Abstract
Smoking-related diseases, such as chronic obstructive pulmonary disease (COPD), are of particular concern in the HIV-infected population. Smoking rates are high in this population, and long-term exposure to cigarette smoke in the setting of HIV infection may increase the number of complications seen. Before the era of combination antiretroviral therapy, HIV-infected persons were noted to have an accelerated form of COPD, with significant emphysematous disease seen in individuals less than 40 years old. Unlike many of the AIDS-defining opportunistic infections, HIV-associated COPD may be more common in the current era of HIV because it is frequently reported in patients without a history of AIDS-related pulmonary complications and because many aging HIV-infected individuals have had a longer exposure to smoking and HIV. In this review, we document the epidemiology of HIV-associated COPD before and after the institution of combination antiretroviral therapy, review data suggesting that COPD is accelerated in those with HIV, and discuss possible mechanisms of HIV-associated COPD, including an increased susceptibility to chronic, latent infections; an aberrant inflammatory response; altered oxidant-antioxidant balance; increased apoptosis associated with HIV; and the effects of antiretroviral therapy.
Keywords: HIV, chronic obstructive pulmonary disease, emphysema, immune reconstitution
The frequency and types of lung diseases encountered in HIV-infected populations have changed many times over the course of the AIDS epidemic. With the development and use of combination antiretroviral therapy (ART), dramatic declines in morbidity and mortality from HIV/AIDS have been seen (1). These improvements do not constitute a cure, however, and over 400,000 people are living with HIV in the United States, and an estimated 33.4 million people are infected worldwide (2). With ART and the resulting increases in life expectancy, the incidence of nonopportunistic lung diseases, such as HIV-associated chronic obstructive pulmonary disease (COPD), may become more common. Respiratory symptoms are common in the HIV-infected population, particularly in HIV-infected persons who smoke, and obstructive lung disease is an increasing cause of morbidity and mortality in the HIV-infected population. Almost 4% of deaths among HIV-infected persons in 1998 were due to obstructive airway disease, representing a 3-fold increase from the preantiretroviral therapy era (3). Rates of hospitalization for asthma, bronchitis, and COPD have also increased since the early 1990s (4). HIV-associated COPD was identified as a critical area for future research by a National Institutes of Health workshop on pulmonary complications of HIV, and several sites in the Lung HIV group have decided to focus on the epidemiology and mechanisms of HIV-associated COPD.
COPD is defined by the Global Initiative on Obstructive Lung Diseases as “a preventable and treatable disease…characterized by airflow obstruction that is not fully reversible” (5). This airflow limitation is accompanied by an abnormal pulmonary inflammatory response. Other phenotypes of obstructive lung disease that are related to COPD include small airways abnormalities and bronchiolitis, increases in airway obstruction and air-trapping, chronic bronchitis, deficits in DlCO, and anatomic and radiographic emphysema (Figure 1). All of these phenotypes have been reported in HIV-infected individuals. In this review, we document the epidemiology of HIV-associated COPD before and after the institution of combination antiretroviral therapy and review data suggesting that COPD is accelerated in individuals with HIV. We then discuss possible mechanisms of COPD in persons with HIV, including the risk factor behavior of the population as well as HIV- and ART-related factors. These factors include an increased susceptibility to chronic subclinical infections, an aberrant inflammatory response, altered oxidant–antioxidant balance, increased apoptosis associated with HIV, and direct effects of antiretroviral therapy. Finally, we discuss the ongoing collaborative efforts of the Lung HIV study sites to understand the prevalence and causes of HIV-associated COPD.
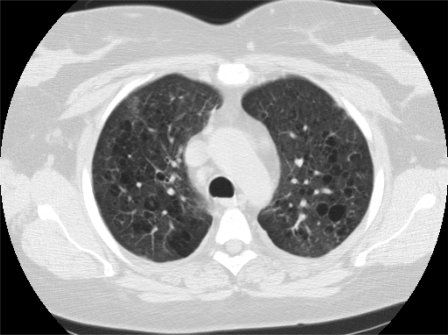
Figure 1.
