained unchanged since the advent of ART (9). Nevertheless, numerous studies have concluded that the impact of antiretroviral drugs has been minimal (10), suggesting that the virus may influence vascular cells in the microenvironment where distribution of antiretroviral drugs is uneven.
CLINICAL PRESENTATION, DIAGNOSIS, AND TREATMENT OF HIV-ASSOCIATED PH
HIV infection is an established risk factor for PH, likely overlooked in the pre-ART era because patients died from opportunistic infections. In general, the presentation and diagnosis of HIV-associated PH is similar to that for sporadic, idiopathic PH. Persons with HIV-PH typically present with progressive subacute dyspnea and occasionally with a nonproductive cough. As the disease progresses and as right ventricular involvement ensues, patients may report pedal edema, fatigue, syncope or near-syncope, and chest pain.
HIV infection may also influence the pathogenesis of several pulmonary disorders. For instance, HIV contributes to accelerated pathogenesis of chronic obstructive pulmonary disease (see the article by Morris and colleagues, in this issue of the Journal, pp. 320–325), and infection with HIV has been associated with a hypercoagulable state that culminates in higher clinically detectable thromboembolic disease (11). Thromboembolic disease is also associated with vascular remodeling and pulmonary hypertension. Common mechanisms include endothelial damage and dysfunction (12).
The diagnosis of HIV-PH is one of exclusion. Secondary causes for pulmonary hypertension should be sought and ruled out. Chest radiographs and chest CT scans demonstrate prominence of the central pulmonary arteries and right ventricular and atrial enlargement. Pulmonary function tests may reveal a mild restrictive pattern with a decreased diffusing capacity for carbon monoxide. Electrocardiogram findings include right ventricular hypertrophy, right axis deviation, right bundle branch block, and right atrial enlargement. Echocardiograms may reveal enlargement of the right heart chambers, decreased right ventricular systolic function, right ventricular hypertrophy, tricuspid regurgitation with a detectable regurgitant jet, and, in severe disease, a paradoxical bulging of the septum into the left ventricle during systole. Right heart catheterization is the gold standard diagnostic test for this condition, which is defined by a mean pulmonary artery pressure greater than 25 mm Hg at rest in the setting of a normal pulmonary capillary wedge pressure (≤ 15 mm Hg) (13).
Because the survival for HIV-infected individuals with PH with advanced symptoms (New York Heart Association, NYHA class III-IV) is worse compared with less symptomatic individuals (NYHA class I-II) (14), identification of asymptomatic individuals is of critical importance. Current guidelines are extrapolated from individuals with idiopathic PH (15, 16). Although in general the treatment for PH in HIV-infected persons is similar to uninfected individuals, possible drug interactions limit the use of sildenafil because its by-products after cytochrome P450 isoform 3A4 metabolism may interact with protease inhibitors (17, 18). Because persons with HIV often have multiple factors independently associated with PH, including lung disease or illicit drug use, distinguishing between the relative contributions of HIV infection and these other risk factors may be impossible. Therefore, identified secondary causes should be treated aggressively. Although some studies suggest that HIV-infected persons treated with ART may experience functional or hemodynamic improvement by Doppler echocardiography, other studies argue that ART does not improve hemodynamic parameters (10). Definitive conclusions will be unlikely given that this disease may not have been properly documented pre- or post-ART.
PATHOGENESIS OF HIV-ASSOCIATED PH
The pathogenesis of HIV-related PH remains to be elucidated completely and is the subject of intense investigation. The pulmonary vasculature is a delicate microenvironment and is susceptible to numerous complex pathogenic events that may trigger pulmonary hypertension. The endothelium is constantly exposed to blood cellular components and interacts with the extracellular matrix. Vasculitides are known outcomes from infectious pathogens in the lung, but whether they are due to persistent viral infection, exposure to toxic viral proteins, or viral-induced immune activation remains undetermined. There is evidence of vascular involvement in HIV/simian immunodeficiency virus (SIV) infection, including arteriopathy with severe intimal and smooth muscle hyperplasia in patients with AIDS and in SIV-infected macaques (19).
