Abstract
When sinusoidal electric stimulation is applied to the intact cochlea, a frequency-specific acoustic emission can be recorded in the ear canal. Acoustic emissions are produced by basilar membrane motion, and have been used to suggest a corresponding acoustic sensation termed “electromotile hearing.” Electromotile hearing has been specifically attributed to electric stimulation of outer hair cells in the intact organ of Corti. To determine the nature of the auditory perception produced by electric stimulation of a cochlea with intact outer hair cells, we tested guinea pigs in a psychophysical task. First, subjects were trained to report detection of sinusoidal acoustic stimuli and dynamic range was assessed using response latency. Subjects were then implanted with a ball electrode placed into scala tympani. Following the surgical implant procedure, subjects were transferred to a task in which acoustic signals were replaced by sinusoidal electric stimulation, and dynamic range was assessed again. Finally, the ability of acoustic pure-tone stimuli to mask the detection of the electric signals was assessed. Based on the masking effects, we conclude that sinusoidal electric stimulation of the intact cochlea results in perception of a tonal (rather than a broad-band or noisy) sound at a frequency of 8 kHz or above.
I. Introduction
That electrical stimulation produces mechanical changes in the organ of Corti was first described in the pioneering studies of Moxon (1971), who reported frequency-specific and place-specific excitation, as well as acoustic masking of electrically-evoked responses. We have more recently learned that sinusoidal electric stimulation of the intact cochlea produces a frequency-specific otoacoustic emission (OAE) in the ear canal (see Hubbard and Mountain, 1983; Xue et al., 1993; Nuttall and Ren, 1995; Ren and Nuttall, 1995; Nakajima et al., 1998; Nuttall et al., 2001; Reyes et al., 2001). These electrically evoked OAEs (EEOAEs) originate near the site of the stimulating electrode (Xue et al., 1993; Ren and Nuttall, 1995; Nuttall et al., 2001), where local electrical stimulation induces motile response (contractile motion, or elongation) of outer hair cells (OHCs). That OHCs change their shape in response to electric stimulation has been shown in cells dissociated from the organ of Corti (Brownell et al., 1985; Kachar et al., 1986; Ashmore, 1987) and cells maintained in a half-turn cochlear explant (Reuter and Zenner, 1990). In vivo electric stimulation of the cochlea results in OHC motile response leading to basilar membrane motion (Nuttall and Dolan, 1993; Nuttall and Ren, 1995; Xue et al., 1995).
The basilar membrane motion observed by Nuttall and colleagues (Nuttall and Dolan, 1993; Nuttall and Ren, 1995) as well as Xue et al. (1995) presumably travels in the reverse direction as OAEs are produced by reverse traveling motion of the basilar membrane (i.e., projecting toward the stapes, through the middle ear, and into the ear canal, see Kemp, 1978; Shera and Guinan, 1999). However, electrically evoked motile response of OHCs also produces forward traveling motion of the basilar membrane (i.e., towards the helicotrema, see Nuttall and Dolan, 1993). Forward traveling waves generated in response to acoustic stimulation result in inner hair cell (IHC) neurotransmitter release, neural activity, and a corresponding auditory sensation (hearing). When a forward traveling wave is generated by electrical stimulation of OHCs, we presume that there is a corresponding auditory percept, which has been proposed as “electromotile hearing” (Nuttall and Ren, 1995). This work uses psychophysical measures of function to directly test the hypothesis that there is an auditory percept associated with electomotile hearing, and further, that the percept is a tonal acoustic sensation. We evaluated EEOAE generation in our subjects to confirm that electrical stimulation produced an EEOAE (which is a less invasive measure of traveling wave generation in the cochlea than direct measurement of basilar membrane motion) in addition to the acoustic sensation assessed with psychophysical procedures. The EEOAE data presented here provide key evidence that the electrical stimulation paradigm we used generated EEOAE responses that were equivalent to those in other studies.
II. MATERIALS AND METHODS
A. Subjects
Adult male guinea pigs (Elm Hill Breeding Labs, Chelmsford, MA) were individually housed with free access to water. All subjects weighed 300-350 grams at the onset of the experimental procedures. Weight gain was continual until subjects achieved a weight of at least 900 grams. Food intake (Purina Guinea Pig Chow) was then moderately restricted. Animal treatment met or exceeded all guidelines in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The University Committee on the Use and Care of Animals of the University of Michigan approved all animal care and testing protocols.
B. Electrophysiology
Anesthetized guinea pigs (40 mg/kg ketamine, 10 mg/kg xylazine) were initially screened for normal acoustic sensitivity using the auditory brainstem response (ABR) to acoustic stimulation as in Le Prell et al. (2004). Threshold sensitivity was determined for 2, 4, 8 and 16 kHz tone bursts (10 msec duration, 0.5 msec rise/fall time) using Tucker Davis Technology (TDT) SigGen software (version 3.2) and TDT System II hardware. Sound-evoked brainstem responses were amplified (10,000x) and filtered (300-3000 Hz), digitized, then averaged (1020 presentations) and viewed using TDT BioSig (version 3.2) software. Thresholds were determined based on visual inspection of the ABR waveforms.
ABR thresholds were typically re-evaluated within two weeks of surgically implanting an electrode inside the cochlea (see below). In addition, the electrical impedance (sinusoid waveform at 1000 Hz) of the electrode was measured and electrically-evoked auditory brainstem responses (eABR) were assessed using the method described by Hall (1990; see also Mitchell et al., 1997). Intra-cochlear stimulation used to evoke an eABR consisted of alternating-polarity monophasic current pulses (50 μsec duration) presented at a rate of 50 pulses per second. Up to 2048 responses were collected for analysis at stimulus currents ranging from 30 to 1000 μA. Responses were amplified (gain=10,000), filtered (0.1 Hz to 3 kHz) and digitized using in-house software. EABR response was used to verify implant function, and to measure direct electrical stimulation of spiral ganglion cells based on visual inspection of wave P3 (see Black et al., 1983; Tykocinski et al., 1995; Shepherd and Javel, 1997). P3 was defined as the third peak of the eABR waveform occurring approximately 1.5 ms post-stimulus onset (see Hall, 1990).
C. Psychophysical Training and Testing Procedures
Subjects were tested 5 days per week for approximately 45 minutes using procedures described in Le Prell et al. (2004). In brief, subjects were trained using positive reinforcement procedures to depress a floor-mounted plastic response button at the start of each test trial; trial onset was signaled by illumination of a cue light mounted in front of the response button. The subjects’ task was to depress the response button during a quiet listening interval (1-9 sec), and release the button upon detecting an acoustic stimulus. Subjects received 45-mg Dustless Precision Pellets (Bio-Serv) for correct release responses. Acoustic stimuli were presented during 75% of the total trials (“test trials”). During the remaining trials, release rates in the absence of the test signal (“catch trials”) were monitored. Subjects were punished with a brief (7 sec) time-out for any incorrect releases. During a time-out, the cue light was extinguished and a new trial could not be initiated.
Acoustic tones were presented free-field via an overhead piezoelectric speaker (Motorola model KSN 1001A) mounted approximately 8 inches above head level. Tone levels were calibrated using a microphone (Bruel & Kjaer type 4136 microphone, type 2619 preamp, type 2804 power supply) placed in the test cage in a position that approximated that of the guinea pig head when the animal was located in the front of the test cage and pressing the response button. Tone frequency (5.6, 8, 11.2, and 16 kHz) and level were varied based on the method of constant stimuli (see Niemiec and Moody, 1995). At each frequency, subjects were presented with one sub-threshold stimulus and 4-5 supra-threshold stimuli. All tones were spaced in 10-dB increments, and each combination of frequency and stimulus level was presented up to 20 times. Threshold was defined as the stimulation level that produced a median response latency of 1500 msec; all thresholds were determined based on linear interpolation between adjacent data points. Response latency provides a sensitive measure of sensory effects, and equal latencies presumably indicate equal sensory effects (for review, see Moody, 1970). Thresholds assigned based on response latency closely corresponded to those assigned using the criteria of 50% correct detection responses.
A reinforcement contingency required subjects to respond rapidly after tone onset to receive a food pellet. This contingency was set such that reinforcement was delivered for approximately 90% of the correct releases, which ensured consistent responses throughout the test session. If the guinea pig did not respond within the trial duration (2550 msec, beginning at tone onset), a response latency of 2550 msec was recorded, the tone was turned off, and a new trial was initiated. If subjects did not complete at least 10 test trials at each stimulus level (with no more than 20% catch trial releases), the daily latency data were discarded.
Initial operant response training required approximately 3 months; baseline latency functions were established within another 2-3 months. Included in the latter 2-3 months was the introduction of the reinforcement contingency, and adjustment of this contingency as animals developed more rapid and more reliable response functions. Once reliable latency functions were established for acoustic stimulation, a ball electrode was implanted into the cochlea (see below). After establishing post-implant acoustic baselines, subjects were transferred to an electric stimulus detection task. We used a battery-powered optically-isolated AC-coupled linear transconductance amplifier to deliver a constant current stimulus to the intra-cochlear electrode. Sinusoidal electric stimulation frequencies were the same as the acoustic test frequencies. Current level ranged from 1 to 374 μA RMS (i.e., 0-52 dB re 1 μA). Because the dynamic range between threshold and the maximum comfortable stimulation level is narrow for electric stimulation, electric stimulation levels were varied in 5-dB increments.