Chest CT of a 44-year-old woman with HIV. She was receiving antiretroviral therapy, and her CD4+ cell count was 479 cells/μl. Note the diffuse emphysematous changes with multiple areas of bullous lung disease. Reproduced with permission from A. Morris.
EPIDEMIOLOGY OF HIV AND COPD BEFORE THE ANTIRETROVIRAL ERA
The first abnormalities of pulmonary function associated with HIV were noted by the Pulmonary Complications of HIV Infection Study (PCHIS). The PCHIS prospectively evaluated over 1,300 HIV-infected and HIV-uninfected persons in a longitudinal, multicenter study (6). HIV-infected participants had more frequent complaints of dyspnea, and those who were also injection drug users (IDUs) had a higher prevalence of cough and sputum production. HIV-infected participants also had lower absolute DlCO and percent predicted DlCO than non–HIV-infected participants. Lower values for DlCO were more common in participants who reported respiratory symptoms and in smokers and IDUs. Some caution is necessary in interpreting these results because 67% of IDUs, 10% of other risk groups, and 18% of the seronegative control subjects were African American, which could affect DlCO comparisons. The DlCO reference values used did not include race; however, an attempt was made to adjust for race in the overall analysis. Although DlCO also appeared to be lower in persons with CD4+ cell counts below 200 cells/μl, the relationship of the observed pulmonary deficits to serum or bronchoalveolar lavage HIV viral levels or to pulmonary inflammation was not examined. In a longitudinal study of 474 HIV-infected persons, DlCO also declined in participants with pulmonary or nonpulmonary Kaposi's sarcoma and in patients developing Pneumocystis (7). Recovery after Pneumocystis was more rapid and complete in nonsmokers. Another study found that a significantly higher number of HIV-infected participants than HIV-uninfected control subjects had evidence of focal air trapping on chest CT scan. The participants with air trapping also had worse obstructive changes in pulmonary function testing, including lower FEV1 and DlCO (8). In these studies, it is difficult to separate the effects of progressive HIV and immunodeficiency from the effects of advancing age because combination antiretroviral therapy was not available.
Diaz and colleagues found that 23% of HIV-infected smokers without a history of pulmonary infections had emphysema, as determined by pulmonary function testing or CT scan, compared with only 2% of control subjects matched for age and smoking (9). Thirty-seven percent of HIV-infected persons with a greater than 12 pack-year smoking history had emphysema, compared with none of the HIV-uninfected control subjects. The mean age in the cohort was 34 years, and participants were relatively healthy (mean CD4 cell count, 320 cells/μl).
Emphysema can occur in HIV-infected persons who are nonsmokers. Diaz reported a series of four HIV-infected nonsmokers who had air-trapping, decreased DlCO, and emphysema on CT scan (10). We have also found emphysema in HIV-infected nonsmokers. In examining autopsy lung specimens, we observed that 16% of HIV-infected individuals who never smoked had anatomic emphysema, a much higher number than would be expected in HIV-negative nonsmokers (A. Morris, unpublished data). These observations suggest that HIV is an additional risk factor for COPD or interacts with other risk factor(s) in the development of COPD.
EPIDEMIOLOGY OF HIV AND COPD IN THE ANTIRETROVIRAL ERA
Unlike many AIDS-defining opportunistic infections, HIV-associated emphysema may be more common in the current era of HIV because it is frequently reported in patients without a history of AIDS-related pulmonary complications and because the aging HIV-infected population has a longer exposure to smoking and HIV. Few studies have examined COPD and emphysema in the era of ART. One large study of HIV-infected and HIV-negative veterans found that COPD, as documented by International Classification of Diseases Ninth Revision (ICD-9) code and self-report, was significantly higher in the HIV-infected population (11). Another chart review of 162 HIV-infected dental patients found that 16.1% reported having a diagnosis of COPD (12). Although these studies reported a high prevalence of COPD diagnoses, both diagnosed COPD based on ICD-9 codes or self-report without measuring pulmonary function directly.