Histologically, lesions in HIV-infected patients with PH are similar to their uninfected counterparts with PH. These features include concentric laminar intimal fibrosis, medial hypertrophy, recanalized thrombi, and plexiform lesions (20). Additional hallmarks include increased expression of smooth muscle cell/fibroblast growth factors, such as platelet-derived growth factor. Similar to severe PH, inflammatory cells are present in the perivasculature of HIV tissues, suggesting that HIV-induced chronic inflammation and immune hyperactivation may enrich the proinflammatory milieu implicated in HIV-PH. Viral proteins such as Nef and Tat have been shown to lead to endothelial dysfunction and increased inflammation through activation of adhesion molecules and production of inflammatory chemokines, independent of virus production (21–25).
Monocytes/macrophages migrate through the endothelium to replenish myeloid cells and to provide immune surveillance. Several studies have shown increased migration of monocytes/macrophages exposed to HIV proteins. However, studies measuring and comparing forward and reverse transmigration showed that chronic exposure of monocytes/macrophages to HIV significantly decreased the emigration of macrophages from tissues by 67% (26), suggesting that HIV-infected macrophages migrate through the endothelium but do not reenter the bloodstream. Even though mechanisms that account for this differential impairment in transendothelial migration remain to be outlined, these observations add important insights to the establishment of tissue HIV reservoirs in macrophage-rich tissues like the lung.
HIV-infected individuals are frequently coinfected with other viruses that may contribute to the development of PH. Hepatitis B and human herpesvirus-8 have been linked to PH, although human herpesvirus-8 infection does not appear to be independently associated with HIV-PH (27, 28).
There is no evidence that HIV infects the pulmonary vascular endothelium, but HIV proteins are noxious to endothelial cells (ECs). HIV proteins interact with molecular partners in the infected host and, therefore, are strong candidates for cause–effect relationships because they may promote apoptosis, growth, and proliferation of a variety of cells in vitro (29, 30).
The HIV proteins Env, Tat and Nef are implicated in cardiopulmomary complications. The HIV envelope glycoprotein-120 (gp-120) present on the surface of virions mediates the attachment and fusion of the virus through the host cell membrane. Cell-free HIV gp-120 can be detected in the blood, cerebrospinal fluid, and brain of patients with HIV/AIDS. Furthermore, it increases production of macrophage-derived proinflammatory cytokines (31), increases the secretion of endothelin-1, and induces apoptosis of human lung ECs (29). HIV Tat protein (trans-activator of transcription) also activates ECs and has angiogenic properties (32, 33).
Among HIV proteins, Nef is the most strongly associated with HIV-related PH. Nef, a misnomer for “negative factor,” is expressed early during viral infection and is fundamental in HIV pathogenesis. In a macaque model, HIV Nef, but not SIV Nef, was associated with obliterative PH-like vascular remodeling and lung lesions. HIV-1 Nef colocalizes with ECs (19). Nef can cross cellular membranes and enter target cells via chemokine receptors such as CXCR4, which is expressed on ECs. Therefore, ECs in the lung may take up extracellular Nef in the absence of infection. Furthermore, Nef interacts with numerous host cell proteins and commandeers trafficking of intracellular vesicles essential in secretory/endocytic pathways (34).
The disruption of subcellular membrane trafficking pathways in ECs and smooth muscle cells warrants further discussion on the pathobiology of PH (35). ECs in plexiform lesions exhibit enlarged endoplasmic reticulum, Golgi stacks, and vacuolation, suggesting aberrant intracellular trafficking as a candidate underlying mechanism. Obliterative-plexiform lesions in patients with idiopathic PH and macaques infected with chimeric SIV/HIV-nef (SHIVnef) present noncharacteristic distribution of the Golgi tethers giantin and p115 at the subcellular level (35), with increased Golgi tethers and matrix proteins per cell in obliterative-plexiform lesions. The definitive role of Nef in Golgi dysfunction in the pathobiology of HIV-PH is a subject of intense investigation. Whether chronic exposure of lung ECs to viral proteins or inflammatory mediators leads to vascular injury and subsequent pulmonary vascular remodeling is a question that deserves further consideration and is being extensively tested.