Electric response functions were first established in a quiet sound booth. Background acoustic stimuli were then added to assess masking efficiency. Order of testing for background tones was randomly selected. Only one background tone was presented during any given test session; multiple electrical sinusoids were tested with each of the acoustic maskers. Each background tone was tested for at least 5 days to verify that all response functions were stable. Based on the frequency-specific pattern of masking we observed, not all background tones were assessed with all test frequencies (i.e., having identified acoustic background tones with no masking effect for a given electrical target, acoustic tones with frequencies further from the electrical target were not probed for masking effects). Background tone level was approximately 10 dB above threshold; thresholds for background tone frequencies were estimated based on linear interpolation of thresholds determined using ABR (2, 4, 8, and 16 kHz) and psychophysical (5.6, 8, 11.2, and 16 kHz) procedures.
Initial subjects were tested with 5.6, 8 and 11.2 kHz electrical target signals and acoustic background tones spaced in approximately 500 Hz increments (5.6, 7, 7.5, 8, 8.5, 9, 10, 10.5, 11.2, 12, 12.3 kHz); completion of testing all desired combinations of acoustic backgrounds and electrical targets and periodic re-evaluation of detection thresholds in quiet required upwards of two years per subject. Results from initial subjects indicated that masking effects were limited to acoustic frequencies within one critical band of the electrical target. Later subjects were therefore tested with 5.6, 8, 11.2, and 16 kHz electrical target signals and acoustic background tones that were at the same frequency as the electrical target, at frequencies that separated by approximately ½ critical band relative to the electrical target, and at frequencies that were separated by approximately 1 critical band relative to the electrical target (5.6, 7, 8, 9, 10, 11.2, 12, 14.5, 16, 17.5, and 19 kHz). Thus, the electrical target and the background acoustic tone were either at the same frequency, closely spaced frequencies, or at frequencies that were perceptually quite distinct. For detailed discussion of cochlear place and critical bandwidth, readers are referred to Greenwood (1961). This modification to the protocol resulted in masking functions with tuning equivalent to those produced by subjects with more densely spaced acoustic background tones and provided the added benefit of reducing the total duration of testing with electrical targets to approximately 1 year.
Because subjects showed continual improvement in electric thresholds throughout the 1-2 year duration of testing, testing in quiet was repeated at approximately bi-monthly intervals. Given that threshold sensitivity changed over time in our subjects, all masking effects were assessed relative to the most temporally proximal set of baselines. Masking effects are reported as shift in electric stimulus detection threshold; masking functions were determined for four subjects.
D. Surgical Procedures
For the current investigation, chronic electrodes were inserted into scala tympani as done by Nuttall et al. (2001). The surgical procedures were closely modeled after those used by Le Prell et al. (2005). In brief, a ball electrode (0.2-0.25 mm diameter, constructed of teflon-coated platinum-iridium wire) was carefully inserted through the wall of the cochlea via a small fenestra slightly lateral to the round window. The site of the electrode was located at approximately 22.4 kHz (based on surgically-induced threshold deficits described in Le Prell et al., 2004). A silastic ball located 0.55 mm away from the end of the electrode prevented over-insertion of the electrode and prevented leaking of the perilymph from the cochlea. Once appropriate placement of the electrode was confirmed, carboxylate cement (Durelon, ESPE, Germany) was used to seal the bulla defect and permanently fix the electrode in place. The opposing end of the electrode was soldered to a two-pin connector (HSS-132-G2, Samtec Inc., IN) prior to the onset of the surgical procedure. A ground wire, connected to the second pin of the connector, was then inserted into neck muscle. Methyl methacrylate cement (Jet Repair Acrylic, Lang Dental Manufacturing, IL) was used to fix the connector to the skull and to seal the tissue edges surrounding the head-mounted connector. The post-auricular incision was then sutured and the incision cleaned. Subjects were treated with a multi-day post-operative regimen of chloramphenical (30 mg/kg) to prevent infection.
Prior to assessing the efficiency of acoustic background stimuli in masking the detection of electrical sinusoids, the subjects used in the masking experiments were unilaterally deafened in the ear contralateral to the implanted electrode (as in Le Prell et al., 2004). The unilateral deafening procedure ensured that subjects listened for electric and acoustic stimulation with the same ear. To deafen the ear, we unilaterally injected neomycin sulfate (10 % solution, 60 μl injection volume) through the round window membrane. Deafening was confirmed using ABR threshold assessment; morphological lesion was confirmed after euthanasia.
E. Electrically Evoked Otoacoustic Emission Assessment
Anesthetized guinea pigs (40 mg/kg ketamine, 10 mg/kg xylazine) were placed on a warmed heating pad. The ear canal and the tympanic membrane were visually inspected (with magnification) prior to insertion and after removal of a microphone (Etymotic Research, ER-10B+ Low Noise Microphone) from the ear canal. A short segment of flexible vinyl tubing selected to be minimally smaller than the guinea pig ear canal was placed around the microphone assembly to seal the ear canal to provide a closed field test condition. Tubing also served to prevent ear wax and other biological debris from directly entering and occluding the microphone itself; the tip of the tubing was close to, but did not contact, the tympanic membrane. Sinusoidal electric stimulation was delivered to the intra-cochlear electrode via the constant current stimulator used during behavioral testing.
Electrically-evoked OAEs were assessed in 9 animals; procedures were typically conducted within the first month post-implant, and repeated prior to euthanizing the animals at the conclusion of the experiments. For 8 of these animals, sinusoidal waveforms were produced by an audio generator (LAG-120B; Leader) and amplified (1000x; amplifier constructed in-house). Current ranged from 0 to 30 μA peak-to-peak (i.e., 0 to 10.6 μA RMS). Current delivery was based on signal voltage, determined using an oscilloscope (40 MHz; Kenwood). Stimulation frequency was fine-tuned such that the emission frequency was 5.6, 8, 11.2, or 16 kHz, and emission frequency and amplitude were determined using a lock-in-amplifier (SR530; Stanford Research Systems). For the ninth animal, current remained fixed between 0 (noise-floor) and 30 μA; however, stimulation frequency (controlled by an SRS 830 lock-in amplifier) was stepped from 1 to 35 kHz in 22 Hz increments (1-sec dwell time per frequency increment), with a time constant of three seconds (as described by Halsey et al., 2006). Development of the latter procedure significantly improved frequency resolution for measuring the EEOAE response.
F. Morphological procedures
To confirm that daily electrical stimulation did not damage cochlear hair cells, organ of Corti tissue was evaluated at the conclusion of the psychophysical testing. Subjects were deeply anesthetized and decapitated, and the ears were harvested and gently perfused with fixative (4% paraformaldehyde in phosphate buffer). Placement of the electrode inside the cochlea was visually confirmed, and the middle ear and inner ear were carefully examined for any evidence of infection or other pathology. The following day, the otic capsule, lateral wall, and tectorial membrane were removed, and the bony modiolus was carefully detached at the base of the cochlea. Organ of Corti tissue, attached to the modiolus, was permeabilized with 0.3% Triton-X (Sigma, St. Louis, MO) in phosphate buffered saline (PBS) for 10 min and then incubated for 30 min with rhodamine phalloidin (Molecular Probes, Eugene, OR) diluted 1:100 in PBS (room temperature). After washing the tissues with PBS, individual turns from the organ of Corti were carefully dissected from the modiolus, mounted on microscope slides with GEL/Mount (Biomedia, Foster City, CA), and examined and photographed using a Leica (Eaton, PA) DMRB epifluorescence microscope.
III. RESULTS
A. Electrophysiology
Complete data sets were collected from 4 guinea pigs. Surgery-induced changes in ABR thresholds were generally 20 dB or less, although hearing loss was greater in one animal (P163, see Figure 1). Surgically-induced threshold deficits were greater than the changes of less than 10 dB in ABR (Prieskorn and Miller, 2000; Le Prell et al., 2004) and psychophysical detection (Le Prell et al., 2004) thresholds reported previously as a consequence of intra-cochlear insertion of polyimide tubing (outer diameter = 0.16 mm). One possibility is that the larger fenestra required for inserting the ball electrode (outer diameter = 0.2-0.25 mm) led to greater surgical trauma. Consistent with this hypothesis, Carvalho and Lalwani (1999) described greater ABR threshold shifts (≥30 dB at 16 kHz and above) after implanting a larger intra-cochlear cannula (0.61 mm diameter).
Figure 1.
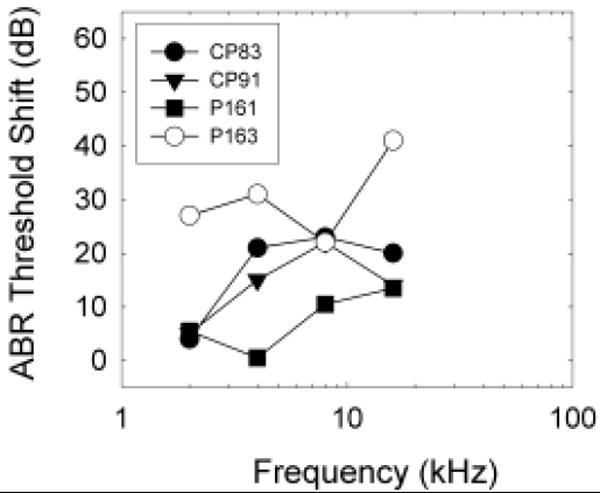
Surgery-induced threshold shift was assessed as change in auditory brainstem response (ABR) threshold at 2, 4, 8 and 16 kHz. Data are shown for individual animals (CP83, CP91, P161, P163) to facilitate comparisons with psychophysical and morphological data. Threshold deficits were typically 20 dB or less, although one animal (P163) had surgically induced threshold deficits of 20-40 dB, suggesting significant trauma during surgery. Consistent with this, we observed significant intra-cochlear bone growth in P163 at the time of euthanasia.