There have been three recent prospective studies that examined respiratory symptoms and measured pulmonary function in the era of combination ART (13–15). The first study performed spirometry in 234 HIV-infected outpatients without a history of acute respiratory disease or asthma (13). Thirty-one percent reported at least one respiratory symptom, and age, smoking history, and history of pneumonia were risk factors for respiratory symptoms and airway obstruction. The prevalence of airway obstruction was 6.8%. The most striking finding of this study was that use of ART was an independent predictor of increased airway obstruction. The association of ART and airway obstruction persisted even after adjustment for other risk factors, such as age and smoking history. Another study of 119 HIV-infected participants also performed spirometry and found that 3.4% had airway obstruction (14). Lung function was worse in smokers, and over half of the participants reported respiratory symptoms. Gingo and colleagues performed the only study that measured spirometry and DlCO and found that 21.0% of HIV-infected participants had airflow obstruction and 64.1% had decreased DlCO (15). In this cohort of HIV-infected outpatients, the authors also found an independent relationship of ART use to increased risk of airway obstruction. Smoking and intravenous drug use were other clinical factors that increased airway obstruction risk. Impairments in DlCO were related only to having a history of ever smoking, suggesting that DlCO abnormalities might represent a different COPD phenotype than airway obstruction.
MECHANISMS RESPONSIBLE FOR HIV-ASSOCIATED COPD
There are multiple hypotheses regarding the pathogenesis of COPD in HIV. Possible causes include the high prevalence of risk behaviors in the HIV-infected population as well as HIV-related and ART-related processes (Table 1). Many of these potential causes may be complementary or additive, and certain COPD phenotypes may involve different pathways. It is likely that many of these processes occur simultaneously and are not exclusive.
TABLE 1.
POTENTIAL MECHANISMS INVOLVED IN HIV-ASSOCIATED CHRONIC OBSTRUCTIVE PULMONARY DISEASE
| Excess risk behaviors (e.g., cigarette smoking) |
| Increased susceptibility to pulmonary infections and colonization |
| Aberrant inflammatory responses |
| Altered oxidant/antioxidant balance |
| Increased apoptosis |
| Effects of antiretroviral therapy |
| Direct effects of antiretroviral drugs |
| Modified immune inflammatory response to colonizing pathogens |
| Autoimmunity |
Risk Behaviors
Many HIV-infected populations have a high degree of behaviors associated with COPD risk, including cigarette and marijuana smoking as well as injection drug use. It is estimated that approximately 75% of HIV-infected persons have smoked at least 100 cigarettes in their lifetime, with roughly half currently smoking cigarettes (16, 17). Injection and inhalational drug use are also common (18). However, it is likely that these risk factors are not the only explanation for accelerated COPD because emphysema and pulmonary function abnormalities are seen in HIV-infected nonsmokers as well. It is likely that these behaviors interact with the effects of HIV and increase the risk of HIV-associated COPD, particularly as individuals with HIV age and experience longer exposures to HIV, smoking, and illicit drug use.
Infections
The “vicious circle” hypothesis has been proposed to explain the mechanism by which infections might act to promote COPD progression (19). In those without HIV infection, smoking leads to structural remodeling, which results in increased risk of microbial colonization or decreased ability to clear subclinical infection. In persons with HIV infection, defective immune responses to infections could also contribute, alone or combined with smoking, to an increased likelihood of developing colonization. Once colonization is established, the organism or organisms recruit white blood cells to the lungs, stimulating the release of inflammatory cytokines and chemokines as well as proteases. The inability to clear the inciting organism perpetuates the cycle, ultimately resulting in tissue destruction, airway thickening, and clinical COPD. In HIV-infected persons, the vicious cycle could be worsened by up-regulation of HIV counts in the lung stimulated by pulmonary colonization. Several studies have shown that pulmonary infections increase lung levels of HIV-1. For example, Koziel and colleagues reported that HIV RNA was detected in 67% of patients with active lung disease compared with 16% of asymptomatic HIV-infected persons, independent of clinical stage of HIV and serum HIV RNA levels (20).