Long-standing pathogenic principles of Nef rely on Nef myristoylation. Myristoylation refers to the acquisition of a myristoyl moiety at the N-terminus, and this reaction is highly dependent on sequence context. Myristoylation targets Nef to the plasma membrane, where it presumably enhances infectivity and down-regulates critical molecules such as major histocompatibility complex (MHC) 1 and CD4 receptors. It is well known that Nef down-regulates CD4 receptor by targeting it to the endocytic degradation pathway in clathrin-coated vesicles and MHC-1 by sequestration in the trans-Golgi. Nef also takes advantage of the normal recycling processes of MHC molecules in monocytes to delay the trafficking of MHC-II to the membrane and relocate it to lysosomes through cholesterol-dependent endocytosis (36). Newer studies demonstrated that Nef dimerizes in vivo, and the dimers are required to down-regulate CD4 and enhance HIV replication (37). On the other hand, dimerization is not required for membrane localization or interactions with SH3-containing cellular proteins. Various cell types transfected with nef fused to green fluorescent protein constructs released at least 3-fold more exosomes (vesicles) compared with controls, and this finding was dependent on an intact myristoylation site. Nef was incorporated into the exosomes, which caused activation-induced apoptosis in resting CD4+ T cells in vitro (38). These studies support the concept that Nef disturbs the subcellular environment relying on different Nef features (e.g., myristoylation and dimerization). Whereas Nef export and apoptotic potential rely on myristoylation, downmodulation of CD4 and MHC molecules (which may affect antigen presentation) can be potentially abrogated by the disruption of Nef dimers.
Nef-transfected lymphocytes displayed profound changes in morphology, including the formation of filopodia, decreased actin-rich membrane protrusions, and impaired adherence to fibronectin (39). This finding may explain the Nef-induced inhibition of T-cell motility, which is dependent on an intact myristoylation site and SH3 binding domain in Nef. Trans-endothelial cell migration of Nef-expressing cells is also highly dependent on an intact Nef myristoylation site (40). Essentially, changes in Nef sequences may affect known (41) and undiscovered Nef protein structure–function relationships.
HIV NEF SEQUENCES: BIOMARKERS FOR EARLY IDENTIFICATION OF HIV-PH?
As part of the Lung HIV Study, we aim to determine whether HIV-PH is associated with specific HIV-1 nef alleles. Peripheral blood mononuclear cells and bronchoalveolar lavage samples have been collected from HIV-infected participants, with and without PH, who are enrolled in an ongoing cohort study at the University of California, San Francisco (UCSF) evaluating the epidemiology and pathogenesis of pulmonary hypertension in HIV-infected individuals (27). The longitudinal collection of samples for the Lung HIV studies is ongoing. In this article, we present the results of our initial cross-sectional analyses performed in a subset of samples.
Study Subjects
We cloned and analyzed HIV-1 nef molecular clones peripheral blood mononuclear cell DNA samples from participants infected with subtype B HIV strains. Participants were enrolled in an ongoing study of the epidemiology and pathogenesis of pulmonary hypertension in HIV-infected individuals being conducted at UCSF. Inclusion criteria for this study included HIV infection for greater than 6 months in duration (as documented by HIV antibody testing or letter of diagnosis) and the ability to provide a reliable HIV medication history or having medical records showing HIV medication history. Exclusion criteria included significant cardiovascular disease, including valvular heart disease, congenital heart disease, symptomatic coronary disease, or known cardiomyopathy; obstructive sleep apnea; known collagen vascular disease; or history of anorexigen use. Participants underwent echocardiography followed by right heart catheterization. Individuals with a mean pulmonary artery pressure (PAP) > 25 mm Hg and pulmonary capillary wedge pressure ≤ 15 mm Hg were identified as having PH, and those with PAP ≤ 25 mm Hg were designated as normotensive. This study was approved by the Lung HIV Data Safety and Monitoring Board, UCSF Committee on Human Research, and the University of Colorado Denver Colorado Multiple Institutional Review Board. The demographics of the participants studied are shown in Table 1. The average duration of antiretroviral medications in the individuals with PH was 4.1 years, which was similar to control subjects (4.91 yr; P = 0.76). With respect to traditional cardiovascular risk factors, a similar proportion of individuals with PH had systemic hypertension, hyperlipidemia, diabetes mellitus, cigarette smoking, and injection drug use history compared with control subjects. HIV-infected individuals with PH were more likely to use methamphetamines than control subjects. Of the individuals with PH, 50% were being treated with PH medication, which included combination therapy with sildenafil and bosentan in all patients, with the median duration of therapy being 0.33 years. The mean PAP obtained by right heart catheterization in the individuals with PH was 41.2 mm Hg, as compared with the individuals without PH (17.6 mm Hg; P < 0.001).