Electrically-evoked ABR neural response thresholds were less than 100 μA-peak, with normal growth of input-output functions observed from 100-1000 μA. In the one animal (P163) in which threshold was carefully measured using 10 μA steps, threshold was 47 μA.
B. Electrically Evoked Otoacoustic Emissions (EEOAEs)
EEOAEs were produced when current was applied to the cochlea for all animals except P163, who had significantly greater hearing loss post surgery. For CP83 and CP91, emissions were produced at current levels as low as 2 μA RMS at all stimulation frequencies (5.6, 8, 11.2, and 16 kHz), and EEOAE amplitude increased with current level. An EEOAE measured using a frequency sweep is illustrated in Figure 2 (P161). Emissions were comparable, in both shape and amplitude, to those recorded from normal animals that have not undergone daily electric stimulation (Halsey et al., 2006). To verify that the emissions we recorded depended on OHC integrity, EEOAEs were assessed in an animal deafened with subcutaneous kanamycin (400 mg/kg) followed 2 hours later by intra-venous ethacrynic acid (40 mg/kg). This treatment elevated ABR thresholds to acoustic stimulation to >100 dB SPL, eliminated virtually all OHCs (evaluated histologically), and depressed EEOAE amplitude as previously described by Nuttall and Ren (1995).
Figure 2.
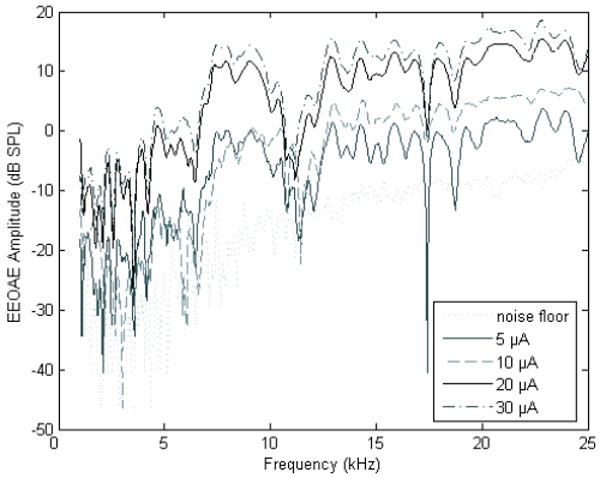
Electrically evoked otoacoustic emissions (EEOAEs) were assessed in response to intra-cochlear electrical stimulation using a frequency sweep paradigm. Emissions were assessed using this paradigm for a single animal (P161); testing was conducted on day 45 post-implant. For all other subjects, testing was limited to 5.6, 8, 11.2, and 16 kHz, with each frequency presented at 0 (noise floor), 5, 10, 20, and 30 μA as shown above using the frequency sweep paradigm. The results were equivalent in that EEOAE amplitude grew with increasing current level.
C. Psychophysical Testing: Baseline Functions
Examples of the dynamic ranges of acoustically and electrically evoked reactions are shown in Figure 3; data are illustrated for animals CP83, CP91, and P163. All subjects readily responded to electrical stimulation when transferred from the acoustic signal detection task to the electrical signal detection task. Acoustic data shown here were collected during the first month post-implant; electrical data shown here were collected during the next one-two months of testing. Dynamic range, i.e., the range of signal levels between maximum safe stimulation level (or, alternatively, the maximum level presented without discomfort) and minimum detectable level, was clearly narrower for electrical signals than for acoustic signals, a result that is consistent with numerous reports of narrower dynamic range for electrical signals. We monitored maximum comfort level by increasing maximum stimulus levels in small increments while carefully monitoring subject responses via a video camera located inside the test chamber with a monitor located remotely, outside the test chamber. Any signal levels that appeared to result in discomfort, evidenced for example, by an animal ‘leaping’ off the response key and hesitating to initiate a new trial, were not repeated.
Figure 3.
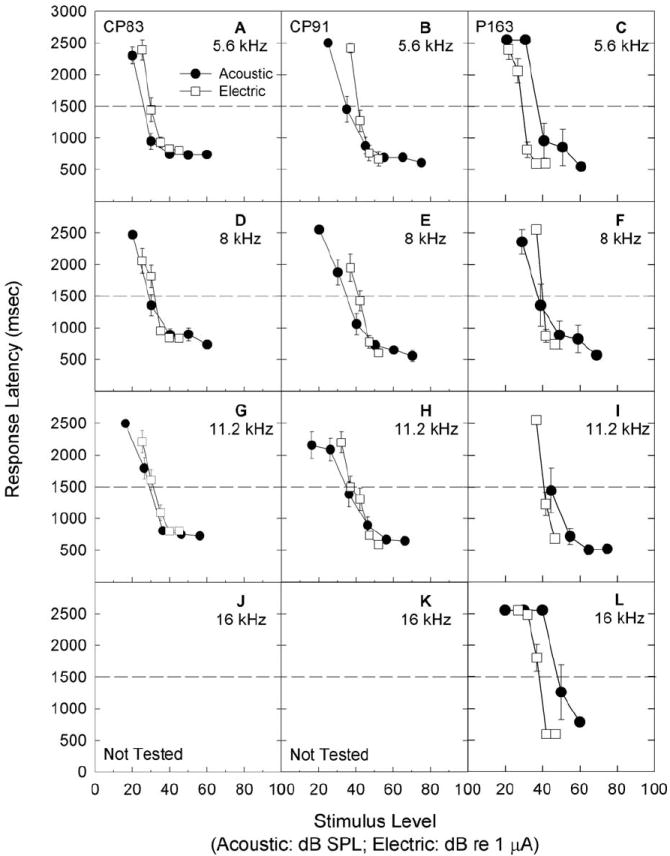
Subjects were trained to report detection of 5.6 (A, B, C), 8 (D, E, F), 11.2 (G, H, I), and 16 (J, K, L) kHz acoustic signals. Once subjects were trained and producing reliable response latency functions, a process which typically required a total of 4-6 months, a ball electrode was implanted through the wall of the cochlea, into scala tympani, for delivery of sinusoidal electrical signals. Here, we illustrate detection response latency for 5.6, 8, 11.2, and 16 kHz acoustic test signals in quiet, with tests conducted over the first month post-surgery. Data are shown for subjects CP83 (A, D, G, J), CP91 (B, E, H, K) and P163 (C, F, I, J). Subjects were then switched to an electrical stimulus detection task. Subjects readily responded to electrical stimulation. Data shown here were collected during the first one-two months of testing; all data were collected in a quiet background. Responses were generally quite consistent, showing little improvement over any given one-two month test window, including the initial test period as illustrated here (response data are mean +/- S.E.). Threshold was defined as the sound level corresponding to a response latency of 1500 ms, see dashed lines.
Although responses indicating detection of electrical signals showed little change within any one-two month test window, detection threshold tended to improve over longer periods time. This result is shown in Figure 4, where response data for 8 kHz electrical signals is illustrated for each animal at multiple times across the one-two year experimental duration. In between the baseline re-evaluation periods shown in each graph, animals were required to detect other electrical targets in quiet, or to detect electrical targets in the presence of a background tone. Because the subjects showed significant improvement over time, in some cases up to 20 dB re 1 μA, the effects of the background acoustic tones were evaluated relative to the most recent tests in quiet.
Figure 4.
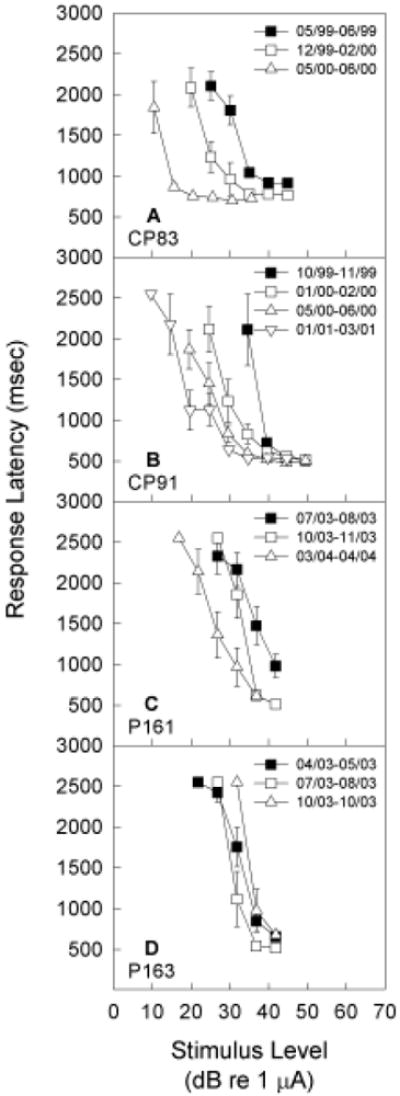
Subjects were re-tested in quiet in the detection task requiring response to 5.6, 8, 11.2, and 16 kHz sinusoidal signals delivered via intra-cochlear electrical stimulation of a ball electrode. Here, we illustrate detection response latency for 8 kHz test signals in quiet, with tests conducted at various times over 1-2 year periods. Pronounced improvement with long-term testing was observed for three subjects (CP83, CP91, P161), with thresholds improving up to 20 dB re 1 μA. All masking effects were therefore assessed relative to the most recent data in quiet.