One pathogen that has been linked to the development of HIV-associated COPD in human and animal studies is Pneumocystis (Pc). Emphysema and COPD-like changes have been found in HIV-infected patients with Pneumocystis pneumonia (PCP). DlCO, FEV1, and peak flow decrease during acute PCP and for at least 3 months after infection (7, 21). These changes may be permanent because PCP leads to accelerated declines in FEV1, FEV1/FVC, and DlCO beyond that expected from age and smoking history (22). We have recently shown that Pc-colonized HIV-infected outpatients have worse airway obstruction than Pc-negative HIV-infected persons, independent of smoking history (23). Sputum matrix metalloprotease (MMP)-12 levels were increased in Pc-colonized participants, suggesting a potential mechanism by which Pc colonization stimulates COPD-like changes. Animal models also support the role of Pc colonization in COPD. Christensen and colleagues recently reported that in immunocompetent mice, exposure to cigarette smoke and Pc colonization resulted in pulmonary function deficits and airspace enlargement greater than that from smoke exposure alone (24). Similar changes have been found in models of Pc colonization in nonhuman primates infected with simian immunodeficiency virus or simian/human immunodeficiency virus. Pc-colonized animals developed airway obstruction and radiographic emphysema, whereas animals with simian immunodeficiency virus or simian/human immunodeficiency virus alone did not (25, 26).
Inflammation
Airflow limitation in non–HIV-associated COPD is progressive and is associated with an abnormal inflammatory response in the lungs. Tissue inflammation in COPD is characterized by a predominant neutrophil, CD8+ lymphocyte, and macrophage infiltration (27–30). The mechanism of tissue damage likely involves the recruitment and activation of these inflammatory cells, with concomitant up-regulation of several cellular proteases and inflammatory cytokines. HIV infection itself is associated with a lymphocytic alveolitis, particularly in individuals with CD4+ cell counts between 200 and 500 cells/μl, suggesting that the virus might act independently to stimulate pulmonary inflammation, particularly increases in CD8+ lymphocytes (9, 31). CD8+ lymphocytes secrete IFN-γ, which induces emphysema in animal models (32). Macrophages may also be important in COPD pathogenesis, and it has been shown that alveolar macrophages from HIV-infected individuals are activated (33, 34). Alveolar macrophage expression of MMP is up-regulated in HIV-infected smokers with early emphysema, and MMP-9 is up-regulated in areas of emphysema in lungs from HIV-infected persons (35, 36).
Altered Antioxidant–Oxidant Balance
Increased oxidative stress is another potential mechanism linking HIV and COPD. Non–HIV-infected persons with COPD have increased markers of oxidative stress systemically and in the lungs (37). Oxidative stress can worsen COPD in several ways. Increased oxidation results in inactivation of antiproteases, activation of MMPs, and direct damage to the lung matrix and decreases the lung's ability to repair itself (37). HIV-infected individuals have altered systemic and lung oxidant/antioxidant balance, with decreases in antioxidant levels such as superoxide dismutase and glutathione and increases in oxidants that may result from HIV proteins (38–41). Smoking may worsen the imbalance between oxidants and antioxidants in HIV-infected persons. Lung and plasma glutathione levels are lower in HIV-infected smokers than in HIV-infected nonsmokers (42, 43). Recent studies demonstrated that, in a rat model, HIV transgene expression resulted in oxidant stress, and alterations in epithelial barrier function via altered expression of tight junction proteins (44). The impact of antiretroviral therapy on oxidative stress is unclear (45, 46).
Apoptosis
It has recently been postulated that lung endothelial and epithelial cell apoptosis are critical steps in COPD development in the non–HIV-infected population (47, 48). HIV-infected persons might have increased susceptibility to apoptosis because the HIV proteins Tat and Nef may induce endothelial cell apoptosis (49). It is also possible that HIV may cause apoptosis directly (50, 51). It has been proposed that apoptosis, oxidative stress, and inflammation work in concert to promote COPD, and this scenario may be true in HIV-associated COPD as well (37).