TABLE 1.
GENERAL DEMOGRAPHICS AND HEMODYNAMICS OF HIV-INFECTED PARTICIPANTS FROM THE SAN FRANCISCO COHORT
| Statistic | Normotensive Group (n = 16) | HIV-PH Group (n = 6) | P Value1 | |
|---|---|---|---|---|
| Sex, male | n (%) | 14 (88) | 5 (83) | 0.80 |
| Age, yr | Mean (SD) | 51.4 (7.6) | 51.1 (9.5) | 0.95 |
| HIV infection, yr | Median (IQR) | 15 (13–17) | 15 (12–20) | 0.85 |
| Receiving ART | n (%) | 12 (75) | 5 (83) | 0.68 |
| CD4+ T cell counts, cells/μl | Mean (IQR) | 468 (366–606) | 423 (236–833) | 0.83 |
| Undetectable HIV VL, < 50 copies/ml | n (%) | 10 (63) | 5 (83) | 0.35 |
| Hypertension | n (%) | 8 (50) | 4 (67) | 0.48 |
| Hyperlipidemia | n (%) | 5 (31) | 3 (50) | 0.42 |
| Diabetes mellitus | n (%) | 2 (13) | 1 (17) | 0.80 |
| Ever smoke | n (%) | 11 (69) | 3 (50) | 0.42 |
| Ever used injection drugs | n (%) | 7 (44) | 4 (67) | 0.34 |
| Ever used methamphetamines | n (%) | 3 (19) | 4 (67) | 0.03* |
| 6MWT, m | Median (IQR) | 503 (434–532) | 423 (314–508) | 0.16 |
| PASP by echocardiography, mm Hg | Mean (SD) | 34.9 (6.3) | 52.5 (26.5) | 0.02* |
| mPAP by RHC, mm Hg | Mean (SD) | 17.6 (2.9) | 41.2 (15.2) | <0.0001* |
Definition of abbreviations: ART = combination antiretroviral therapy; HIV-PH = HIV-associated pulmonary hypertension; IQR = interquartile ratio; mPAP = mean pulmonary artery pressure; PASP = pulmonary artery systolic pressure; RHC = right heart catheterization; 6MWT = 6-min walk test; VL = viral load.
1Statistical approaches used to calculate P values were Chi square for categorical variables, t tests for comparing mean values (assuming equal distribution), and Wilcoxon rank sum for comparing median values (assuming nonequal distribution).
Statistically significant.
HIV-1 Nef Sequence Analyses
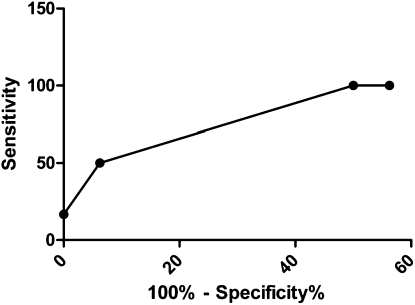
The full-length HIV-1 nef I cloned, and nucleotide sequences were aligned using BioEdit (43), translated into proteins, and realigned after visual inspection. Phylogenetic analyses of the full-length Nef protein demonstrated that individual sequences clustered specific to each participant, ruling out contamination at any level of sampling (not shown). Overall, individuals with HIV-PH harbored nef alleles with variations in Nef functional domains associated with CD4 and MHC-1 down-regulation, compared with normotensives. Receiver-operator characteristic curve analyses revealed that participants with more than three mutations were 12 times more likely to exhibit HIV-PH compared with those not having the mutations (Figure 1). Based on these analyses, mutations in three functional domains have a true positive value (sensitivity) of 50% (95% confidence interval [CI], 12–88) and a true negative value (specificity) of 94% (95% CI, 70–100). These data have an area under the curve of 0.85 (95% CI, 0.68–1; P = 0.014).
Figure 1.
Sensitivity and specificity analyses for an HIV-PH diagnosis based on the number of Nef functional domains with mutations. Data were summarized with a receiver-operator characteristic (ROC) curve; overall discrimination was quantified with area under the curve (AUC). Amino acid residues at each position were analyzed per clone. ROC curve analyses revealed that patients with mutations in Nef were 12 times more likely to exhibit HIV-PH compared with those not having the mutations; AUC of 0.85 (95% confidence interval, 0.68–1.00; P = 0.014).