D. Psychophysical Testing: Masking Functions
Preliminary manipulations conducted with two subjects (CP83, CP91) revealed that masking of the electric signal by an acoustic background was level-dependant. That is, when the acoustic stimulus and the electric target were the same frequency, increasing the level of the background tone increased masking (not depicted, see Le Prell et al., 2000). During the remainder of testing, masker level was fixed at 10-dB SL and masker frequency was varied. Figure 5 depicts the frequency specificity and magnitude of the masking effect, assessed as shift in electric threshold. Background tones produced frequency-specific masking of electric sinusoids in animals with normal acoustic thresholds when the electric sinusoid was at least 8 kHz (see Figure 5, panels D-L). Background tones had the greatest effect when the background tone and the electric stimulation fell within the same critical band (following Greenwood, 1961). These frequency-specific masking functions are consistent with characterizations of auditory nerve tuning that show very narrowly tuned (frequency specific) sound-driven responses at low signal levels, and broadly tuned responses across a wider range of frequencies at higher signal levels (Liberman, 1978; Winter et al., 1990; Wang et al., 1997). When tested with 10-dB SL background tones, the animal with high-frequency hearing loss (P163, see Figure 1) did not show frequency-specific masking effects with any electrical targets (Figure 5, panels N-P). The 5.6 kHz electric target was not masked by acoustic pure-tones for any of the animals (Figure 5, panels A-C and M).
Figure 5.
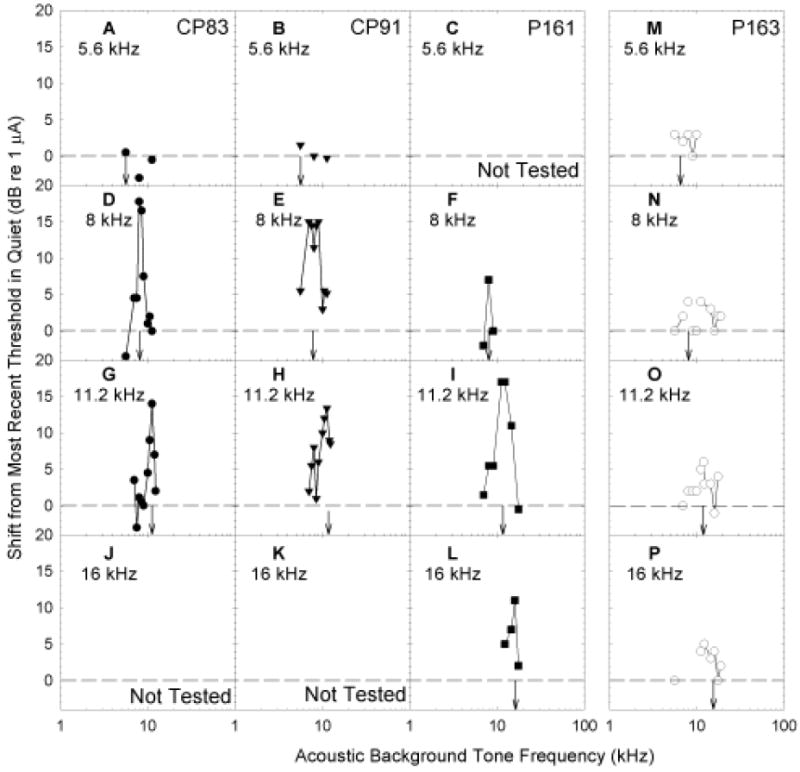
Subjects were trained to report detection of 5.6 (A, B, C, M), 8 (D, E, F, N), 11.2 (G, H, I, O), and 16 (J, K, L, P) kHz sinusoidal signals. Sinusoidal signals were acoustic during initial training, and later delivered using intra-cochlear electrical stimulation. Here, we illustrate the shift in electrical sinusoid detection thresholds when 10-dB SL acoustic pure-tone background maskers were presented (see x-axis). Target signal frequency is indicated in each panel using downwards arrows. Shift in detection threshold was calculated as the background-induced change from the most temporally proximate detection thresholds assessed in quiet; threshold shifts were greatest at frequencies closest to the electrical stimulation frequency except when the electrical target was a 5.6 kHz sinusoid. Anatomical evaluations were relatively normal in two animals (CP83, P161). The third animal (CP91) developed a sudden elevation in both acoustic and electrical signal detection thresholds and was found to have corresponding hair cell loss. In the fourth subject (P163, see right panels), the electrode was encased in bone.
E. Morphology
The organ of Corti from the implanted ears of two subjects (CP83, P161) exhibited only mild and scattered OHC loss (see Figure 6), consistent with age-related cell death (as described by Coleman, 1976). Little IHC loss was observed throughout the cochlear duct. Thus, the process of introducing the electrode into ears with intact hair cell populations and the daily electrical stimulation over extended temporal periods had no apparent negative consequences in these animals.
Figure 6.
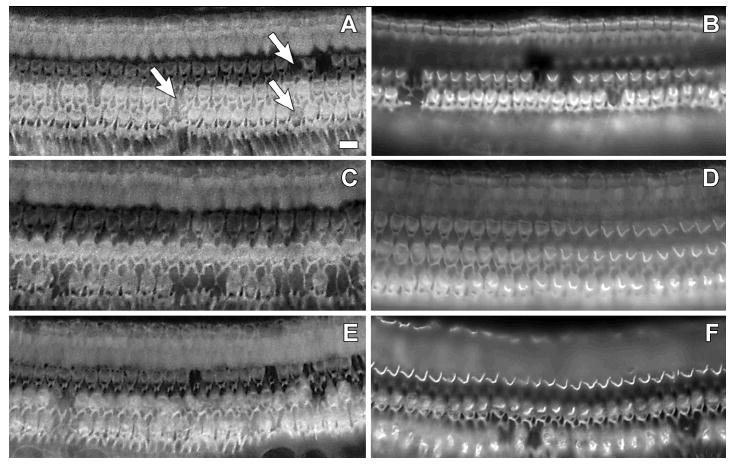
Whole-mount surface preparations of cochlear tissues from CP83 (left) and P161 (right). Actin filaments in the organ of Corti were labeled using rhodamine-phalloidin and visualized under epifluorescence. Tissues are from the basal turn (A, B), second turn (C, D), and third turn (E, F). Images were focused at the level of the OHC apical surface. Only mild and scattered OHC loss, consistent with age-related cell death (see Coleman 1976), was observed in these tissues (for examples, see arrows in panel A, where each arrow points at a site of a single missing OHC). IHC loss was generally not observed.
OHC loss for a third subject that underwent long-term daily electrical stimulation (CP91) was more widespread. While OHC loss was fairly limited in the basal turn (approximately 10%), scars indicating missing OHCs ranged from approximately 50% in the upper second turn to 85%-90% in the third turn and apex. This animal developed a hearing loss characterized by an abrupt and pronounced shift in electrical and acoustical detection thresholds approximately 21 months after the start of the daily electrical stimulation experiments. There was no evidence that this pathology progressed over time with the daily delivery of electric stimulation, as detection thresholds improved slowly over time throughout the first 21 months of testing (i.e., prior to the onset of the sudden and severe deficits). Two months later, after using behavioral techniques to characterize the hearing loss for both electrical and acoustic stimulation in this animal, it was euthanized. At that time, the OHC scars appeared thick and mature as previously described by Raphael and Altschuler (1991). IHC loss was limited to about 5% in the base and second turn, and 10% in the third turn and apex.
In the final animal to undergo long-term daily electrical stimulation tests (P163), the ball electrode was completely encased in bone inside the cochlea at the conclusion of the experiments. This bone appeared to project from the modiolus, such that a portion of the organ of Corti was also encased in bone. Evaluation of the organ of Corti revealed the first and second turns to have normal hair cell populations. In the third turn, there was approximately 15% OHC loss, predominantly within the third row of OHCs. IHC loss was limited to 8% in the third turn. Given the significant bone growth in this animal, in which the electrode was found to be encased in bone, it appears that surgical trauma clearly can induce a biological response. However, implants that are minimally invasive generally do not induce significant tissue response (see for example Brown et al., 1993; Le Prell et al., 2004); the current report of minimal tissue response across animals is consistent with results from earlier investigations using similarly minimally invasive implants.
IV. DISCUSSION
A. Electrical stimulation of ears with intact hair cells produces electrically-evoked otoacoustic emissions
EEOAEs, generated by forward and reverse traveling motion along the basilar membrane, have been interpreted by others as likely to be accompanied by an acoustic percept termed ‘electromotile hearing’ (Nuttall and Ren, 1995). In the current study, we used EEOAE measures primarily to confirm that sinusoidal electrical stimulation of our implants in our animals produces EEOAEs as described by others. For all but one test subject, EEOAE amplitude grew with current level, and with frequency; results that are equivalent to those described in detail by others. Although EEOAEs were smaller in amplitude at the lowest test frequency (5.6 kHz) for those animals tested at a restricted set of frequencies (i.e., 5.6, 8, 11.2, and 16 kHz); responses were clearly above the noise floor even at the lowest frequency, thus there was not a clear relationship between EEOAE presence and efficacy of background masking signals; i.e., the lack of frequency specific masking at 5.6 kHz was not accompanied by lack of an EEOAE in response to 5.6 kHz stimulation. This is clearly illustrated in Figure 2, which illustrates EEOAE responses at frequencies as low as 1 kHz. Because EEOAEs were evaluated in all subjects, we were also able to confirm that the single animal lacking an EEOAE in response to electrical stimulation (P163) had acoustic percepts that were distinctly different from those of other animals in which EEOAEs were generated by electrical stimulation of the implant.