Effects of Antiretroviral Therapy
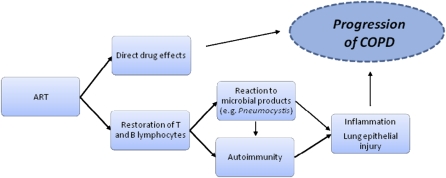
Although ART decreases infectious and noninfectious complications of HIV, ART may also be a risk factor in the development of several chronic medical conditions, such as cardiovascular disease, the metabolic syndrome, and osteoporosis (52–54). These conditions may be directly related to antiretroviral agents, particularly protease inhibitors, or to prolonged exposure to HIV (54, 55). Studies linking ART and airway obstruction suggest that airway obstruction may be the latest in the series of chronic conditions linked to ART, although it is possible that unmeasured confounders may account for the association, and studies thus far have been cross-sectional (13, 15). The mechanistic link between ART and airway obstruction is not known, but potential explanations, outlined below and in Figure 2, include direct effects of antiretroviral drugs, restoration of the immune system allowing for an increased inflammatory response after ART is initiated, or the development of autoimmunity.
Figure 2.
Potential mechanisms of antiretroviral-mediated lung damage in HIV.
In cardiovascular disease, protease inhibitors in particular have effects on the endothelium. There are no published reports suggesting a direct effect of antiretroviral agents on airway obstruction, but it is possible that similar effects occur in the lung. Endothelial damage to the pulmonary capillary bed or capillary destruction could reduce the effective blood volume for gas exchange, thus reducing the DlCO. Both the membrane (Dm) and capillary blood volume components of the DlCO are reduced in HIV-infected persons in the lowest quartile of DlCO values (56).
ART initiation can lead to the immune reconstitution inflammatory syndrome (IRIS). It is believed that the restoration of T lymphocytes, when combined with an antigenic stimulus (e.g., Mycobacteria, Pneumocystis, or other antigens), initiates an inflammatory cascade producing this clinical syndrome. We have proposed that a “modified” IRIS might be involved in COPD pathogenesis in HIV. In modified IRIS, immune restoration might not produce obvious clinical symptoms but might result in a chronic inflammatory response. This response could be initiated or propagated by the colonizing organisms discussed above or by autoantigens. It is also possible that HIV could act with persistent, albeit lower, organism burden, as a nidus of chronic inflammation in the context of improved immune function after starting ART.
Autoimmunity has been linked to COPD in the non–HIV-infected population and could occur in response to infectious agents or other triggers (57). It has been proposed that an acquired immune response to self-epitopes contributes to the inflammation and pulmonary damage seen in COPD (57, 58). Non–HIV-infected patients with COPD have increased antielastin and antiepithelial antibodies, which may be involved in disease pathogenesis (57, 58). Organ-specific autoimmunity has been demonstrated as a complication of ART and seems to occur later than infection-associated IRIS (59, 60). An increase in autoimmune conditions has also been noted in HIV-infected persons after beginning ART (4, 51–54). Detectable antithyroid antibodies appear after initiation of ART, and clinical thyroid disease is associated with a lower pre-ART CD4+ cell count (61, 62). Development of autoimmunity during immune restoration likely involves the release of naive T cells and generally occurs more than 6 months after ART initiation (61). Autoreactive T cells are subsequently more likely to be activated in the setting of infections, thymic dysfunction, or altered cytokine profiles associated with HIV infection and ART (61, 63). The susceptibility of HIV-infected individuals on ART to developing autoimmunity in the setting of immune restoration, combined with the findings supporting an autoimmune pathogenesis in COPD, suggest that autoimmune mechanisms may be important in HIV-related COPD.
LUNG HIV INVESTIGATIONS OF COPD
Given the increasing importance of obstructive lung disease and the lack of large-scale epidemiologic studies comparing COPD prevalence, incidence, and progression between HIV-infected and HIV-negative persons, several sites in the Lung HIV consortium are performing longitudinal studies of COPD using pulmonary function testing and chest CT. Sites 2 (Baltimore), 4 (Ohio), 7 (Pittsburgh), and 8 (Washington) are following cohorts of HIV-infected and HIV-negative participants to better define the epidemiology of HIV-associated COPD and to explore potential causal mechanisms, including the role of latent infections, inflammation, immune restoration, and altered antioxidant levels. Individual sites are performing longitudinal spirometry and DlCO measurements, chest CT scans for qualitative and quantitative emphysema, clinical data collection, and banking of blood and bronchoalveolar lavage samples.