The functional consequences of these mutations are a subject of intense investigation in our laboratory. However, based on the nature of Nef as a molecular adaptor and highly immunogenic viral protein, we can speculate that certain alleles drive immune responses that are dependent on specific amino acids in functional domains or that alleles may disrupt intracellular trafficking pathways in a selective manner and influence vascular cell physiology. Finally, through our Lung HIV longitudinal studies, we are sampling peripheral blood and lung cells to test whether common mutations emerge in patients with HIV-PH compared with HIV normotensive patients. These studies will allow further dissection of whether certain functional domains are mutated preferentially.
CONCLUSIONS
HIV evolutionary patterns in the lungs may play a role in HIV-PH. The high mutational profile of HIV may set the stage for selection of specific HIV quasispecies at certain anatomic sites like the lung, where the presence of the virus may trigger disease phenotypes. In our study cohort, a similar proportion of individuals with and without PH were treated with ART, suggesting that genetic mutations introduced by ART were less likely to confound the results of our findings. HIV-1 Nef, as a broad-spectrum adaptor, may affect infected and uninfected cells. The finding of nef mutations in individuals with HIV-PH compared with normotensives together with the tight sequence-functions affiliation inherent in Nef suggest that mutations in Nef could be used as screening tools for HIV-PH. Besides the longitudinal aspect of the Lung HIV studies, our enterprise is now focused on merging basic science and bioinformatics approaches to understand the consequences of mutations in HIV Nef in the context of pulmonary vascular biology.
Supported by the NIH/NHLBI grants R01 HL90480 and HL90480-03S1 (S.C.F.), R01HL091526 (P.H.), K24 HL087713, HL090335, and HL090335-02S1 (L.H.).
Author Disclosure: S.A., P.H., and J.M. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.H. received grant support from the Foundation for Innovative New Diagnostics (FIND). S.C.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Joint United Nations Program on HIV/AIDS and World Health Organization. Global Report: UNAIDS report on the global AIDS epidemic 2010 (accessed March 2011). Available from: http://www.unaids.org.
- 2.Braithwaite RS, Justice AC, Chang CC, Fusco JS, Raffanti SR, Wong JB, Roberts MS. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med 2005;118:890–898. [DOI] [PubMed] [Google Scholar]
- 3.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med 2005;6:99–106. [DOI] [PubMed] [Google Scholar]
- 4.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41:194–200. [DOI] [PubMed] [Google Scholar]
- 5.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–372. [DOI] [PubMed] [Google Scholar]
- 6.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–1333. [DOI] [PubMed] [Google Scholar]
- 7.Kirk GD, Merlo C, O' Driscoll P, Mehta SH, Galai N, Vlahov D, Samet J, Engels EA. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007;45:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest 2000;118:1133–1141. [DOI] [PubMed] [Google Scholar]
- 9.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS 2008;22:S35–S40. [DOI] [PubMed] [Google Scholar]
- 10.Degano B, Guillaume M, Savale L, Montani D, Jais X, Yaici A, Le PJ, Humbert M, Simonneau G, Sitbon O. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS 2010;24:67–75. [DOI] [PubMed] [Google Scholar]
- 11.Malek J, Rogers R, Kufera J, Hirshon JM. Venous thromboembolic disease in the HIV-infected patient. Am J Emerg Med 2011;29:278–282. [DOI] [PubMed] [Google Scholar]
- 12.Humbert M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur Respir Rev 2010;19:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–S66. [DOI] [PubMed] [Google Scholar]
- 14.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Herve P, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2003;167:1433–1439. [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, et al. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. Eur Heart J 2004;25:2243–2278. [DOI] [PubMed] [Google Scholar]
- 16.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest 2007;131:1917–1928. [DOI] [PubMed] [Google Scholar]
- 17.Merry C, Barry MG, Ryan M, Tjia JF, Hennessy M, Eagling VA, Mulcahy F, Back DJ. Interaction of sildenafil and indinavir when co-administered to HIV-positive patients. AIDS 1999;13:F101–F107. [DOI] [PubMed] [Google Scholar]