B. Electrical stimulation of ears with intact hair cells produces frequency-specific masking, suggesting a tone-like sensation at higher frequencies
Anecdotal descriptions from human patients implanted with a cochlear prosthesis suggest the auditory percept associated with direct depolarization of auditory neurons is not tone-like (Jones et al., 1940; House and Urban, 1973; Bilger, 1977b; 1977a; Bilger and Black, 1977; Eddington et al., 1978; Tong et al., 1982; Watson et al., 1991; Dorman et al., 1994; Blamey et al., 1995; Collins et al., 1997). In contrast, preliminary data from a study in which electrical current was applied to a cochlea with intact hair cells suggest that electric stimulation of intact hair cells results in a tonal percept. In a pitch-matching experiment using a single human subject, sinusoidal electric stimulation (8 kHz) of an electrode placed on the promontory was matched in pitch to approximately 8 kHz acoustic stimulation in the contralateral ear (A. Nuttall, personal communication).
Interactions of electric and acoustic stimuli have been shown at the single-fiber level (Moxon, 1971; von Ilberg et al., 1999) and with the whole-nerve compound action potential (CAP). Specific evidence includes masking of the acoustically evoked CAP by electric pulses (McAnally et al., 1993; Kirk and Yates, 1994; McAnally and Clark, 1994; McAnally et al., 1997c; 1997a; 1997b) and masking of the electrically evoked CAP by acoustic stimuli (Aran et al., 1986; Kirk and Yates, 1994; James et al., 2001). Our experiments revealed an interaction of electric and acoustic stimulation at the perceptual level. Background acoustic pure-tones masked the detection of electrical stimulation provided that the stimulation frequencies were similar (i.e., within the same critical band) and that the electrical test frequency was 8 kHz or greater.
C. Does electrical stimulation of ears with intact hair cells produce an acoustic percept at lower frequencies?
There are several possible explanations for the observation that the 5.6 kHz electric sinusoid was not masked by acoustic stimulation. First, the 5.6 kHz electric stimulation may have resulted in an acoustic sensation that was not tone-like, such as would be expected if the auditory nerve were directly stimulated. A noise-like sensation would not be masked by pure-tone acoustic background tones. Alternatively, the 5.6 kHz electric stimulation may have produced a non-auditory sensation concurrent with, or, in the absence of, an auditory sensation. Thus, in the presence of the 5.6 kHz acoustic background, detection of an acoustic percept may have been masked but a nonauditory cue would provide a discriminative cue to perform the release response. Consistent with this suggestion, we observed a single guinea pig subject react to 4 kHz low-current electric stimulation as though the stimulation was painful. This stimulation was discontinued for this subject, and was not repeated with additional animals. Bipolar stimulation of the cochlea (i.e., stimulation of 2 intra-cochlear electrodes) was unpleasant when lower rates of pulsatile stimulation were applied near the round window (Fearn and Wolfe, 2000), and a single human subject described lower-frequency (2 kHz) electric stimulation delivered to the promontory as aversive (A. Nuttall, personal communication). Thus, some unknown tactile sensation may accompany lower frequency electric stimulation. That there may be an anomalous non-acoustic component to electric stimulation of the cochlea has been proposed previously, based on a single subject with consistent psychophysical detection responses in the absence of a reliable eABR (Miller et al., 1995a).
D. Electromotile hearing?
Acoustic percepts may have been elicited through one or more of four distinct mechanisms. First, the tonotopically resonant basilar membrane motion that produced the EEOAE may have resulted in deflection of IHC stereocilia, release of excitatory neurotransmitter substance by the IHCs (for review, see Le Prell et al., 2001), and auditory nerve activity (e.g., electromotile hearing). Alternatively, current injected into the cochlea may have directly stimulated spiral ganglion cells within the broad vicinity of the electrode (for discussion, see Spelman et al., 1980; 1982; Clopton and Spelman, 1995; Spelman et al., 1995; Kral et al., 1998). Data from Cohen et al. (2003) suggest that spread of excitation falls off fairly sharply, with most probe/masker electrode interactions occurring within a region of about 10% of the length of the organ of Corti either basal to or apical to the site of stimulation. The placement of the electrode at approximately 22.4 kHz can be presumed to be located approximately 15% of the total cochlear length measured from the base, and the highest electrical stimulation frequency of 16 kHz can be presumed to be located approximately 20% of the total cochlear length measured from the base (from Tsuji and Liberman, 1997, percent distance from base = 66.4 - 38.2 * log (kHz)). Thus, it is possible that there was some direct stimulation of auditory neurons located in the vicinity of the organ of Corti that would best respond to 16 kHz stimulation. However, it is unlikely that there was any significant direct neural excitation of neurons responding best to the 11.2 kHz or lower test frequencies, which are located 26% (11.2 kHz), 32% (8 kHz), and 38% (5.6 kHz) of the total cochlear length measured from the base. Arguing against the notion that there was significant direct electrical neural excitation at the 16 kHz place in the cochlea, we note that direct electrical stimulation is typically not described as tonal by human listeners, whereas the 16 kHz stimulation was masked only by background tones that were similar in frequency relative to the 16 kHz electrical target. While it is conceivable that stimulation of the IHCs by the intra-cochlear electrical current triggered the release of excitatory neurotransmitter and the consequent auditory nerve activity and perception of sound, electrical stimulation of the IHCs would be subject to the same spatial constraints imposed by limited current spread as described for direct neural stimulation. Finally, while it is conceivable that stimulation of the facial nerve or vestibular system could occur, sensations generated via this mechanism would not be masked by pure-tone acoustic signals.
Based on the frequency map of the guinea pig cochlea provided by Tsuji and Liberman (1997), and the demonstration by Cohen et al. (2003) that spread of excitation falls off fairly sharply at distance extending beyond about 10% of the length of the organ of Corti, we consider it unlikely that direct electrical stimulation of auditory hair cells or auditory neurons generated the tone-like percepts associated with electrical stimulation at the higher stimulation frequencies. We can further attempt to discriminate among these mechanisms by estimating the thresholds for direct electrical stimulation of auditory neurons. Thresholds for detection of neural stimulation, in the absence of hair cell stimulation, are available from animals in which hair cell populations were eliminated with ototoxic drugs prior to implant with a cochlear prosthesis. eABR thresholds for our animals (with intact hair cell populations) were generally consistent with those reported by A. L. Miller and colleagues (1999), and somewhat lower than those reported by C. A. Miller and colleagues (1995b). From the limited existing data sets, we know that guinea pig eABR thresholds are approximately 16 μA for 3 kHz stimulation (Miller et al., 1999), and that neural stimulation thresholds increase by approximately 4 dB/octave (in monkeys, see Pfingst, 1984). Based on these data sets, we calculate that average detection thresholds for auditory nerve stimulation (in the absence of intact hair cell populations) might be expected to be approximately 25 μA RMS at 6 kHz, 40 μA RMS at 12 kHz, and 63 μA RMS at 24 kHz. Electric detection thresholds for 8 kHz (bipolar) sinusoidal stimulation in a single cat (see Smith et al., 1995) are consistent with the predicted thresholds (see Figure 7). Although the data sets are disparate, including psychophysical and electrophysiological thresholds for sinusoidal, as well as monophasic/monopolar and biphasic/bipolar signals, psychophysical and electrophysiological thresholds are typically well-correlated (Borg and Engstrom, 1983; Miller et al., 1995b; Szymanski et al., 1999; Wolski et al., 2003; Le Prell et al., 2004).
Figure 7.
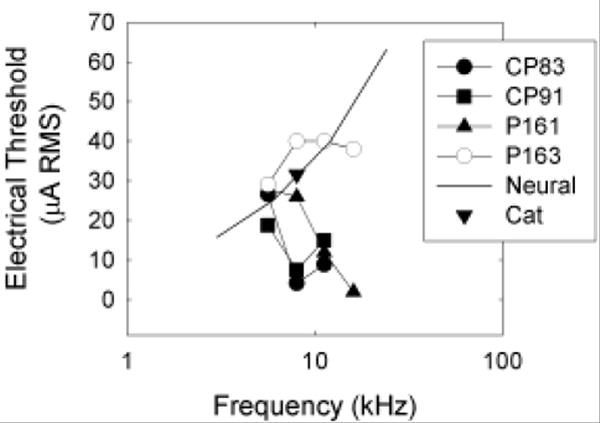
Detection thresholds for electrical stimulation are plotted at all test frequencies (5.6, 8, 11.2, and 16 kHz) for each of our subjects (CP83, CP91, P161, P163). In addition, we plot the lowest current level predicted to result in detectable electrical stimulation of auditory neurons in animals without outer hair cells (“Neural”). Electric detection thresholds for 8 kHz (bipolar) sinusoidal stimulation in a single deafened cat (taken from Smith et al., 1995) are consistent with the “predicted” neural thresholds. Empirically determined thresholds lower than the predicted neural stimulation thresholds are interpreted as the first direct perceptual evidence for the psychological phenomena termed “electromotile hearing.”