CONCLUSIONS
Many questions remain regarding HIV-associated COPD. Whether the disease begins earlier or progresses more quickly in those with HIV infection has not been prospectively studied in the current era. Much remains to be learned about the mechanisms involved in the pathogenesis of HIV-associated COPD, and the role of standard COPD treatments has not been examined in the HIV-infected population. Respiratory symptoms and COPD remain common in HIV-infected persons, even in the current era where obstructive lung disease appears to be an increasing cause of morbidity and mortality. The results of the Lung HIV studies will provide us with much needed insights regarding the characteristics and pathogenesis of COPD among HIV-infected individuals.
Supported by NHLBI grants R01 HL 090339 (A.M., E.K.), R01 HL 090342 (K.C.), R01 HL 090335 (L.H.), HL 090335–02S1 (L.H.), and K24 HL 087713 (L.H.).
Author Disclosure: A.M. received grant support from the NIH, Gilead, and Roche. M.P.G. received grant support from Parker B. Davis and the Vascular Medicine Institute, University of Pittsburgh. K.C. received grant support from the NIH. L.H. received grant support from the NIH and the Foundation for Innovative New Diagnostics (FIND). L.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.C.K. received or has pending institutional grant support from the NHLBI, Actillion, Almirall, Altana, AstraZeneca, Boehringer Ingelheim, Cheisi, Dey, Eumedics, GlaxoSmithKline, IVAX, Nabi, Novartis, Osiris, Pfizer, Roche, Schering Plough, and Sepracor. His institution received lecture fees from TEVA and payment for travel accommodations from Chiesi and Novartis.
References
- 1.Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.(UNAIDS) Joint UN programme on HIV/AIDS. Global facts and figures. 2009.
- 3.Louie JK, Hsu LC, Osmond DH, Katz MH, Schwarcz SK. Trends in causes of death among persons with acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy, San Francisco, 1994–1998. J Infect Dis 2002;186:1023–1027. [DOI] [PubMed] [Google Scholar]
- 4.Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS 2006;20:1095–1107. [DOI] [PubMed] [Google Scholar]
- 5.Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO workshop report. Bethesda, National Heart, Lung, and Blood Institute, December 2009. Available from: wwwgoldCOPDcom.
- 6.Rosen MJ, Lou Y, Kvale PA, Rao AV, Jordan MC, Miller A, Glassroth J, Reichman LB, Wallace JM, Hopewell PC. Pulmonary Complications of HIV Infection Study Group. Pulmonary function tests in HIV-infected patients without AIDS. Am J Respir Crit Care Med 1995;152:738–745. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell DM, Fleming J, Pinching AJ, Harris JR, Moss FM, Veale D, Shaw RJ. Pulmonary function in human immunodeficiency virus infection: a prospective 18-month study of serial lung function in 474 patients. Am Rev Respir Dis 1992;146:745–751. [DOI] [PubMed] [Google Scholar]
- 8.Gelman M, King MA, Neal DE, Pacht ER, Clanton TL, Diaz PT. Focal air trapping in patients with HIV infection: CT evaluation and correlation with pulmonary function test results. Am J Roentgenol 1999;172:1033–1038. [DOI] [PubMed] [Google Scholar]
- 9.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, Drake J, Clanton TL. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–372. [DOI] [PubMed] [Google Scholar]
- 10.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med 1992;116:124–128. [DOI] [PubMed] [Google Scholar]
- 11.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–1333. [DOI] [PubMed] [Google Scholar]
- 12.Magalhaes MG, Greenberg B, Hansen H, Glick M. Comorbidities in older patients with HIV: a retrospective study. J Am Dent Assoc 2007;138:1468–1475. [DOI] [PubMed] [Google Scholar]
- 13.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS ONE 2009;4:e6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, Smieja M. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther 2009;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingo M, George MP, Kessinger C, Lucht L, Rissler B, Weinmann R, Slivka W, McMahon D, Wenzel S, Sciurba FC, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med 2010;182:790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: The time is now. Clin Infect Dis 2000;31:808–812. [DOI] [PubMed] [Google Scholar]