- 18.Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol 2000;50:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med 2006;174:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petitpretz P, Brenot F, Azarian R, Parent F, Rain B, Herve P, Simonneau G. Pulmonary hypertension in patients with human immunodeficiency virus infection: comparison with primary pulmonary hypertension. Circulation 1994;89:2722–2727. [DOI] [PubMed] [Google Scholar]
- 21.Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol. J Neurochem 2001;78:1315–1324. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann MH, Walter S, Ylisastigui L, Striebel F, Ovod V, Geyer M, Gluckman JC, Erfle V. Extracellular HIV-1 Nef increases migration of monocytes. Exp Cell Res 2006;312:3659–3668. [DOI] [PubMed] [Google Scholar]
- 23.Alexander L, Weiskopf E, Greenough TC, Gaddis NC, Auerbach MR, Malim MH, O'Brien SJ, Walker BD, Sullivan JL, Desrosiers RC. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol 2000;74:4361–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, et al. HIV-1 Tat protein mimicry of chemokines. 1. Proc Natl Acad Sci USA 1998;95:13153–13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cota-Gomez A, Flores NC, Cruz C, Casullo A, Aw TY, Ichikawa H, Schaack J, Scheinman R, Flores SC. The human immunodeficiency virus-1 Tat protein activates human umbilical vein endothelial cell E-selectin expression via an NF-kappa B-dependent mechanism. J Biol Chem 2002;277:14390–14399. [DOI] [PubMed] [Google Scholar]
- 26.Westhorpe CL, Zhou J, Webster NL, Kalionis B, Lewin SR, Jaworowski A, Muller WA, Crowe SM. Effects of HIV-1 infection in vitro on transendothelial migration by monocytes and monocyte-derived macrophages. J Leukoc Biol 2009;85:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, Ellman AB, Huang L, Dollard SC, Martin JN. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS 2008;22:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montani D, Marcelin AG, Sitbon O, Calvez V, Simonneau G, Humbert M. Human herpes virus 8 in HIV and non-HIV infected patients with pulmonary arterial hypertension in France. AIDS 2005;19:1239–1240. [DOI] [PubMed] [Google Scholar]
- 29.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun 2005;333:1107–1115. [DOI] [PubMed] [Google Scholar]
- 30.Rusnati M, Presta M. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis 2002;5:141–151. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Ruff M, Karwatowska-Prokopczuk E, Hunt L, Ji H, Pert CB, Zukowska-Grojec Z. HIV envelope protein gp120 induces neuropeptide Y receptor-mediated proliferation of vascular smooth muscle cells: relevance to AIDS cardiovascular pathogenesis. Regul Pept 1998;75–76:201–205. [DOI] [PubMed]
- 32.Albini A, Barillari G, Benelli R, Gallo RC, Ensoli B. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci USA 1995;92:4838–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, et al. The angiogenesis induced by HIV-1 Tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med 1996;2:1371–1375. [DOI] [PubMed] [Google Scholar]
- 34.Roeth JF, Collins KL. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol Mol Biol Rev 2006;70:548–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehgal PB, Mukhopadhyay S, Patel K, Xu F, Almodovar S, Tuder RM, Flores SC. Golgi dysfunction is a common feature in idiopathic human pulmonary hypertension and vascular lesions in SHIV-nef-infected macaques. Am J Physiol Lung Cell Mol Physiol 2009;297:L729–L737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhry A, Verghese DA, Das SR, Jameel S, George A, Bal V, Mayor S, Rath S. HIV-1 Nef promotes endocytosis of cell surface MHC class II molecules via a constitutive pathway. J Immunol 2009;183:2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poe JA, Smithgall TE. HIV-1 Nef dimerization is required for Nef-mediated receptor downregulation and viral replication. J Mol Biol 2009;394:329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010;11:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nobile C, Rudnicka D, Hasan M, Aulner N, Porrot F, Machu C, Renaud O, Prevost MC, Hivroz C, Schwartz O, et al. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. J Virol 2010;84:2282–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park IW, He JJ. HIV-1 Nef-mediated inhibition of T cell migration and its molecular determinants. J Leukoc Biol 2009;86:1171–1178. [DOI] [PubMed] [Google Scholar]
- 41.Geyer M, Fackler OT, Peterlin BM. Structure–function relationships in HIV-1 Nef. EMBO Rep 2001;2:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999;41:95–98. [Google Scholar]