At our lowest test frequency (5.6 kHz), guinea pig thresholds were generally equivalent to predicted thresholds for detection of direct neural stimulation (see Figure 7). Thus, direct neural stimulation probably provided a salient cue for detection of 5.6 kHz electric sinusoids. This effect is consistent with the failure of pure-tone acoustic signals to mask detection of the 5.6 kHz electric sinusoid (see Figure 5A-C, 5M). At higher test frequencies, most subjects (CP83, CP91, P161) detected sinusoidal electric stimulation at current levels well below those predicted to result in neural stimulation (see Figure 7). These responses we interpret as electromotile hearing. Consistent with the purported nature of electromotile hearing, pure-tone acoustic stimuli had frequency-specific masking effects at 8, 11.2, and 16 kHz (see Figure 5D-L). In contrast, our subject P163, for whom the electrode was found to be encased in bone, had thresholds that were approximately equal to the predicted neural stimulation thresholds (Figure 7). Consistent with the premise that direct neural stimulation does not result in a tone-like percept, we did not observe strong evidence of frequency specific masking for this subject (see Figure 5M-P).
An alternative experimental approach that would have allowed more direct comparison of electrophonic responses and those driven by direct depolarization would include ototoxic drug treatment following the collection of baseline electrical signal detection data. Ototoxic drugs would eliminate the electrophonic response, without disrupting the neural response driven by direct depolarization of auditory neurons. We considered this approach for our investigation; however, this approach does not permit us to identify any disruption of hair cell survival as a consequence of chronic electrical stimulation. Therefore, we did not choose to use this experimental approach. Such manipulations may represent an interesting approach for future investigations designed to clarify the mechanisms of perception across stimulation levels.
E. Electrical stimulation of ears with intact hair cells does not damage these cells
Although some patterns of electrical stimulation (i.e., continuous low-frequency stimulation) can be detrimental to the survival of cochlear hair cells (Duckert, 1983; Duckert and Miller, 1984), other stimulation patterns do not produce functional or morphological deficits (Ni et al., 1992). One animal in the present study demonstrated a sudden shift in thresholds following 21 months of testing (see section III-E, Morphology), but there was no evidence of any progressive deficits prior to this time. Data from our experiments with guinea pigs thus importantly suggest that intact hair cell populations are not damaged by daily electrical stimulation at levels that result in acoustic sensations. These data significantly extend the duration of testing from 85 days (von Ilberg et al., 1999), during which time no changes in CAP were observed, to approximately two years (i.e., in the current experiments).
F. Clinical Utility
The treatment of choice for those with profound hearing loss is the cochlear prosthesis; a device that directly stimulates the auditory nerve using electrical signals. Users of this device can comprehend open-set speech information without the aid of lip-reading (Skinner et al., 1994; 1997; Waltzman et al., 1997; Firszt et al., 2004). Historically, the benefits of the implant have been dependant on the density and excitability of surviving auditory nerve fibers (Clopton et al., 1980; Nadol et al., 1989; Incesulu and Nadol, 1998; see also Skinner et al., 2002). However, as the criteria for implanting human patients with cochlear prostheses relax (NIH Consensus Statement, 1995), patients with greater residual hearing are being implanted with intra-cochlear electrode arrays.
Gantz and colleagues recently began to implant shorter (10 mm) intracochlear electrode arrays that preserve intact low-frequency hearing. They demonstrated that electrical stimulation of the base of the cochlea combined with acoustic stimulation of the intact apical cochlea (via hearing aid use) enhances perceptual experience for cochlear implant users (Gantz et al., 2000; Tyler et al., 2000; Gantz and Turner, 2003; 2004; Turner et al., 2004). Similar results have been described in a case study by Skarzynski et al. (2003) and also by Kiefer and colleagues (von Ilberg et al., 1999; Kiefer et al., 2005), who report that patients can ‘merge’ acoustic and electric stimuli into a unified sensation that is more pleasant than either the electric or acoustic component alone.
The suggestion that cochlear implant users with some residual hearing could benefit from cochlear stimulation strategies that take advantage of intact OHC populations is increasingly popular (Risberg et al., 1990; McAnally and Clark, 1994; Nuttall and Ren, 1995; McAnally et al., 1997b). By minimizing surgical trauma during cochlear implant procedures, residual populations of hair cells may be retained, resulting in superior implant performance. This concept of “soft surgery” for cochlear implants was first articulated by Lehnhardt (1993), and later described by Cohen (1997). In a population of 26 human patients implanted using the soft surgery technique, 62% retained their residual hearing 1-month post-operative whereas 5% lost all residual hearing (Skarzynski et al., 2002). The morphology of OHCs was normal following surgery designed to minimize trauma in guinea pigs euthanized 2 to 6 weeks post-implant (Rogowski et al., 1995).
The current data set extends our understanding of interactions occurring as a consequence of combined use of acoustic and electric signals. Specifically, the current data illustrate the potential for generation of tone-like percepts with electromotile stimulation in the patient with a sufficient population of surviving hair cells. Improving the perception of tonal signals would have significant benefit for perception of music, which is typically quite poor in implant users (McDermott, 2004; Rubinstein, 2004; e.g., Gantz et al., 2005; Gfeller et al., 2005; Laneau et al., 2006). Presumably, benefits of electromotile stimulation would also include improved speech discrimination by speakers of tonal languages (Huang et al., 1995; 1996; Fu et al., 1998; Lan et al., 2004; Huang et al., 2005; Lee and van Hasselt, 2005).
G. Conclusions
Psychophysical tuning curves are the gold standard for frequency-specific processing in the auditory periphery. Thus, these data provide clear and robust evidence of frequency-specific perception with electrical stimulation of the intact cochlea. Taken together, our results indicate intra-cochlear electric stimulation of OHCs in intact ears produces an acoustic sensation that is tone-like at least at higher frequencies (i.e., at or above 8 kHz). This behavioral result implies an electrically evoked traveling wave that is “acoustic-like.” Our results suggest that if intact OHCs are present in cochleae with residual hearing, then stimulation of these hair cells may result in an acoustic sensation. These results may help to explain the recent observations that the perceptual experience of cochlear prosthesis users is improved by stimulation of remaining OHCs in patients that use both a hearing aid, and a cochlear prosthesis (von Ilberg et al., 1999; Kiefer et al., 2005).
Acknowledgments
This research was supported by the National Organization for Hearing Research (CGL) and the Royal National Institute for the Deaf (YR) and by grants from the National Institutes of Health-National Institute of Deafness and Other Communication Disorders [NIH-NIDCD]: P01-DC00078 (DFD, DBM, YR), F32-DC00367 (CGL), and P30-DC05188]. We are grateful to David Moody for his expertise and assistance with the conduct of these experiments. We thank Masahiko Izumikawa and Ryosei Minoda for their expert assistance with histological procedures. We thank Kathryn Abele, Rebecca Diener, Elizabeth Hand, Edwin Labut, and Catherine Thompson, for participating in the daily care and testing of these animals. We thank Amy Miller, John Middlebrooks, and Alfred Nuttall for valuable comments on earlier versions of this manuscript. Finally, we thank James Beals, Chris Ellinger, Kärin Halsey, Robert Masta, Alice Mitchell, Diane Prieskorn, and Donald Swiderski, for technical contributions.
Footnotes
Portions of this research were presented at the Midwinter Meetings of the Association for Research in Otolaryngology (for abstracts, see Le Prell et al., 2000; 2002).