- 17.Gritz ER, Vidrine DJ, Lazev AB, Amick BC III, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res 2004;6:71–77. [DOI] [PubMed] [Google Scholar]
- 18.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care 2006;44:S52–S60. [DOI] [PubMed] [Google Scholar]
- 19.Sethi S. Bacterial infection and the pathogenesis of COPD. Chest 2000;117:286S–291S. [DOI] [PubMed] [Google Scholar]
- 20.Koziel H, Kim S, Reardon C, Li X, Garland R, Pinkston P, Kornfeld H. Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 1999;160:2048–2055. [DOI] [PubMed] [Google Scholar]
- 21.Shaw RJ, Roussak C, Forster SM, Harris JR, Pinching AJ, Mitchell DM. Lung function abnormalities in patients infected with the human immunodeficiency virus with and without overt pneumonitis. Thorax 1988;43:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris AM, Huang L, Bacchetti P, Turner J, Hopewell PC, Wallace JM, Kvale PA, Rosen MJ, Glassroth J, Reichman LB, et al. The Pulmonary Complications of HIV Infection Study Group. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. Am J Respir Crit Care Med 2000;162:612–616. [DOI] [PubMed] [Google Scholar]
- 23.Morris A, Alexander T, Radhi S, Lucht L, Sciurba FC, Kolls JK, Srivastava R, Steele C, Norris KA. Airway obstruction is increased in Pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol 2009;47:3773–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen PJ, Preston AM, Ling T, Du M, Fields WB, Curtis JL, Beck JM. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect Immun 2008;76:3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris KA, Morris A, Patil S, Fernandes E. Pneumocystis colonization, airway inflammation, and pulmonary function decline in acquired immunodeficiency syndrome. Immunol Res 2006;36:175–187. [DOI] [PubMed] [Google Scholar]
- 26.Shipley T, Kling H, Morris A, Patil S, Kristoff J, Guyach S, Murphy J, Shao X, Sciurba F, Rogers R, et al. Persistent Pneumocystis colonization leads to the development of chronic obstructive pulmonary disease (COPD) in a non-human primate model of AIDS. J Infect Dis (In press) [DOI] [PMC free article] [PubMed]
- 27.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996;153:530–534. [DOI] [PubMed] [Google Scholar]
- 28.Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Mapp CE, Fabbri LM, Donner CF, Saetta M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 1998;158:1277–1285. [DOI] [PubMed] [Google Scholar]
- 29.Pesci A, Balbi B, Majori M, Cacciani G, Bertacco S, Alciato P, Donner CF. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur Respir J 1998;12:380–386. [DOI] [PubMed] [Google Scholar]
- 30.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. Cd8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:822–826. [DOI] [PubMed] [Google Scholar]
- 31.Twigg HL, Soliman DM, Day RB, Knox KS, Anderson RJ, Wilkes DS, Schnizlein-Bick CT. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med 1999;159:1439–1444. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med 2000;192:1587–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twigg HL III, Iwamoto GK, Soliman DM. Role of cytokines in alveolar macrophage accessory cell function in HIV-infected individuals. J Immunol 1992;149:1462–1469. [PubMed] [Google Scholar]
- 34.Buhl R, Jaffe HA, Holroyd KJ, Borok Z, Roum JH, Mastrangeli A, Wells FB, Kirby M, Saltini C, Crystal RG. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol 1993;150:1019–1028. [PubMed] [Google Scholar]
- 35.Yearsley MM, Diaz PT, Knoell D, Nuovo GJ. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol 2005;14:48–52. [DOI] [PubMed] [Google Scholar]
- 36.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV-1(+) smokers with early emphysema. J Leukoc Biol 2009;86:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:527–531. [DOI] [PubMed] [Google Scholar]
- 38.Buhl R, Meyer A, Vogelmeier C. Oxidant-protease interaction in the lung: rospects for antioxidant therapy. Chest 1996;110:267S–272S. [DOI] [PubMed] [Google Scholar]
- 39.Treitinger A, Spada C, Verdi JC, Miranda AF, Oliveira OV, Silveira MV, Moriel P, Abdalla DS. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest 2000;30:454–459. [DOI] [PubMed] [Google Scholar]