References
- Aran JM, Erre JP, Hiel H, Charlet de Sauvage R, Goeury P, Rouanet JF. Investigation of cochlear mechanisms using combined acoustical and electrical stimulations. Scand Audiol Suppl. 1986;25:63–69. [PubMed] [Google Scholar]
- Ashmore JF. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol (Lond) 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger RC. Psycoacoustic evaluation of current prostheses. Ann Otol Rhinol Laryngol Suppl. 1977a;86:92–140. [PubMed] [Google Scholar]
- Bilger RC. Electrical stimulation of the auditory nerve and auditory prostheses: a review of the literature. Ann Otol Rhinol Laryngol Suppl. 1977b;86:11–20. doi: 10.1177/00034894770860s302. [DOI] [PubMed] [Google Scholar]
- Bilger RC, Black FO. Auditory prostheses in perspective. Ann Otol Rhinol Laryngol Suppl. 1977;86:3–10. doi: 10.1177/00034894770860s301. [DOI] [PubMed] [Google Scholar]
- Black RC, Clark GM, O’Leary SJ, Walters C. Intracochlear electrical stimulation of normal and deaf cats investigated using brainstem response audiometry. Acta Otolaryngol Suppl (Stockh) 1983;399:5–17. doi: 10.3109/00016488309105588. [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Parisi ES, Clark GM. Pitch matching of electric and acoustic stimuli. Ann Otol Rhinol Laryngol Suppl. 1995;166:220–222. [PubMed] [Google Scholar]
- Borg E, Engstrom B. Hearing thresholds in the rabbit. A behavioral and electrophysiological study. Acta Otolaryngol (Stockh) 1983;95:19–26. doi: 10.3109/00016488309130911. [DOI] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20:87–90. [PubMed] [Google Scholar]
- Clopton BM, Spelman FA, Miller JM. Estimates of essential neural elements for stimulation through a cochlear prosthesis. Ann Otol Rhinol Laryngol Suppl. 1980;89:5–7. doi: 10.1177/00034894800890s202. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Spelman FA. Electrode configuration and spread of neural excitation: compartmental models of spiral ganglion cells. Ann Otol Rhinol Laryngol Suppl. 1995;166:115–118. [PubMed] [Google Scholar]
- Cohen LT, Richardson LM, Saunders E, Cowan RSC. Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hear Res. 2003;179:72–87. doi: 10.1016/s0378-5955(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg. 1997;117:214–216. doi: 10.1016/s0194-5998(97)70176-1. [DOI] [PubMed] [Google Scholar]
- Coleman JW. Hair cell loss as a function of age in the normal cochlea of the guinea pig. Acta Otolaryngol (Stockh) 1976;82:33–40. doi: 10.3109/00016487609120860. [DOI] [PubMed] [Google Scholar]
- Collins LM, Zwolan TA, Wakefield GH. Comparison of electrode discrimination, pitch ranking, and pitch scaling data in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am. 1997;101:440–455. doi: 10.1121/1.417989. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Smith M, Smith L, Parkin JL. The pitch of electrically presented sinusoids. J Acoust Soc Am. 1994;95:1677–1679. doi: 10.1121/1.408558. [DOI] [PubMed] [Google Scholar]
- Duckert LG. Morphological changes in the normal and neomycin-perfused guinea pig cochlea following chronic prosthetic implantation. Laryngoscope. 1983;93:841–855. doi: 10.1288/00005537-198307000-00001. [DOI] [PubMed] [Google Scholar]
- Duckert LG, Miller JM. Morphological changes following cochlear implantation in the animal model. Acta Otolaryngol Suppl (Stockh) 1984;411:28–37. [PubMed] [Google Scholar]
- Eddington DK, Dobelle WH, Brackmann DE, Mladejovsky MG, Parkin JL. Auditory prostheses research with multiple channel intracochlear stimulation in man. Ann Otol Rhinol Laryngol. 1978;87:1–39. [PubMed] [Google Scholar]
- Fearn R, Wolfe J. Relative importance of rate and place: experiments using pitch scaling techniques with cochlear implants recipients. Ann Otol Rhinol Laryngol Suppl. 2000;185:51–53. doi: 10.1177/0003489400109s1221. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Skinner MW, Tobey EA, Peterson A, Gaggl W, Runge-Samuelson CL, Wackym PA. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear. 2004;25:375–387. doi: 10.1097/01.aud.0000134552.22205.ee. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Zeng FG, Shannon RV, Soli SD. Importance of tonal envelope cues in Chinese speech recognition. J Acoust Soc Am. 1998;104:505–510. doi: 10.1121/1.423251. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Rubinstein JT, Tyler RS, Teagle HF, Cohen NL, Waltzman SB, Miyamoto RT, Kirk KI. Long-term results of cochlear implants in children with residual hearing. Ann Otol Rhinol Laryngol Suppl. 2000;185:33–36. doi: 10.1177/0003489400109s1214. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol (Stockh) 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gfeller K, Olszewski C, Rychener M, Sena K, Knutson JF, Witt S, Macpherson B. Recognition of “real-world” musical excerpts by cochlear implant recipients and normal-hearing adults. Ear Hear. 2005;26:237–250. doi: 10.1097/00003446-200506000-00001. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. Critical bandwidth and the frequency coordinates of the basilar membrane. J Acoust Soc Am. 1961;33:1344–1356. [Google Scholar]
- Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res. 1990;49:155–168. doi: 10.1016/0378-5955(90)90102-u. [DOI] [PubMed] [Google Scholar]
- Halsey K, Fegelman K, Raphael Y, Grosh K, Dolan DF. Long-term effects of acoustic trauma on the electrically-evoked otoacoustic emission. J Assoc Res Otolaryngol. 2006;6:324–340. doi: 10.1007/s10162-005-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House WF, Urban J. Long term results of electrode implantation and electronic stimulation of the cochlea in man. Ann Otol. 1973;82:504–517. doi: 10.1177/000348947308200408. [DOI] [PubMed] [Google Scholar]
- Huang CY, Yang HM, Sher YJ, Lin YH, Wu JL. Speech intelligibility of Mandarin-speaking deaf children with cochlear implants. Int J Pediatr Otorhinolaryngol. 2005;69:505–511. doi: 10.1016/j.ijporl.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Huang TS, Wang NM, Liu SY. Tone perception of Mandarin-speaking postlingually deaf implantees using the Nucleus 22-Channel Cochlear Mini System. Ann Otol Rhinol Laryngol Suppl. 1995;166:294–298. [PubMed] [Google Scholar]
- Huang TS, Wang NM, Liu SY. Nucleus 22-channel cochlear mini-system implantations in Mandarin-speaking patients. Am J Otol. 1996;17:46–52. [PubMed] [Google Scholar]
- Hubbard AE, Mountain DC. Alternating current delivered into the scala media alters sound pressure at the eardrum. Science. 1983;222:510–512. doi: 10.1126/science.6623090. [DOI] [PubMed] [Google Scholar]
- Incesulu A, Nadol JB., Jr Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1998;107:906–911. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- James C, Blamey P, Shallop JK, Incerti PV, Nicholas AM. Contralateral masking in cochlear implant users with residual hearing in the non-implanted ear. Audiol Neurootol. 2001;6:87–97. doi: 10.1159/000046814. [DOI] [PubMed] [Google Scholar]
- Jones RC, Stevens SS, Lurie MH. Three mechanisms of hearing by electrical stimulation. J Acoust Soc Am. 1940;12:281–290. [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Pok M, Adunka O, Sturzebecher E, Baumgartner W, Schmidt M, Tillein J, Ye Q, Gstoettner W. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol. 2005;10:134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Kirk DL, Yates GK. Evidence for electrically evoked travelling waves in the guinea pig cochlea. Hear Res. 1994;74:38–50. doi: 10.1016/0378-5955(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hear Res. 1998;121:11–28. doi: 10.1016/s0378-5955(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Lan N, Nie KB, Gao SK, Zeng FG. A novel speech-processing strategy incorporating tonal information for cochlear implants. IEEE Trans Biomed Eng. 2004;51:752–760. doi: 10.1109/TBME.2004.826597. [DOI] [PubMed] [Google Scholar]
- Laneau J, Wouters J, Moonen M. Improved music perception with explicit pitch coding in cochlear implants. Audiol Neurootol. 2006;11:38–52. doi: 10.1159/000088853. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Dolan D, Moody DB. Electromotile hearing: Evidence that tone-like percepts are produced by electrical stimulation in the cochlea. Abs Assoc Res Otolaryngol. 2000;23:259. [Google Scholar]
- Le Prell CG, Bledsoe SC, Jr, Bobbin RP, Puel JL. Neurotransmission in the inner ear: Functional and molecular analyses. In: Jahn AF, Santos-Sacchi J, editors. Physiology of the Ear. Singular Publishing; New York: 2001. pp. 575–611. [Google Scholar]
- Le Prell CG, Kawamoto K, Raphael Y, Dolan D, Moody DB. Electromotile hearing: Evidence that tone-like percepts are produced by electrical stimulation of cochlear outer hair cells. Abs Assoc Res Otolaryngol. 2002;25:165. [Google Scholar]
- Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan D, Bledsoe SC, Jr, Moody DB. Chronic infusion of AMPA into the guinea pig cochlea induces temporary functional deficits and long-term morphological trauma. J Acoust Soc Am. 2004;116:1044–1056. doi: 10.1121/1.1772395. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Halsey K, Hughes LF, Dolan DF, Bledsoe SC., Jr Disruption of lateral olivocochlear neurons via a dopaminergic neurotoxin depresses sound-evoked auditory nerve activity. J Assoc Res Otolaryngol. 2005;6:48–62. doi: 10.1007/s10162-004-5009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, van Hasselt CA. Spoken word recognition in children with cochlear implants: a five-year study on speakers of a tonal language. Ear Hear. 2005;26 doi: 10.1097/00003446-200508001-00005. [DOI] [PubMed] [Google Scholar]
- Lehnhardt E. Intracochleare plazierung der cochlear-implant-elektroden in soft surgery technique. HNO. 1993;41:356–359. [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Clark GM, Syka J. Hair cell mediated responses of the auditory nerve to sinusoidal electrical stimulation of the cochlea in the cat. Hear Res. 1993;67:55–68. doi: 10.1016/0378-5955(93)90232-p. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Clark GM. Stimulation of residual hearing in the cat by pulsatile electrical stimulation of the cochlea. Acta Otolaryngol (Stockh) 1994;114:366–372. doi: 10.3109/00016489409126071. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Brown M, Clark GM. Estimating mechanical responses to pulsatile electrical stimulation of the cochlea. Hear Res. 1997a;106:146–153. doi: 10.1016/s0378-5955(97)00012-9. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Brown M, Clark GM. Comparison of current waveforms for the electrical stimulation of residual low frequency hearing. Acta Otolaryngol (Stockh) 1997b;117:831–835. doi: 10.3109/00016489709114209. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Brown M, Clark GM. Acoustic and electric forward-masking of the auditory nerve compound action potential: evidence for linearity of electromechanical transduction. Hear Res. 1997c;106:137–145. doi: 10.1016/s0378-5955(97)00011-7. [DOI] [PubMed] [Google Scholar]
- McDermott HJ. Music perception with cochlear implants: a review. Trends in Amplification. 2004;8:49–82. doi: 10.1177/108471380400800203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Smith DW, Pfingst BE. Across-species comparisons of psychophysical detection thresholds for electrical stimulation of the cochlea: II. Strength-duration functions for single, biphasic pulses. Hear Res. 1999;135:47–55. doi: 10.1016/s0378-5955(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Miller CA, Faulkner MJ, Pfingst BE. Functional responses from guinea pigs with cochlear implants. II. Changes in electrophysiological and psychophysical measures over time. Hear Res. 1995a;92:100–111. doi: 10.1016/0378-5955(95)00205-7. [DOI] [PubMed] [Google Scholar]
- Miller CA, Woodruff KE, Pfingst BE. Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures. Hear Res. 1995b;92:85–99. doi: 10.1016/0378-5955(95)00204-9. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Moody DB. Reaction time as an index of sensory function. In: Stebbins WC, editor. Animal Psychophysics: The Design and Conduct of Psychophysical Experiments. Appleton-Century-Crofts; New York: 1970. pp. 277–302. [Google Scholar]
- Moxon EC. Submitted to the Department of Electrical Engineering. Massachusetts Institute of Technology; Cambridge: 1971. Neural and Mechanical Responses to Electrical Stimulation of the Cat’s Inner Ear. [Google Scholar]
- Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nakajima HH, Mountain DC, Hubbard AE. Nonlinear characteristics of electrically evoked otoacoustic emissions. Hear Res. 1998;122:109–118. doi: 10.1016/s0378-5955(98)00094-x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington D.C: 1996. [Google Scholar]
- Ni D, Shepherd RK, Seldon HL, Xu SA, Clark GM, Millard RE. Cochlear pathology following chronic electrical stimulation of the auditory nerve. I: Normal hearing kittens. Hear Res. 1992;62:63–81. doi: 10.1016/0378-5955(92)90203-y. [DOI] [PubMed] [Google Scholar]
- Niemiec AJ, Moody DB. Constant stimulus and tracking procedures for measuring sensitivity. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Vol. 6. Birkhauser Verlag, Basel; 1995. pp. 65–77. [Google Scholar]
- NIH Consensus Statement. Cochlear implants in adults and children. NIH Consens Statement. 1995;13:1–30. [PubMed] [Google Scholar]
- Nuttall AL, Dolan DF. Basilar membrane velocity responses to acoustic and intracochlear electric stimuli. In: Duifuis H, Horst JW, van Dijk P, van Netten SM, editors. Biophysics of Hair Cell Sensory Systems. World Scientific; Singapore: 1993. pp. 288–295. [Google Scholar]
- Nuttall AL, Ren T. Electromotile hearing: evidence from basilar membrane motion and otoacoustic emissions. Hear Res. 1995;92:170–177. doi: 10.1016/0378-5955(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Nuttall AL, Zheng J, Ren T, de Boer E. Electrically evoked otoacoustic emissions from apical and basal perilymphatic electrode positions in the guinea pig cochlea. Hear Res. 2001;152:77–89. doi: 10.1016/s0378-5955(00)00238-0. [DOI] [PubMed] [Google Scholar]
- Pfingst BE. Operating ranges and intensity psychophysics for cochlear implants. Implications for speech processing strategies. Arch Otolaryngol. 1984;110:140–144. doi: 10.1001/archotol.1984.00800290004002. [DOI] [PubMed] [Google Scholar]
- Prieskorn DM, Miller JM. Technical report: chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140:212–215. doi: 10.1016/s0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton. 1991;18:215–227. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- Ren T, Nuttall AL. Extracochlear electrically evoked otoacoustic emissions: a model for in vivo assessment of outer hair cell electromotility. Hear Res. 1995;92:178–183. doi: 10.1016/0378-5955(95)00217-0. [DOI] [PubMed] [Google Scholar]
- Reuter G, Zenner HP. Active radial and transverse motile responses of outer hair cells in the organ of Corti. Hear Res. 1990;43:219–230. doi: 10.1016/0378-5955(90)90230-m. [DOI] [PubMed] [Google Scholar]
- Reyes S, Ding D, Sun W, Salvi R. Effect of inner and outer hair cell lesions on electrically evoked otoacoustic emissions. Hear Res. 2001;158:139–150. doi: 10.1016/s0378-5955(01)00309-4. [DOI] [PubMed] [Google Scholar]
- Risberg A, Agelfors E, Lindstrom B, Bredberg G. Electrophonic hearing and cochlear implants. Acta Otolaryngol Suppl (Stockh) 1990;469:156–163. [PubMed] [Google Scholar]
- Rogowski M, Reiss G, Lehnhardt E. Morphologic study of the guinea pig cochlea after cochlear implantation using the “soft surgery” technique. Ann Otol Rhinol Laryngol Suppl. 1995;166:434–436. [PubMed] [Google Scholar]
- Rubinstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004;12:444–448. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, D’Haese P, Walkowiak A, Piotrowska A, Sliwa L, Anderson I. Preservation of residual hearing in children and post-lingually deafened adults after cochlear implantation: an initial study. ORL J Otorhinolaryngol Relat Spec. 2002;64:247–253. doi: 10.1159/000064134. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9:CS20–24. [PubMed] [Google Scholar]
- Skinner MW, Clark GM, Whitford LA, Seligman PM, Staller SJ, Shipp DB, Shallop JK, Everingham C, Menapace CM, Arndt PL, et al. Evaluation of a new spectral peak coding strategy for the Nucleus 22 Channel Cochlear Implant System. Am J Otol. 1994;15(Suppl 2):15–27. [PubMed] [Google Scholar]
- Skinner MW, Holden LK, Holden TA, Demorest ME, Fourakis MS. Speech recognition at simulated soft, conversational, and raised-to-loud vocal efforts by adults with cochlear implants. J Acoust Soc Am. 1997;101:3766–3782. doi: 10.1121/1.418383. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Ketten DR, Holden LK, Harding GW, Smith PG, Gates GA, Neely JG, Kletzker GR, Brunsden B, Blocker B. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3:332–350. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DW, Watt S, Konrad KE, Olszyk VB. Behavioral auditory thresholds for sinusoidal electrical stimuli in the cat. J Acoust Soc Am. 1995;98:211–220. doi: 10.1121/1.413755. [DOI] [PubMed] [Google Scholar]
- Spelman FA, Pfingst BE, Miller JM, Hassul M, Powers WE, Clopton BM. Biophysical measurements in the implanted cochlea. Otolaryngol Head Neck Surg. 1980;88:183–187. [PubMed] [Google Scholar]
- Spelman FA, Clopton BM, Pfingst BE. Tissue impedance and current flow in the implanted ear. Implications for the cochlear prosthesis. Ann Otol Rhinol Laryngol Suppl. 1982;98:3–8. [PubMed] [Google Scholar]
- Spelman FA, Pfingst BE, Clopton BM, Jolly CN, Rodenhiser KL. Effects of electrical current configuration on potential fields in the electrically stimulated cochlea: field models and measurements. Ann Otol Rhinol Laryngol Suppl. 1995;166:131–136. [PubMed] [Google Scholar]
- Szymanski MD, Bain DE, Kiehl K, Pennington S, Wong S, Henry KR. Killer whale (Orcinus orca) hearing: auditory brainstem response and behavioral audiograms. J Acoust Soc Am. 1999;106:1134–1141. doi: 10.1121/1.427121. [DOI] [PubMed] [Google Scholar]
- Tong YC, Clark GM, Blamey PJ, Busby PA, Dowell RC. Psychophysical studies for two multiple-channel cochlear implant patients. J Acoust Soc Am. 1982;71:153–160. doi: 10.1121/1.387342. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Turner CW, Gantz BJ, Vidal C, Behrens A, Henry BA. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115:1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- Tykocinski M, Shepherd RK, Clark GM. Electrophysiologic effects following acute intracochlear direct current stimulation of the guinea pig cochlea. Ann Otol Rhinol Laryngol Suppl. 1995;166:68–71. [PubMed] [Google Scholar]
- Tyler RS, Kelsay DM, Teagle HF, Rubinstein JT, Gantz BJ, Christ AM. 7-year speech perception results and the effects of age, residual hearing and preimplant speech perception in prelingually deaf children using the Nucleus and Clarion cochlear implants. Adv Otorhinolaryngol. 2000;57:305–310. doi: 10.1159/000059134. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebecher E, Klinke R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61:334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Gomolin RH, Green JE, Shapiro WH, Hoffman RA, Roland JT., Jr Open-set speech perception in congenitally deaf children using cochlear implants. Am J Otol. 1997;18:342–349. [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107:67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Watson CS, Dobie RA, Durlach N, Humes LE, Levitt H, Miller JD, Sherrick CE, Simmons FB, Studebaker GA, Tyler RS, Widin GP. Speech-perception aids for hearing-impaired people: current status and needed research. Working Group on Communication Aids for the Hearing-Impaired. J Acoust Soc Am. 1991;90:637–683. [PubMed] [Google Scholar]
- Winter IM, Robertson D, Yates GK. Diversity of characteristic frequency rate-intensity functions in guinea pig auditory nerve fibres. Hear Res. 1990;45:191–202. doi: 10.1016/0378-5955(90)90120-e. [DOI] [PubMed] [Google Scholar]
- Wolski LF, Anderson RC, Bowles AE, Yochem PK. Measuring hearing in the harbor seal (Phoca vitulina): Comparison of behavioral and auditory brainstem response techniques. J Acoust Soc Am. 2003;113:629–637. doi: 10.1121/1.1527961. [DOI] [PubMed] [Google Scholar]
- Xue S, Mountain DC, Hubbard AE. Acoustic enhancement of electrically-evoked otoacoustic emissions reflects basilar membrane tuning: experiment results. Hear Res. 1993;70:121–126. doi: 10.1016/0378-5955(93)90056-7. [DOI] [PubMed] [Google Scholar]
- Xue S, Mountain DC, Hubbard AE. Electrically evoked basilar membrane motion. J Acoust Soc Am. 1995;97:3030–3041. doi: 10.1121/1.413103. [DOI] [PubMed] [Google Scholar]