- 40.Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res 2003;47:217–224. [DOI] [PubMed] [Google Scholar]
- 41.Shatrov VA, Ratter F, Gruber A, Droge W, Lehmann V. HIV type 1 glycoprotein 120 amplifies tumor necrosis factor-induced NF-kappa b activation in jurkat cells. AIDS Res Hum Retroviruses 1996;12:1209–1216. [DOI] [PubMed] [Google Scholar]
- 42.Pacht ER, Diaz P, Clanton T, Hart J, Gadek JE. Alveolar fluid glutathione is not reduced in asymptomatic HIV-seropositive subjects. Am J Respir Crit Care Med 1997;155:374–377. [DOI] [PubMed] [Google Scholar]
- 43.Cole SB, Langkamp-Henken B, Bender BS, Findley K, Herrlinger-Garcia KA, Uphold CR. Oxidative stress and antioxidant capacity in smoking and nonsmoking men with HIV/acquired immunodeficiency syndrome. Nutr Clin Pract 2005;20:662–667. [DOI] [PubMed] [Google Scholar]
- 44.Lassiter C, Fan X, Joshi PC, Jacob BA, Sutliff RL, Jones DP, Koval M, Guidot DM. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aukrust P, Muller F, Svardal AM, Ueland T, Berge RK, Froland SS. Disturbed glutathione metabolism and decreased antioxidant levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: potential immunomodulatory effects of antioxidants. J Infect Dis 2003;188:232–238. [DOI] [PubMed] [Google Scholar]
- 46.Hulgan T, Morrow J, D'Aquila RT, Raffanti S, Morgan M, Rebeiro P, Haas DW. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis 2003;37:1711–1717. [DOI] [PubMed] [Google Scholar]
- 47.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 49.Tuder RM, McGrath S, Neptune E. The pathobiological mechanisms of emphysema models: what do they have in common? Pulm Pharmacol Ther 2003;16:67–78. [DOI] [PubMed] [Google Scholar]
- 50.Micoli KJ, Mamaeva O, Piller SC, Barker JL, Pan G, Hunter E, McDonald JM. Point mutations in the c-terminus of HIV-1 gp160 reduce apoptosis and calmodulin binding without affecting viral replication. Virology 2006;344:468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Micoli KJ, Pan G, Wu Y, Williams JP, Cook WJ, McDonald JM. Requirement of calmodulin binding by HIV-1 gp160 for enhanced fas-mediated apoptosis. J Biol Chem 2000;275:1233–1240. [DOI] [PubMed] [Google Scholar]
- 52.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 53.Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, Cooper DA, Emery S. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS 2007;21:2445–2453. [DOI] [PubMed] [Google Scholar]
- 54.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20:2165–2174. [DOI] [PubMed] [Google Scholar]
- 55.Koppel K, Bratt G, Schulman S, Bylund H, Sandstrom E. Hypofibrinolytic state in HIV-1-infected patients treated with protease inhibitor-containing highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002;29:441–449. [DOI] [PubMed] [Google Scholar]
- 56.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Neal D, Nagaraja HN, Drake J, Clanton TL. The pathophysiology of pulmonary diffusion impairment in human immunodeficiency virus infection. Am J Respir Crit Care Med 1999;160:272–277. [DOI] [PubMed] [Google Scholar]
- 57.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax 2003;58:832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 2007;13:567–569. [DOI] [PubMed] [Google Scholar]
- 59.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev 2002;1:329–337. [DOI] [PubMed] [Google Scholar]
- 60.Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthitis Rheum 2005;35:166–174. [DOI] [PubMed] [Google Scholar]
- 61.Chen F, Day SL, Metcalfe RA, Sethi G, Kapembwa MS, Brook MG, Churchill D, de Ruiter A, Robinson S, Lacey CJ, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine 2005;84:98–106. [DOI] [PubMed] [Google Scholar]
- 62.Jubault V, Penfornis A, Schillo F, Hoen B, Izembart M, Timsit J, Kazatchkine MD, Gilquin J, Viard JP. Sequential occurrence of thyroid autoantibodies and Graves' disease after immune restoration in severely immunocompromised human immunodeficiency virus-1-infected patients. J Clin Endocrinol Metab 2000;85:4254–4257. [DOI] [PubMed] [Google Scholar]
- 63.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998;396:690–695. [DOI] [PubMed] [Google Scholar]